Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review
Abstract
1. Introduction
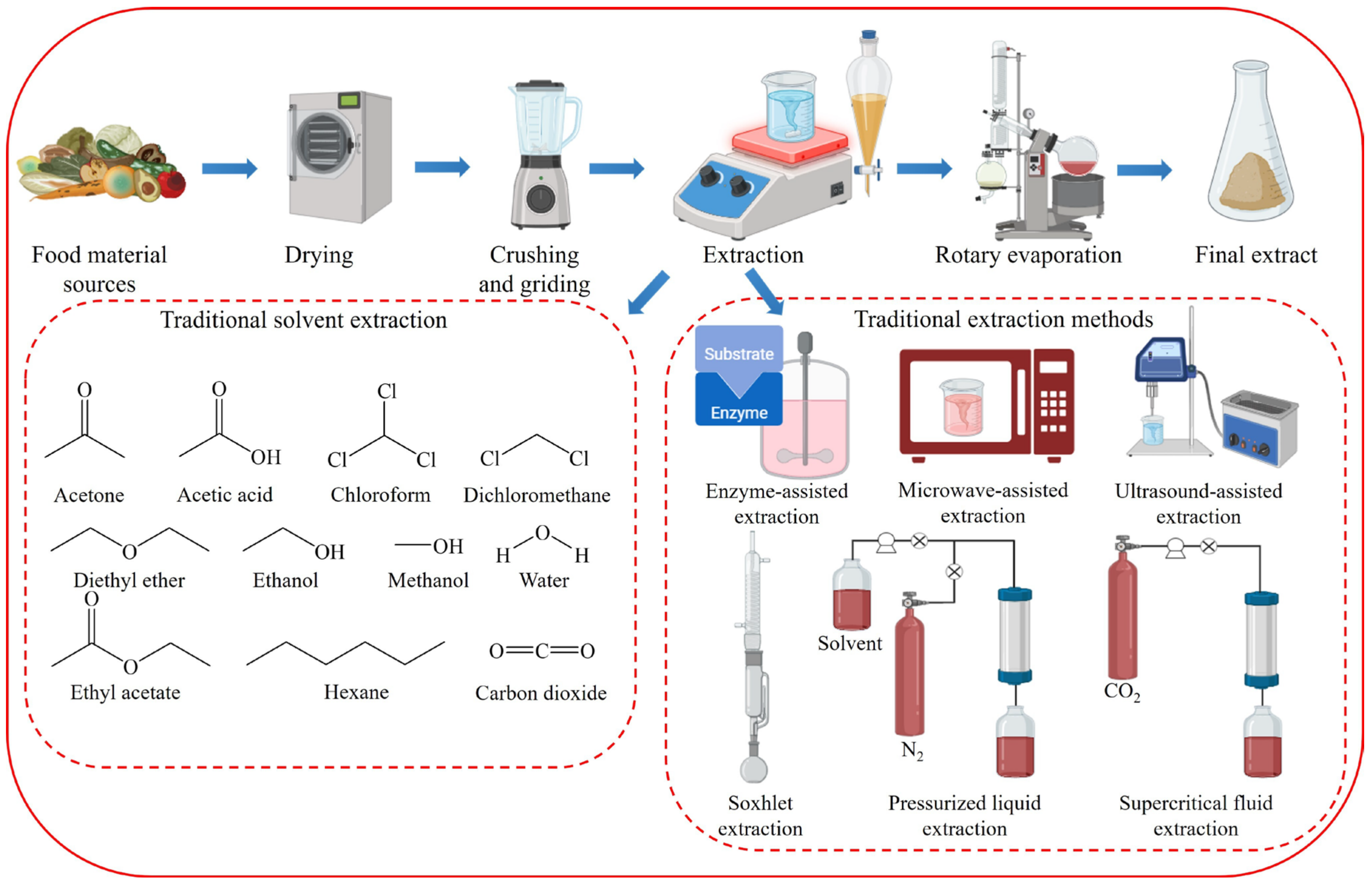
2. Disadvantages of Conventional Extraction Techniques
2.1. Disadvantages of Traditional Solvent Extraction
| Solvent | Extraction Method | Extraction, Temperature, and Time | Boiling Point of Solvent [°C] | Compound Type | Reference |
|---|---|---|---|---|---|
| Acetone | Ultrasound-assisted extraction | 45 °C, 20 min | 56.0 | Tannins | [58] |
| Acetic acid | Ultrasound-assisted extraction | 50 °C, 30 min | 118 | Flavonoids | [59] |
| Chloroform | Homogenization and drying at 65 °C overnight | 25 °C, | 61.0 | Lipids | [60] |
| Dichloromethane | Dichloromethane/methanol solvent system; freeze-drying at 80 °C for 24 h | 50 °C, 6 h | 39.6 | Lipids | [61] |
| Diethyl ether | Soxhlet extraction; rinsing bath at room temperature for 12 h | 5 h | 34.0 | Lipids | [62] |
| DME | Flow extraction | 25 °C, | −24.8 | Lipids | [63] |
| Ethanol | Soxhlet extraction | 100 °C, 8 h | 78.4 | Rice bran oil | [64] |
| Ethyl acetate | Soxhlet extraction | 8 h | 77.1 | Phenolic compounds | [65] |
| Hexane | Soxhlet extraction | 60 °C, 11 h | 68.7 | Phenolic compounds | [66] |
| Methanol | Homogenization | 60 °C, 24 h | 64.7 | Phenolics, alkaloids, flavonoids, and terpenoids | [67] |
| Water | Shaking incubation | 25 °C, 24 h | 100 | Phenolic compounds, flavonoids, anthocyanins, and antioxidants | [68] |
2.2. Disadvantages of Traditional Extraction Methods
3. Advantages of Liquefied DME as an Extractant
3.1. Physical Properties of DME
3.2. Cell Destruction and Drying-Free Extraction Techniques
3.3. Safety of Liquefied DME as an Extraction Solvent
3.4. Environmental Issues Caused by Liquefied DME Extraction
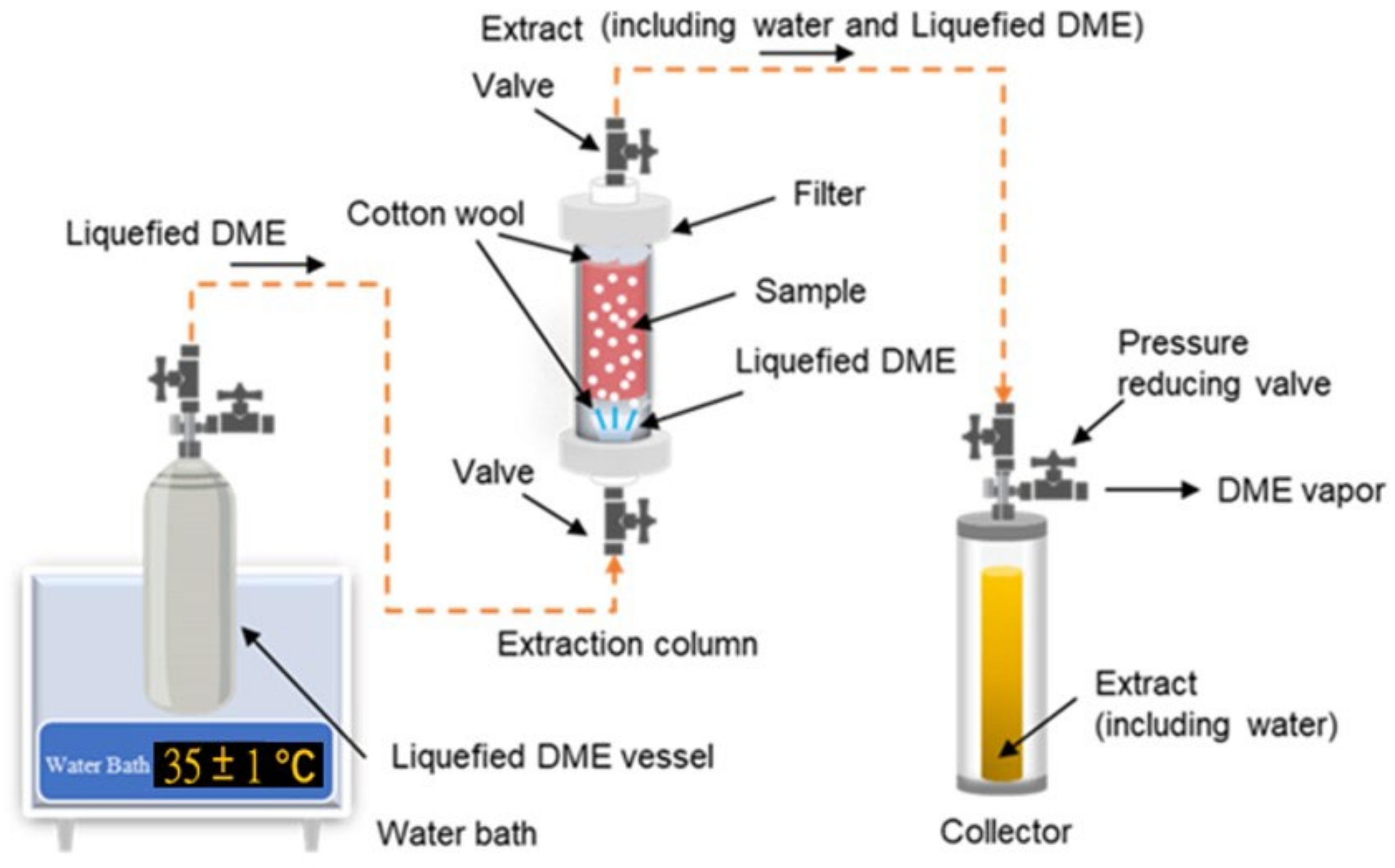
3.5. Liquefied DME Extraction
4. Applications of Liquefied DME Extraction
4.1. Lipid Extraction from Microalgae
| Authors | Resource | Lipid Extraction Yield (%) | Water Content (%) | Dewatering Rate (%) |
|---|---|---|---|---|
| Kanda et al., 2011 [161] | Natural blue–green microalgae | 40.1 | 91.0 | 68.1 |
| Kanda et al., 2012 [162] | Natural blue–green microalgae | 9.9–23.2 | 78.2–93.4 | 83–91 |
| Kanda et al., 2013 [167] | Botryococcus braunii Race B paste | 48.9 | 74.3 | – |
| Boonnoun et al., 2014 [168] | Haematococcus pluvialis | 30.0 | 82.1 | – |
| Kanda et al., 2015 [169] | Euglena gracilis | 32.5 | 80.3 | 92.0 |
| Sakuragi et al., 2016 [170] | Euglena gracilis | 19.7 | 95.0 | – |
| Hoshino et al., 2016 [171] | Aurantiochytrium limacinum | 46.1 | 67.9 | – |
| Hoshino et al., 2017 [172] | Arthrospira platensis | 9.8 | 80.1 | 94.2 |
| Kanda et al., 2020 [24] | Chaetoceros gracilis Pleurochrysis carterae | 22.0 11.6 | 88.5 62.0 | 81 – |
| Wang et al., 2020 [173] | Tetradesmus obliquus | 21.9–29.5 | 65.0–85.0 | 100 |
| Wang et al., 2021 [174] | Nannochloropsis oculata | 23.3 | 94.8 | 100 |
| Bauer et al., 2022 [175] | Phaeodactylum tricornutum | 9.2 | 10.0–80.0 | – |
| Myint et al., 2023 [176] | Haematococcus pluvialis | 290.1 mg g−1 dry extract | 75.7 | 99.3 |
| Bauer et al., 2023 [177] | Four common microalgae and cyanobacteria | 1.7–5.6 | 4.70–2.51 | – |
| Kanda et al., 2023 [178] | Chaetoceros simplex var. calcitrans | 22.7 | 90 | 100 |
4.2. Extraction of Functional Components from Natural Resources
| Authors | Resource | Specific Ingredients | Extraction Solvent | Lipid Extraction Yield (Dry Weight of the Microalgae) |
|---|---|---|---|---|
| Catchpole et al., 2003 [179] | Chili, black pepper, and ginger | Capsaicin | Liquefied DME scCO2 Propane Acetone | 19 g/kg 19 g/kg 11 g/kg 20 g/kg |
| Kanda et al., 2013 [181] | Green tea | Caffeine | Liquefied DME | 47 μg/g |
| Billakanti et al., 2013 [187] | Macroalgae (Undaria pinnatifida) | Fucoxanthin | Liquefied DME Ethanol | 0.066 mg/g 0.060 mg/g |
| Hoshino et al., 2014 [197] | Citrus Leaves and Peels | Citrus flavonoids | Liquefied DME | 6.6–49.9 mg/g |
| Boonnoun et al., 2014 [168] | Microalgae (Haematococcus pluvialis) | Astaxanthin | Liquefied DME Acetone | 0.33% 1.82% |
| Kanda et al., 2014 [111] | Macroalgae (Undaria pinnatifida) | Fucoxanthin | Liquefied DME | 390 μg/g |
| Goto et al., 2015 [190] | Macroalgae (Undaria pinnatifida) | Fucoxanthin | Liquefied DME scCO2 | 390 μg/g 58 μg/g |
| Noriyasu et al., 2015 [192] | Japanese squash peel | Chlorophylls and carotenoids | Liquefied DME | 0–300 μg/g fresh weight |
| Nerome et al., 2016 [198] | Garcinia Mangostana Linn | Mangostin | Liquefied DME Ethanol | 42.9 mg/g 41.14 mg/g |
| Boonnoun et al., 2017 [193] | Marigold flowers | Lutein | Liquefied DME | 20.65 mg/g |
| Furukawa et al., 2016 [199] | Vegetable | Proteins | Liquefied DME | – |
| Nakamura et al., 2017 [200] | Lemon peel tissue | Citric acid Vitamin C Essential oils | Liquefied DME | 10.75 mg/100 g 43 mg/100 g 4% |
| Fang et al., 2018 [201] | Tuna liver | Fish oil | Liquefied DME scCO2 | 17.46 ± 0.23% 17.51 ± 0.11% |
| Kerdsiri et al., 2020 [117] | Jasmine rice bran | γ-oryzanol Linoleic acid Oleic acid | Liquefied DME | 2.47% 22.4% 39.5% |
| Rice berry and rice bran | γ-oryzanol Linoleic acid Oleic acid | Liquefied DME | 6.01% 20.0% 33.5% | |
| Fang et al., 2019 [202] | Tuna livers | n-3 Polyunsaturated fatty acids | Liquefied DME Wet reduction Enzymatic extraction scCO2 | 98.57 ± 0.60% 56.76 ± 1.57% 85.25 ± 1.29% 98.45 ± 1.04% |
| Vitamins | Liquefied DME Wet reduction Enzymatic extraction scCO2 | 37.91 μg/g 17.99 μg/g 24.43 μg/g 40.26 μg/g | ||
| Wongwaiwech et al., 2020 [196] | Rice bran oil | γ-oryzanol Phytosterol Policosanol | Liquefied DME | 924.51 mg/100 g 367.54 mg/100 g 30,787 mg/100 g |
| Babadi et al., 2020 [191] | Chlorococcum humicola | Carotenoids Chlorophylls | Liquefied DME | 4.14 mg/g 8.45 mg/g |
| Kanda et al., 2020 [203] | Macroalgae | Lutein | Liquefied DME | 0.30 mg/g |
| Chloroform−methanol extraction | 0.24 mg/g | |||
| Kanda et al., 2022 [204] | Japanese knotweed rhizome | Resveratrol and glycoside | Liquefied DME Ethanol | 0.342 and 2.57 mg/g 0.215 and 2.01 mg/g |
| Pingyod et al., 2021 [205] | Centella asiatica leaves | Triterpenoid | Liquefied DME and ethanol | 18.80% |
| Bizaj et al., 2021 [206] | Hops | α-Acids | Liquefied DME Propane scCO2 Sulfur hexafluoride | 9.6% 8.7% 7.9% 0.1% |
| β-Acids | Liquefied DME Propane scCO2 Sulfur hexafluoride | 4.5% 4.3% 3.8% 0.1% | ||
| Li et al., 2021 [207] | Cyanobacteria | Fatty acids | Liquefied DME | 8.72–21.15% |
| Kamchonemenukool et al., 2022 [208] | Sugar mill waste | Policosanol | Liquefied DME | 2888 mg/100 g |
| Phytosterol | 10,147.75–20,878.75 mg/100 g | |||
| Ciulla et al., 2023 [184] | Coffee beans and powder | Caffeine | Liquefied DME scCO2 | 0.479 mg/g 0.32 mg/g |
| Kamchonemenukool et al., 2023 [45] | Rice bran acid oil | γ-oryzanol | Liquefied DME scCO2 | 4865.25 mg/100 g, 2569.04 mg/100 g |
| Kanda et al., 2023 [138] | Curcuma longa L. | Curcumin | Liquefied DME | 7.94 mg/g |
| Ethanol | 6.77 mg/g | |||
| Kanda et al., 2023 [178] | Chaetoceros simplex var. calcitrans | Fucoxanthin | Liquefied DME | 9.2 mg/g |
| Ethanol | 11.9 mg/g |
5. Theoretical Study of Liquefied DME
6. Bioactive Extraction to Biomedical Advances
7. Future Trends
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural Plant Chemicals: Sources of Industrial and Medicinal Materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health-a comprehensive review. Vet. Q. 2020, 41, 1–29. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Vieira da Silva, B.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, P.; Wang, D.; Mocan, A.; Waltenberger, B.; Breuss, J.M.; Tewari, D.; Mihaly-Bison, J.; Huminiecki, Ł.; Starzyński, R.R.; Tzvetkov, N.T.; et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: Natural products inhibiting proliferation. Biotechnol. Adv. 2018, 36, 1608–1621. [Google Scholar] [CrossRef]
- Waltenberger, B.; Mocan, A.; Šmejkal, K.; Heiss, E.H.; Atanasov, A.G. Natural Products to Counteract the Epidemic of Cardiovascular and Metabolic Disorders. Molecules 2016, 21, 807. [Google Scholar] [CrossRef]
- Vijaykrishnaraj, M.; Wang, K. Dietary natural products as a potential inhibitor towards advanced glycation end products and hyperglycemic complications: A phytotherapy approaches. Biomed. Pharmacother. 2021, 144, 112336. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Lordan, R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic—Implications for consumers and regulatory oversight. PharmaNutrition 2021, 18, 100282. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The current use and evolving landscape of nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.d.S.; Maciel Filho, R.; Mantelatto, P.E.; Cavalett, O.; Rossell, C.E.V.; Bonomi, A.; Leal, M.R.L.V. Sugarcane processing for ethanol and sugar in Brazil. Environ. Dev. 2015, 15, 35–51. [Google Scholar] [CrossRef]
- Rajapaksha, D.S.W.; Shimizu, N. Valorization of spent black tea by recovery of antioxidant polyphenolic compounds: Subcritical solvent extraction and microencapsulation. Food Sci. Nutr. 2020, 8, 4297–4307. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Buszewski, B.; Szultka, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Adeyi, O.; Oke, E.O.; Okolo, B.I.; Adeyi, A.J.; Otolorin, J.A.; Nwosu-Obieogu, K.; Adeyanju, J.A.; Dzarma, G.W.; Okhale, S.; Ogu, D.; et al. Process optimization, scale-up studies, economic analysis and risk assessment of phenolic rich bioactive extracts production from Carica papaya L. leaves via heat-assisted extraction technology. Heliyon 2022, 8, e09216. [Google Scholar] [CrossRef]
- Martinez-Fernandez, J.S.; Gu, X.; Chen, S. Techno-economic assessment of bioactive compound recovery from potato peels with sequential hydrothermal extraction. J. Clean. Prod. 2021, 282, 124356. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latham, L.Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Egea, M.B.; de Oliveira Filho, J.G.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds from Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2021, 9, 802543. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Hoshino, R.; Murakami, K.; Wahyudiono; Zheng, Q.; Goto, M. Lipid extraction from microalgae covered with biomineralized cell walls using liquefied dimethyl ether. Fuel 2020, 262, 116590. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Lemmon, E.W. An Equation of State for the Thermodynamic Properties of Dimethyl Ether. J. Phys. Chem. Ref. Data 2011, 40, 023104. [Google Scholar] [CrossRef]
- Commission Directive (EU) 2016/1855 of 19 October 2016 Amending Directive 2009/32/EC of the European Parliament and of the Council on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients (Text with EEA Relevance). Available online: http://data.europa.eu/eli/dir/2016/1855/oj (accessed on 4 December 2023).
- Food and Drug Administration. GRAS Notice for the Use of Dimethyl Ether as an Extraction Solvent. Notice No, G.R.A.S. GRN 000741. 2017. Available online: https://www.fda.gov/media/113335/download (accessed on 4 December 2023).
- Patrice Didion, Y.; Gijsbert Tjalsma, T.; Su, Z.; Malankowska, M.; Pinelo, M. What is next? the greener future of solid liquid extraction of biobased compounds: Novel techniques and solvents overpower traditional ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- Medina-Medrano, J.R.; Torres-Contreras, J.E.; Valiente-Banuet, J.I.; Mares-Quiñones, M.D.; Vázquez-Sánchez, M.; Álvarez-Bernal, D. Effect of the solid–liquid extraction solvent on the phenolic content and antioxidant activity of three species of Stevia leaves. Sep. Sci. Technol. 2019, 54, 2283–2293. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods 2022, 11, 3412. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.-P.; Bernard, O. Life-Cycle Assessment of Biodiesel Production from Microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Chien, I.L. Design and Control of Ethanol/Benzene Separation by Energy-Saving Extraction–Distillation Process Using Glycerol as an Effective Heavy Solvent. Ind. Eng. Chem. Res. 2019, 58, 14295–14311. [Google Scholar] [CrossRef]
- Ethanol Content in Herbal Medicinal Products and Traditional Herbal Medicinal Products Used in Children-Scientific Guideline. Available online: https://www.ema.europa.eu/en/ethanol-content-herbal-medicinal-products-traditional-herbal-medicinal-products-used-children (accessed on 4 December 2023).
- Over-the-Counter Drug Products Intended for Oral Ingestion That Contain Alcohol. Available online: https://www.federalregister.gov/documents/1995/03/13/95-6128/over-the-counter-drug-products-intended-for-oral-ingestion-that-contain-alcohol (accessed on 4 December 2023).
- Vojvodić, S.; Čonić, B.S.; Torović, L. Safety assessment of herbal food supplements: Ethanol and residual solvents associated risk. J. Food Compost. Anal. 2023, 122, 105483. [Google Scholar] [CrossRef]
- Goto, M. Chemical recycling of plastics using sub- and supercritical fluids. J. Supercrit. Fluids 2009, 47, 500–507. [Google Scholar] [CrossRef]
- Gopalan, B.; Goto, M.; Kodama, A.; Hirose, T. Supercritical Carbon Dioxide Extraction of Turmeric (Curcuma longa). J. Agric. Food Chem. 2000, 48, 2189–2192. [Google Scholar] [CrossRef]
- Goto, M.; Sato, M.; Hirose, T. Extraction of Peppermint Oil by Supercritical Carbon Dioxide. J. Chem. Eng. Jpn. 1993, 26, 401–407. [Google Scholar] [CrossRef]
- Hoshino, Y.; Wahyudiono; Machmudah, S.; Hirayama, S.; Kanda, H.; Hoshino, M.; Goto, M. Extraction of Functional Components from Freeze-Dried Angelica furcijuga Leaves Using Supercritical Carbon Dioxide. ACS Omega 2022, 7, 5104–5111. [Google Scholar] [CrossRef]
- Triques, C.C.; da Silva, E.A.; Santos, K.A.; Klein, E.J.; Slusarski-Santana, V.; Fagundes-Klen, M.R.; Fiorese, M.L. Supercritical Fluid Extraction of Essential Oils from Natural Sources. In Essential Oils: Extraction Methods and Applications; Inamuddin, Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 707–739. [Google Scholar] [CrossRef]
- Kamchonemenukool, S.; Wongwaiwech, D.; Thongsook, T.; Weerawatanakorn, M. Subcritical liquified dimethyl ether and supercritical fluid carbon dioxide extraction of gamma oryzanol from rice bran acid oil. J. Agric. Food Res. 2023, 14, 100672. [Google Scholar] [CrossRef]
- Milovanovic, S.; Grzegorczyk, A.; Świątek, Ł.; Grzęda, A.; Dębczak, A.; Tyskiewicz, K.; Konkol, M. A Novel Strategy for the Separation of Functional Oils from Chamomile Seeds. Food Bioprocess Technol. 2023, 16, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Gevinski, E.V.; da Silva, D.M.; Júnior, P.A.L.; Bandiera, V.J.; Lohmann, A.M.; Rigo, D.; Duarte, P.F.; Franceschi, E.; Zandoná, G.P.; et al. Extraction of hops pelletized (Humulus lupulus) with subcritical CO2 and hydrodistillation: Chemical composition identification, kinetic model, and evaluation of antioxidant and antimicrobial activity. Food Res. Int. 2023, 167, 112712. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I.; Riemma, S.; Iannone, R. Life cycle assessment of supercritical CO2 extraction of caffeine from coffee beans. J. Supercrit. Fluids 2018, 133, 393–400. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical Carbon Dioxide Extraction of Molecules of Interest from Microalgae and Seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Santana, A.; Jesus, S.; Larrayoz, M.A.; Filho, R.M. Supercritical Carbon Dioxide Extraction of Algal Lipids for the Biodiesel Production. Procedia Eng. 2012, 42, 1755–1761. [Google Scholar] [CrossRef]
- Lorenzen, J.; Igl, N.; Tippelt, M.; Stege, A.; Qoura, F.; Sohling, U.; Brück, T. Extraction of microalgae derived lipids with supercritical carbon dioxide in an industrial relevant pilot plant. Bioprocess Biosyst. Eng. 2017, 40, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Soh, L.; Zimmerman, J. Biodiesel production: The potential of algal lipids extracted with supercritical carbon dioxide. Green Chem. 2011, 13, 1422–1429. [Google Scholar] [CrossRef]
- Hedayati, A.; Ghoreishi, S.M. Supercritical carbon dioxide extraction of glycyrrhizic acid from licorice plant root using binary entrainer: Experimental optimization via response surface methodology. J. Supercrit. Fluids 2015, 100, 209–217. [Google Scholar] [CrossRef]
- Anderson, K.E.; Siepmann, J.I. Solubility in Supercritical Carbon Dioxide: Importance of the Poynting Correction and Entrainer Effects. J. Phys. Chem. B 2008, 112, 11374–11380. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Hedayati, A.; Mohammadi, S. Optimization of periodic static-dynamic supercritical CO2 extraction of taxifolin from Pinus nigra bark with ethanol as entrainer. J. Supercrit. Fluids 2016, 113, 53–60. [Google Scholar] [CrossRef]
- Shimizu, S.; Abbott, S. How Entrainers Enhance Solubility in Supercritical Carbon Dioxide. J. Phys. Chem. B 2016, 120, 3713–3723. [Google Scholar] [CrossRef]
- Pilařová, V.; Al Hamimi, S.; Cunico, L.P.; Nováková, L.; Turner, C. Extending the design space in solvent extraction—From supercritical fluids to pressurized liquids using carbon dioxide, ethanol, ethyl lactate, and water in a wide range of proportions. Green Chem. 2019, 21, 5427–5436. [Google Scholar] [CrossRef]
- Rifna, E.J.; Dwivedi, M. Effect of pulsed ultrasound assisted extraction and aqueous acetone mixture on total hydrolysable tannins from pomegranate peel. Food Biosci. 2022, 45, 101496. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.; Zhao, Y.; Qin, X.; Li, J.; Park, J.H.; et al. Ultrasonic-assisted extraction of flavonoids from peanut leave and stem using deep eutectic solvents and its molecular mechanism. Food Chem. 2024, 434, 137497. [Google Scholar] [CrossRef]
- Axelsson, M.; Gentili, F. A Single-Step Method for Rapid Extraction of Total Lipids from Green Microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef]
- Cequier-Sánchez, E.; Rodríguez, C.; Ravelo, Á.G.; Zárate, R. Dichloromethane as a Solvent for Lipid Extraction and Assessment of Lipid Classes and Fatty Acids from Samples of Different Natures. J. Agric. Food Chem. 2008, 56, 4297–4303. [Google Scholar] [CrossRef]
- Juhaimi, F.A.; Uslu, N.; Babiker, E.E.; Ghafoor, K.; Ahmed, I.A.M.; Özcan, M.M. The Effect of Different Solvent Types and Extraction Methods on Oil Yields and Fatty Acid Composition of Safflower Seed. J. Oleo Sci. 2019, 68, 1099–1104. [Google Scholar] [CrossRef]
- Tallon, S.J.; Catchpole, O.J.; Eltringham, W. Development and scale up of new compressed gas extraction technologies for food and natural product processing. In Proceedings of the AIChE Centennial: Chemical Engineering Education: Past and Future, AIChE Annual Meeting, Conference Proceedings, AIChE 100, Philadelphia, PA, USA, 16–21 November 2008; ISBN 978–081691050–2. [Google Scholar]
- Fornasari, C.H.; Secco, D.; Santos, R.F.; da Silva, T.R.B.; Galant Lenz, N.B.; Tokura, L.K.; Lenz, M.L.; de Souza, S.N.M.; Zanão, L.A., Jr.; Gurgacz, F. Efficiency of the use of solvents in vegetable oil extraction at oleaginous crops. Renew. Sustain. Energy Rev. 2017, 80, 121–124. [Google Scholar] [CrossRef]
- Akkol, E.K.; Göger, F.; Koşar, M.; Başer, K.H.C. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008, 108, 942–949. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Alwazeer, D.; Elnasanelkasim, M.A. Hydrogen-rich water as a green solvent for the extraction of phytochemicals from agri-food wastes. Sustain. Chem. Pharm. 2023, 33, 101035. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Integrated strategies for water removal and lipid extraction from coffee industry residues. Sustain. Energy Technol. Assess. 2018, 29, 26–35. [Google Scholar] [CrossRef]
- Gómez-de la Cruz, F.J.; Cruz-Peragón, F.; Casanova-Peláez, P.J.; Palomar-Carnicero, J.M. A vital stage in the large-scale production of biofuels from spent coffee grounds: The drying kinetics. Fuel Process. Technol. 2015, 130, 188–196. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.-H.; Lee, K.-C.; Ameer, K.; Eun, J.-B. Comparison of freeze-drying and hot air-drying on Asian pear (Pyrus pyrifolia Nakai ‘Niitaka’) powder: Changes in bioaccessibility, antioxidant activity, and bioactive and volatile compounds. J. Food Sci. Technol. 2019, 56, 2836–2844. [Google Scholar] [CrossRef]
- Periche, A.; Castelló, M.L.; Heredia, A.; Escriche, I. Influence of drying method on steviol glycosides and antioxidants in Stevia rebaudiana leaves. Food Chem. 2015, 172, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Rao, M.A.; Lima, J.E.; Torloni, M. Energy consumption and the potential for conservation in a spray-dried coffee plant. J. Food Sci. 1980, 45, 685–688. [Google Scholar] [CrossRef]
- Ramírez, C.A.; Patel, M.; Blok, K. From fluid milk to milk powder: Energy use and energy efficiency in the European dairy industry. Energy 2006, 31, 1984–2004. [Google Scholar] [CrossRef]
- Oancea, S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef]
- Yeh, H.-F.; Luo, C.-Y.; Lin, C.-Y.; Cheng, S.-S.; Hsu, Y.-R.; Chang, S.-T. Methods for Thermal Stability Enhancement of Leaf Essential Oils and Their Main Constituents from Indigenous Cinnamon (Cinnamomum osmophloeum). Agric. Food Chem. 2013, 61, 6293–6298. [Google Scholar] [CrossRef] [PubMed]
- Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods 2022, 11, 17755. [Google Scholar] [CrossRef]
- Li, J.; Sobańtka, A. A Systematic Analysis of the Effect of Extraction Solvents on the Chemical Composition of Extraction Solutions and the Analytical Implications in Extractables and Leachables Studies. J. Pharm. Biomed. Anal. 2023, 222, 115081. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. TrAC Trends Anal. Chem. 2019, 119, 115612. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Gori, A.; Boucherle, B.; Rey, A.; Rome, M.; Fuzzati, N.; Peuchmaur, M. Development of an innovative maceration technique to optimize extraction and phase partition of natural products. Fitoterapia 2021, 148, 104798. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Singh, R.; Vijayraghavan, R.; Arora, A. From waste to wealth: High recovery of nutraceuticals from pomegranate seed waste using a green extraction process. Ind. Crops Prod. 2018, 112, 790–802. [Google Scholar] [CrossRef]
- Weggler, B.A.; Gruber, B.; Teehan, P.; Jaramillo, R.; Dorman, F.L. Chapter 5—Inlets and sampling. In Separation Science and Technology; Snow, N.H., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 12, pp. 141–203. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Mahmudati, N.; Wahyono, P.; Djunaedi, D. Antioxidant activity and total phenolic content of three varieties of Ginger (Zingiber officinale) in decoction and infusion extraction method. J. Phys. Conf. Ser. 2020, 1567, 022028. [Google Scholar] [CrossRef]
- Perera, P.R.D.; Ekanayake, S.; Ranaweera, K.K.D.S. Antidiabetic Compounds in Syzygium cumini Decoction and Ready to Serve Herbal Drink. Evid. Based Complement. Altern. Med. 2017, 2017, 1083589. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Rafińska, K.; Wrona, O.; Krakowska-Sieprawska, A.; Walczak-Skierska, J.; Kiełbasa, A.; Rafiński, Z.; Pomastowski, P.; Kolankowski, M.; Buszewski, B. Enzyme-assisted extraction of plant material—New functional aspects of the process on an example of Medicago sativa L. Ind. Crops Prod. 2022, 187, 115424. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 335–344. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Sparr Eskilsson, C.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Abdurahman Hamid, N.; Alara Ruth, O.; Azhari Hamid, N.; Manal Suliman, O.; Noormazlinah, A. Microwave-Assisted Extraction of Bioactive Compounds (Review). In Microwave Heating; Gennadiy, I.C., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 1. [Google Scholar] [CrossRef]
- Kaya, M.; Cam, M. Eritadenine: Pressurized liquid extraction from Lentinula edodes and thermal degradation kinetics. Sustain. Chem. Pharm. 2022, 29, 100809. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Makoś, P.; Słupek, E.; Sobczak, J.; Zabrocki, D.; Hupka, J.; Rogala, A. Dimethyl ether (DME) as potential environmental friendly fuel. In Proceedings of the International Conference on Advances in Energy Systems and Environmental Engineering (ASEE19), Wroclaw, Poland, 9–12 June 2019. [Google Scholar]
- Tamagawa, K.; Takemura, M.; Konaka, S.; Kimura, M. Molecular structure of dimethylether as determined by a joint analysis of gas electron diffraction and microwave spectroscopic data. J. Mol. Struct. 1984, 125, 131–142. [Google Scholar] [CrossRef]
- Che, C.; Li, Y.; Zhang, G.; Deng, D. Doped Amorphous Carbon Films Prepared by Liquid Phase Electrodeposition. Open J. Appl. Sci. 2014, 3, 5–13. [Google Scholar] [CrossRef][Green Version]
- Ascenzi, D.; Cernuto, A.; Balucani, N.; Tosi, P.; Ceccarelli, C.; Martini, L.M.; Pirani, F. Destruction of dimethyl ether and methyl formate by collisions with He+. Astron. Astrophys. 2019, 625, A72. [Google Scholar] [CrossRef]
- Zheng, Q.; Watanabe, M. Advances in low-temperature extraction of natural resources using liquefied dimethyl ether. Resour. Chem. Mater. 2022, 1, 16–26. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Combustion performance and emission reduction characteristics of automotive DME engine system. Prog. Energy Combust. Sci. 2013, 39, 147–168. [Google Scholar] [CrossRef]
- Thomas, G.; Feng, B.; Veeraragavan, A.; Cleary, M.J.; Drinnan, N. Emissions from DME combustion in diesel engines and their implications on meeting future emission norms: A review. Fuel Process. Technol. 2014, 119, 286–304. [Google Scholar] [CrossRef]
- Sezer, İ. Thermodynamic, performance and emission investigation of a diesel engine running on dimethyl ether and diethyl ether. Int. J. Therm. Sci. 2011, 50, 1594–1603. [Google Scholar] [CrossRef]
- Eltringham, W.; Catchpole, O.J. Relative Permittivity Measurements of Gaseous, Liquid, and Supercritical Dimethyl Ether. J. Chem. Eng. Data 2007, 52, 363–367. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and Efficient Extraction Methods for Marine-Derived Compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono; Goto, M. Extraction of Fucoxanthin from Raw Macroalgae excluding Drying and Cell Wall Disruption by Liquefied Dimethyl Ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef]
- He, X.; Liu, J.; Van Vleck, E. Preface-SI: SIAM-2015. J. Comput. Appl. Math. 2016, 307, 1. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Catchpole, O.J.; Tallon, S.J.; Grey, J.B.; Fletcher, K.; Fletcher, A.J. Extraction of lipids from a specialist dairy stream. J. Supercrit. Fluids 2008, 45, 314–321. [Google Scholar] [CrossRef]
- Catchpole, O.; Ryan, J.; Zhu, Y.; Fenton, K.; Grey, J.; Vyssotski, M.; MacKenzie, A.; Nekrasov, E.; Mitchell, K. Extraction of lipids from fermentation biomass using near-critical dimethylether. J. Supercrit. Fluids 2010, 53, 34–41. [Google Scholar] [CrossRef]
- Liu, S.; Hu, W.; Fang, Y.; Cai, Y.; Zhang, J.; Liu, J.; Ding, Y. Extraction of oil from wet Antarctic krill (Euphausia superba) using a subcritical dimethyl ether method. RSC Adv. 2019, 9, 34274–34282. [Google Scholar] [CrossRef]
- Kerdsiri, J.; Wisuitiprot, W.; Boonnoun, P.; Chantakul, R.; Netsopa, S.; Nuengchamnong, N.; Waranuch, N. Effect of extraction methods on biological activities of Thai rice bran extracts. Songklanakarin J. Sci. Technol. 2020, 42, 1007–1015. [Google Scholar] [CrossRef]
- Kanda, H.; Shimakata, M.; Wang, T.; Zhu, L.; Wahyudiono; Goto, M. Supercritical methanol-induced esterification of microalgal lipids employing biomineralized cell walls as the catalyst. Fuel 2022, 330, 125707. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Kitamura, Y. Intensification of microalgae drying and oil extraction process by vapor recompression and heat integration. Bioresour. Technol. 2016, 207, 67–75. [Google Scholar] [CrossRef]
- Sato, S.; Matsumura, A. Extraction of Phenol in Water Phase Using Liquefied Dimethyl Ether. J. Jpn. Pet. Inst. 2003, 46, 375–378. [Google Scholar] [CrossRef]
- Kanda, H.; Makino, H. Environmental Cleanup Technology by Using Liquefied Dimethyl Ether. J. Soc. Powder Technol. Jpn. 2009, 46, 825–827. [Google Scholar] [CrossRef]
- Catchpole, O.; Moreno, T.; Montañes, F.; Tallon, S. Perspectives on processing of high value lipids using supercritical fluids. J. Supercrit. Fluids 2018, 134, 260–268. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand, Application A1056, Dimethyl Ether as a Processing Aid for Dairy Ingredients & Products Approval Report. 2012. Available online: https://www.foodstandards.gov.au/sites/default/files/food-standards-code/applications/Documents/A1056%20DME%20as%20a%20PA%20(dairy)%20AR%20FINAL1.pdf (accessed on 18 December 2023).
- U.S. Environmental Protection Agency (EPA). Dimethyl Ether; Exemption from the Requirement of a Tolerance. Available online: https://www.federalregister.gov/documents/2005/05/18/05-9475/dimethyl-ether-exemption-from-the-requirement-of-a-tolerance (accessed on 4 December 2023).
- Bohnenn, L.J.M. SPC. Soap, Perfumery, and Cosmetics; United Trade Press: London, UK, 1979; p. 300. [Google Scholar]
- Bohnenn, L.J.M. DME-A Promising Alternative. Propellant in the Fluorocarbon Crisis. Aerosol Rep. 1979, 18, 70–79. [Google Scholar]
- Davidson, B.M. Studies of intoxication VI. The action of methyl ether. J. Pharmacol. Exp. Ther. 1925, 26, 43–48. Available online: https://jpet.aspetjournals.org/content/26/1/43 (accessed on 18 December 2023).
- Occupational Alliance for Risk Science Workplace Environment Exposure Level (WEEL) Committee. Dimethyl ether (DME). Toxicol. Ind. Health 2022, 38, 713–716. [Google Scholar] [CrossRef]
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 2021, 58, 55–77. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2022, 7, 32–37. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Basu, A.; Sills, R.A. Introduction and advancement of a new clean global fuel: The status of DME developments in China and beyond. J. Nat. Gas Sci. Eng. 2012, 9, 94–107. [Google Scholar] [CrossRef]
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl. Catal. B 2017, 217, 494–522. [Google Scholar] [CrossRef]
- Good, D.A.; Francisco, J.S.; Jain, A.K.; Wuebbles, D.J. Lifetimes and global warming potentials for dimethyl ether and for fluorinated ethers: CH3OCF3 (E143a), CHF2OCHF2 (E134), CHF2OCF3 (E125). J. Geophys. Res. Atmos. 1998, 103, 28181–28186. [Google Scholar] [CrossRef]
- Brady, R.N. Internal Combustion (Gasoline and Diesel) Engines. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Liao, H.; Kang, S.; Hansen, N.; Zhang, F.; Yang, B. Influence of ozone addition on the low-temperature oxidation of dimethyl ether in a jet-stirred reactor. Combust. Flame 2020, 214, 277–286. [Google Scholar] [CrossRef]
- Kanda, H.; Fukuta, Y.; Wahyudiono; Goto, M. Enhancement of Lipid Extraction from Soya Bean by Addition of Dimethyl Ether as Entrainer into Supercritical Carbon Dioxide. Foods 2021, 10, 1223. [Google Scholar] [CrossRef]
- Kanda, H.; Zhu, L.; Zhu, W.; Wang, T. Ethanol-free extraction of curcumin and antioxidant activity of components from wet Curcuma longa L. by liquefied dimethyl ether. Arab. J. Chem. 2023, 16, 104585. [Google Scholar] [CrossRef]
- Kanda, H. Super-Energy-Saving Dewatering Method for High-Specific-Surface-Area Fuels by Using Dimethyl Ether. Adsorpt. Sci. Technol. 2008, 26, 345–349. [Google Scholar] [CrossRef]
- Kanda, H. Final Report 2022, Production of Biofuels Using Algal Biomass, Science and Technology Research Partnership for Sustainable Development (SATREPS), Japan Science and Technology Agency. Available online: https://www.jst.go.jp/global/kadai/pdf/h2705_final.pdf (accessed on 18 December 2023).
- Dexso Butanex 345/600 mm Extractor. Available online: https://verdampftnochmal.de/products/en/Dexso-Butanex-345-/-600mm-Extractor_2 (accessed on 12 December 2023).
- Liquid Gas Extraction Technology Uses Dimethyl Ether to Extract Organic Material. Available online: https://www.pcne.eu/article/liquid-gas-extraction-technology/ (accessed on 12 December 2023).
- High Quality Butane Hexane Ethanol Solvent Extraction Herb Equipment/Oil Extraction Machine/Subcritical Extraction Equipment. Available online: https://www.alibaba.com/product-detail/High-quality-Butane-hexane-ethanol-solvent_62547269161.html?spm=a2700.details.0.0.106e350agQzf21 (accessed on 12 December 2023).
- Mini Solvent Extraction Unit for Lab. Available online: https://www.bestextractionmachine.com/products/pilot-solvent-extraction-unit.html (accessed on 12 December 2023).
- Tanaka, K.; Higashi, Y. Measurements of the Isobaric Specific Heat Capacity and Density for Dimethyl Ether in the Liquid State. J. Chem. Eng. Data 2010, 55, 2658–2661. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86, 848–863. [Google Scholar] [CrossRef]
- Zhen, X.; Wang, Y. An overview of methanol as an internal combustion engine fuel. Renew. Sustain. Energy Rev. 2015, 52, 477–493. [Google Scholar] [CrossRef]
- Machmudah, S.; Diono, W.; Kanda, H.; Goto, M. Supercritical fluids extraction of valuable compounds from algae: Future perspectives and challenges. Eng. J. 2018, 22, 13–30. [Google Scholar] [CrossRef]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Santigosa, E.; Brambilla, F.; Milanese, L. Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals 2021, 11, 971. [Google Scholar] [CrossRef]
- Volkman, J.K.; Jeffrey, S.W.; Nichols, P.D.; Rogers, G.I.; Garland, C.D. Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128, 219–240. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Xue, S.; Wang, M.; Cong, W. The Potential of Microalgae Lipids for Edible Oil Production. Appl. Biochem. Biotechnol. 2016, 180, 438–451. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Screening of algae for potential alternative sources of eicosapentaenoic acid. Phytochemistry 1991, 30, 2963–2967. [Google Scholar] [CrossRef]
- Gu, W.; Kavanagh, J.M.; McClure, D.D. Photoautotrophic production of eicosapentaenoic acid. Crit. Rev. Biotechnol. 2021, 41, 731–748. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.-V.; Parry, D.L. The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 1999, 170, 147–159. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Jesionowska, M.; Ovadia, J.; Hockemeyer, K.; Clews, A.C.; Xu, Y. EPA and DHA in microalgae: Health benefits, biosynthesis, and metabolic engineering advances. J. Am. Oil Chem. Soc. 2023, 100, 831–842. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P. Simple extraction method of green crude from natural blue-green microalgae by dimethyl ether. Fuel 2011, 90, 1264–1266. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Ikehara, T.; Yasumoto-Hirose, M. Lipids extracted from several species of natural blue–green microalgae by dimethyl ether: Extraction yield and properties. Fuel 2012, 95, 88–92. [Google Scholar] [CrossRef]
- Bae, Y.J.; Ryu, C.; Jeon, J.-K.; Park, J.; Suh, D.J.; Suh, Y.-W.; Chang, D.; Park, Y.-K. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Suzuki, R.; Ito, N.; Uno, Y.; Nishii, I.; Kagiwada, S.; Okada, S.; Noguchi, T. Transformation of Lipid Bodies Related to Hydrocarbon Accumulation in a Green Alga, Botryococcus braunii (Race B). PLoS ONE 2013, 8, e81626. [Google Scholar] [CrossRef]
- Hirano, K.; Hara, T.; Ardianor; Nugroho, R.A.; Segah, H.; Takayama, N.; Sulmin, G.; Komai, Y.; Okada, S.; Kawamura, K. Detection of the oil-producing microalga Botryococcus braunii in natural freshwater environments by targeting the hydrocarbon biosynthesis gene SSL-3. Sci. Rep. 2019, 9, 16974. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Yoshimura, T.; Okada, S. Wet extraction of hydrocarbons from Botryococcus braunii by dimethyl ether as compared with dry extraction by hexane. Fuel 2013, 105, 535–539. [Google Scholar] [CrossRef]
- Boonnoun, P.; Kurita, Y.; Kamo, Y.; Wahyudiono; Machmudah, S.; Okita, Y.; Ohashi, E.; Kanda, H.; Goto, M. Wet Extraction of Lipids and Astaxanthin from Haematococcus pluvialis by Liquefied Dimethyl Ether. J. Nutr. Food Sci. 2014, 4, 1000305. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Goto, M.; Makino, H. Energy-Saving Lipid Extraction from Wet Euglena gracilis by the Low-Boiling-Point Solvent Dimethyl Ether. Energies 2015, 8, 610–620. [Google Scholar] [CrossRef]
- Sakuragi, K.; Li, P.; Aoki, N.; Otaka, M.; Makino, H. Oil recovery from wet Euglena gracilis by shaking with liquefied dimethyl ether. Fuel Process. Technol. 2016, 148, 184–187. [Google Scholar] [CrossRef]
- Hoshino, R.; Murakami, K.; Wahyudiono; Machmudah, S.; Okita, Y.; Ohashi, E.; Kanda, H.; Goto, M. Economical Wet Extraction of Lipid from labyrinthula Aurantiochytrium limacinum by Using Liquefied Dimethyl Ether. Eng. J. 2016, 20, 145–153. [Google Scholar] [CrossRef]
- Hoshino, R.; Ogawa, M.; Murakami, K.; Wahyudiono; Kanda, H.; Goto, M. Extraction of Lipids from Wet Arthrospira platensis by Liquefied Dimethyl Ether. Solvent Extr. Res. Dev. Jpn. 2017, 24, 47–60. [Google Scholar] [CrossRef]
- Wang, Q.; Oshita, K.; Takaoka, M.; Shiota, K. Influence of water content and cell disruption on lipid extraction using subcritical dimethyl ether in wet microalgae. Bioresour. Technol. 2021, 329, 124892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Oshita, K.; Takaoka, M. Effective lipid extraction from underwatered microalgae liquid using subcritical dimethyl ether. Biotechnol. Biofuels 2021, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.C.; Becker, G.C.; Kruse, A. Influence of Water Content and Upstream Processing on the Liquefied Dimethyl Ether Batch Extraction of Phaeodactylum tricornutum. ACS Sustain. Chem. Eng. 2022, 10, 16250–16260. [Google Scholar] [CrossRef]
- Myint, A.A.; Wulandari, S.; Choi, J.; Sim, S.J.; Kim, J. High-yield dimethyl ether-based recovery of astaxanthin and fatty acids directly from wet Haematococcus pluvialis. Sep. Purif. Technol. 2023, 320, 124226. [Google Scholar] [CrossRef]
- Bauer, M.C.; Konnerth, P.; Kruse, A. Extraction of common microalgae by liquefied dimethyl ether: Influence of species and pretreatment on oil yields and composition. Biomass Convers. Biorefin. 2023, 13, 141–158. [Google Scholar] [CrossRef]
- Kanda, H.; Zhu, L.; Xu, B.; Kusumi, K.; Wang, T. Extraction of fucoxanthin, antioxidants and lipid from wet diatom Chaetoceros simplex var. calcitrans by liquefied dimethyl ether. Arab. J. Chem. 2024, 17, 105538. [Google Scholar] [CrossRef]
- Catchpole, O.J.; Grey, J.B.; Perry, N.B.; Burgess, E.J.; Redmond, W.A.; Porter, N.G. Extraction of Chili, Black Pepper, and Ginger with Near-Critical CO2, Propane, and Dimethyl Ether: Analysis of the Extracts by Quantitative Nuclear Magnetic Resonance. J. Agric. Food Chem. 2003, 51, 4853–4860. [Google Scholar] [CrossRef]
- Daood, H.G.; Illés, V.; Gnayfeed, M.H.; Mészáros, B.; Horváth, G.; Biacs, P.A. Extraction of pungent spice paprika by supercritical carbon dioxide and subcritical propane. J. Supercrit. Fluids 2002, 23, 143–152. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Makino, H. Production of decaffeinated green tea leaves using liquefied dimethyl ether. Food Bioprod. Process. 2013, 91, 376–380. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and Caffeine Content of Green Tea Dietary Supplements and Correlation with Antioxidant Capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef]
- Ito, M.; Ando, T.; Yamamoto, K.; Ishido, A. Caffeine intoxication as a result of excessive consumption of bottled coffee products: A case report. J. Rural Med. 2023, 18, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.; Canale, V.; Wolicki, R.D.; Ferrone, V.; Carlucci, G.; Fontana, A.; Siani, G.; D’Alessandro, N.; Di Profio, P. Comparison of extraction methods for active biomolecules using sub-critical dimethyl ether and n-butane. Eur. Food Res. Technol. 2023, 249, 367–374. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Avila-Peltroche, J.; Won, B.Y.; Cho, T.O. An improved protocol for protoplast production, culture, and whole plant regeneration of the commercial brown seaweed Undaria pinnatifida. Algal Res. 2022, 67, 102851. [Google Scholar] [CrossRef]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Robert Waaland, J.; Rabiei, R. Fatty Acids, Amino Acids, Mineral Contents, and Proximate Composition of Some Brown Seaweeds. J. Phycol. 2012, 48, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Eghbali Babadi, F.; Boonnoun, P.; Nootong, K.; Powtongsook, S.; Goto, M.; Shotipruk, A. Identification of carotenoids and chlorophylls from green algae Chlorococcum humicola and extraction by liquefied dimethyl ether. Food Bioprod. Process. 2020, 123, 296–303. [Google Scholar] [CrossRef]
- Noriyasu, A.; Furukawa, H.; Kikuchi, A.; Takaichi, H.; Bouteau, F.; Li, X.; Nishihama, S.; Yoshizuka, K.; Kawano, T. Use of liquefied cold temperature dimethyl ether for extraction of pigments from fresh vegetable tissues. Adv. Hortic. Sci. 2015, 29, 48–52. [Google Scholar] [CrossRef]
- Boonnoun, P.; Tunyasitikun, P.; Clowutimon, W.; Shotipruk, A. Production of free lutein by simultaneous extraction and de-esterification of marigold flowers in liquefied dimethyl ether (DME)–KOH–EtOH mixture. Food Bioprod. Process. 2017, 106, 193–200. [Google Scholar] [CrossRef]
- Xu, Z.; Godber, J.S. Purification and Identification of Components of γ-Oryzanol in Rice Bran Oil. J. Agric. Food Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, E.; Akaberi, M.; Emami, A.S.; Tayarani-Najaran, Z. Biological and Pharmacological Effects of Gamma-oryzanol: An Updated Review of the Molecular Mechanisms. Curr. Pharm. Des. 2021, 27, 2299–2316. [Google Scholar] [CrossRef]
- Wongwaiwech, D.; Weerawatanakorn, M.; Boonnoun, P. Subcritical dimethyl ether extraction as a simple method to extract nutraceuticals from byproducts from rice bran oil manufacture. Sci. Rep. 2020, 10, 21007. [Google Scholar] [CrossRef]
- Hoshino, R.; Wahyudiono; Machmudah, S.; Kanda, H.; Goto, M. Simultaneous Extraction of Water and Essential Oils from Citrus Leaves and Peels Using Liquefied Dimethyl Ether. J. Nutr. Food Sci. 2014, 4, 1000301. [Google Scholar] [CrossRef]
- Nerome, H.; Hoshino, R.; Ito, S.; Esaki, R.; Eto, Y.; Wakiyama, S.; Sharmin, T.; Goto, M.; Kanda, H.; Mishima, K. Functional Ingredients Extraction from Garcinia mangostana Pericarp by Liquefied Dimethyl Ether. Eng. J. 2016, 20, 155–162. [Google Scholar] [CrossRef]
- Furukawa, H.; Kikuchi, A.; Noriyasu, A.; Bouteau, F.; Nishihama, S.; Yoshizuka, K.; Li, X.; Kawano, T. Use of Liquefied Dimethyl Ether for the Extraction of Proteins from Vegetable Tissues. Solvent Extr. Res. Dev. Jpn. 2016, 23, 127–135. [Google Scholar] [CrossRef][Green Version]
- Nakamura, A.; Hara, Y.; Kawano, T. Dewatering and Extraction of Hydrophilic Solutes and Essential Oils from Cryo-preserved Lemon Peels Using Liquefied Dimethyl Ether. Solvent Extr. Res. Dev. Jpn. 2017, 24, 37–45. [Google Scholar] [CrossRef][Green Version]
- Fang, Y.; Gu, S.; Liu, S.; Zhang, J.; Ding, Y.; Liu, J. Extraction of oil from high-moisture tuna liver by subcritical dimethyl ether: Feasibility and optimization by the response surface method. RSC Adv. 2018, 8, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, S.; Hu, W.; Zhang, J.; Ding, Y.; Liu, J. Extraction of Oil from High-Moisture Tuna Livers by Subcritical Dimethyl Ether: A Comparison with Different Extraction Methods. Eur. J. Lipid Sci. Technol. 2019, 121, 1800087. [Google Scholar] [CrossRef]
- Kanda, H.; Wahyudiono; Machmudah, S.; Goto, M. Direct Extraction of Lutein from Wet Macroalgae by Liquefied Dimethyl Ether without Any Pretreatment. ACS Omega 2020, 5, 24005–24010. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Oishi, K.; Machmudah, S.; Wahyudiono; Goto, M. Ethanol-free extraction of resveratrol and its glycoside from Japanese knotweed rhizome by liquefied dimethyl ether without pretreatments. Asia-Pac. J. Chem. Eng. 2021, 16, e2600. [Google Scholar] [CrossRef]
- Pingyod, C.; Waranuch, N.; Phrompittayarat, W.; Boonnoun, P.; Ingkaninan, K. Extraction of Centella asiatica leaves using a mixture ofsubcritical dimethyl ether and ethanol: Optimization of conditionsby response surface methodology. Songklanakarin J. Sci. Technol. 2021, 43, 711–718. [Google Scholar] [CrossRef]
- Bizaj, K.; Škerget, M.; Košir, I.J.; Knez, Ž. Sub- and Supercritical Extraction of Slovenian Hops (Humulus lupulus L.) Aurora Variety Using Different Solvents. Plants 2021, 10, 1137. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Zhu, W.; Mu, B.; Chen, S.; Sun, J. A Study of Salvaged Cyanobacteria Slurry Treatment Using Liquefied Dimethyl Ether: Dehydration and Organic Matter Extraction. ACS Sustain. Chem. Eng. 2021, 9, 14644–14652. [Google Scholar] [CrossRef]
- Kamchonemenukool, S.; Ho, C.-T.; Boonnoun, P.; Li, S.; Pan, M.-H.; Klangpetch, W.; Weerawatanakorn, M. High Levels of Policosanols and Phytosterols from Sugar Mill Waste by Subcritical Liquefied Dimethyl Ether. Foods 2022, 11, 2937. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibáñez, E. Hansen solubility parameters for selection of green extraction solvents. TrAC Trends Anal. Chem. 2019, 118, 227–237. [Google Scholar] [CrossRef]
- Zhou, K.-G.; Mao, N.-N.; Wang, H.-X.; Peng, Y.; Zhang, H.-L. A Mixed-Solvent Strategy for Efficient Exfoliation of Inorganic Graphene Analogues. Angew. Chem. Int. 2011, 50, 10839–10842. [Google Scholar] [CrossRef] [PubMed]
- Terrell, E. Estimation of Hansen solubility parameters with regularized regression for biomass conversion products: An application of adaptable group contribution. Chem. Eng. Sci. 2022, 248, 117184. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Monrad, J.K.; Howard, L.R.; Hansen, C.M. Optimization of Subcritical Fluid Extraction of Bioactive Compounds Using Hansen Solubility Parameters. J. Food Sci. 2009, 74, E342–E354. [Google Scholar] [CrossRef] [PubMed]
- Louwerse, M.J.; Maldonado, A.; Rousseau, S.; Moreau-Masselon, C.; Roux, B.; Rothenberg, G. Revisiting Hansen Solubility Parameters by Including Thermodynamics. ChemPhysChem 2017, 18, 2999–3006. [Google Scholar] [CrossRef]
- Venkatram, S.; Kim, C.; Chandrasekaran, A.; Ramprasad, R. Critical Assessment of the Hildebrand and Hansen Solubility Parameters for Polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Turpin, E.R.; Taresco, V.; Al-Hachami, W.A.; Booth, J.; Treacher, K.; Tomasi, S.; Alexander, C.; Burley, J.; Laughton, C.A.; Garnett, M.C. In Silico Screening for Solid Dispersions: The Trouble with Solubility Parameters and χFH. Mol. Pharm. 2018, 15, 4654–4667. [Google Scholar] [CrossRef]
- De La Peña-Gil, A.; Toro-Vazquez, J.F.; Rogers, M.A. Simplifying Hansen Solubility Parameters for Complex Edible Fats and Oils. Food Biophys. 2016, 11, 283–291. [Google Scholar] [CrossRef]
- Lara, J.; Zimmermann, F.; Drolet, D.; Hansen, C.M.; Chollot, A.; Monta, N. The use of the Hansen solubility parameters in the selection of protective polymeric materials resistant to chemicals. Int. J. Curr. Res. 2017, 9, 47860–47867. Available online: https://hal.science/hal-01639526 (accessed on 18 December 2023).
- Liu, S.S.; Li, X.P.; Qi, P.J.; Song, Z.J.; Zhang, Z.; Wang, K.; Qiu, G.X.; Liu, G.Y. Determination of three-dimensional solubility parameters of styrene butadiene rubber and the potential application in tire tread formula design. Polym. Test. 2020, 81, 106170. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Hansen, C.M. Elastomer swelling and Hansen solubility parameters. Polym. Test. 2005, 24, 1054–1061. [Google Scholar] [CrossRef]
- Liu, G.; Hoch, M.; Wrana, C.; Kulbaba, K.; Qiu, G. A new way to determine the three-dimensional solubility parameters of hydrogenated nitrile rubber and the predictive power. Polym. Test. 2013, 32, 1128–1134. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Bi, L.Y.; Zhang, H.J.; Zhu, X.T.; Liu, G.Y.; Qiu, G.X.; Liu, S.S. Predictive power in oil resistance of fluororubber and fluorosilicone rubbers based on three-dimensional solubility parameter theory. Polym. Test. 2019, 75, 380–386. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, G.; Wang, Y. Hansen Solubility Parameters of Coal Tar-Derived Typical PAHs Using Turbidimetric Titration and an Extended Hansen Approach. J. Chem. Eng. Data 2017, 62, 954–960. [Google Scholar] [CrossRef]
- Nakamura, D.; Hirano, M.; Ohta, R. Nontoxic organic solvents identified using an a priori approach with Hansen solubility parameters. Chem. Commun. 2017, 53, 4096–4099. [Google Scholar] [CrossRef] [PubMed]
- Nardella, F.; Prothmann, J.; Sandahl, M.; Spégel, P.; Ribechini, E.; Turner, C. Native lignin extraction from soft- and hardwood by green and benign sub/supercritical fluid extraction methodologies. RSC Adv. 2023, 13, 21945–21953. [Google Scholar] [CrossRef] [PubMed]
- Paseta, L.; Potier, G.; Abbott, S.; Coronas, J. Using Hansen solubility parameters to study the encapsulation of caffeine in MOFs. Org. Biomol. Chem. 2015, 13, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Cunico, L.P.; Acosta, M.C.; Turner, C. Experimental measurements and modeling of curcumin solubility in CO2-expanded ethanol. J. Supercrit. Fluids 2017, 130, 381–388. [Google Scholar] [CrossRef]
- Daisuk, P.; Shotipruk, A. Recovery of γ-oryzanol from rice bran oil soapstock derived calcium soap: Consideration of Hansen solubility parameters and preferential extractability in various solvents. LWT 2020, 134, 110238. [Google Scholar] [CrossRef]
- Díaz de los Ríos, M.; Hernández Ramos, E.; González Canavaciolo, V.; Vicente Murillo, R.; Pérez Carrión, K.; Zumalacarregui de Cárdenas, L. Obtaining a Fraction of Sugarcane Wax Rich in Policosanol by Using Ethanol as Solvent: Results Interpretation through Hansen’s Solubility Theory. ACS Omega 2022, 7, 27324–27333. [Google Scholar] [CrossRef] [PubMed]
- Ghazwani, M.; Alam, P.; Alqarni, M.H.; Yusufoglu, H.S.; Shakeel, F. Solubilization of Trans-Resveratrol in Some Mono-Solvents and Various Propylene Glycol + Water Mixtures. Molecules 2021, 26, 3019. [Google Scholar] [CrossRef] [PubMed]
- Nerome, H.; Machmudah, S.; Wahyudiono; Fukuzato, R.; Higashiura, T.; Kanda, H.; Goto, M. Effect of Solvent on Nanoparticle Production of β-Carotene by a Supercritical Antisolvent Process. Chem. Eng. Technol. 2016, 39, 1771–1777. [Google Scholar] [CrossRef]
- Hernández, E.; Díaz, M.; Pérez, K. Determination of Hansen solubility parameters for sugarcane oil. Use of ethanol in sugarcane wax refining. Grasas Aceites 2021, 72, e408. [Google Scholar] [CrossRef]
- Tirado, D.F.; Tenorio, M.J.; Cabañas, A.; Calvo, L. Prediction of the best cosolvents to solubilise fatty acids in supercritical CO2 using the Hansen solubility theory. Chem. Eng. Sci. 2018, 190, 14–20. [Google Scholar] [CrossRef]
- Cosby, L.E.; Lee, K.H.; Knobloch, T.J.; Weghorst, C.M.; Winter, J.O. Comparative Encapsulation Efficiency of Lutein in Micelles Synthesized via Batch and High Throughput Methods. Int. J. Nanomed. 2020, 15, 8217–8230. [Google Scholar] [CrossRef] [PubMed]
- Del Pilar Sánchez-Camargo, A.; Pleite, N.; Herrero, M.; Cifuentes, A.; Ibáñez, E.; Gilbert-López, B. New approaches for the selective extraction of bioactive compounds employing bio-based solvents and pressurized green processes. J. Supercrit. Fluids. 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Wulandari, S.; Choi, J.; Kurniawan, R.G.; Sugiarto, J.R.; Myint, A.A.; Kwak, S.K.; Kim, J. Synthesis of highly stable encapsulated astaxanthin/β-cyclodextrin microparticles using supercritical CO2 as an antisolvent. J. CO2 Util. 2023, 75, 102575. [Google Scholar] [CrossRef]
- Surface Free Energy Components by Polar/Dispersion and Acid—Base Analyses; and Hansen Solubility Parameters for Various Polymers. Available online: https://www.accudynetest.com/polytable_02.html?sortby=sort_hansen_disp#a (accessed on 4 December 2023).
- Shinohara, K.; Yanagisawa, M.; Makida, Y. Direct Observation of Long-Chain Branches in a Low-Density Polyethylene. Sci. Rep. 2019, 9, 9791. [Google Scholar] [CrossRef]
- Lehnert, R.J.; Kandelbauer, A. Comments on “Solubility parameter of chitin and chitosan” Carbohydrate Polymers 36 (1998) 121–127. Carbohydr. Polym. 2017, 175, 601–602. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Okochi, Y.; Ohshimo, S.; Shimose, T.; Ashida, H.; Sato, T.; Ando, Y. Lipid and fatty acid dynamics by maternal Pacific bluefin tuna. PLoS ONE 2019, 14, e0222824. [Google Scholar] [CrossRef]
- Fraterrigo Garofalo, S.; Cavallini, N.; Demichelis, F.; Savorani, F.; Mancini, G.; Fino, D.; Tommasi, T. From tuna viscera to added-value products: A circular approach for fish-waste recovery by green enzymatic hydrolysis. Food Bioprod. Process. 2023, 137, 155–167. [Google Scholar] [CrossRef]
- Hilborn, R.; Costello, C. The potential for blue growth in marine fish yield, profit and abundance of fish in the ocean. Mar. Policy 2018, 87, 350–355. [Google Scholar] [CrossRef]
- Lynch, S.A.; Álvarez, C.; O’Neill, E.E.; Keenan, D.F.; Mullen, A.M. Optimization of protein recovery from bovine lung by pH shift process using response surface methodology. J. Sci. Food Agric. 2018, 98, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Vuong, Q.V.; Schreider, M.J.; Bowyer, M.C.; Altena, I.A.V.; Scarlett, C.J. The Effects of Drying on Physico-Chemical Properties and Antioxidant Capacity of the Brown Alga (Hormosira banksii (Turner) Decaisne). J. Food Process. Preserv. 2017, 41, e13025. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, J.; Li, J.; Chen, W.; Huang, G.; Ding, Y. Rapid preparation of protein powder from high-moisture tuna liver: New insight into subcritical dimethyl ether. LWT 2020, 124, 109179. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, Q.; Lin, Y.; Ge, H.; Huang, G.; Jiang, H. Physicochemical, digestive and rheological properties of protein from tuna by subcritical dimethyl ether: Focus on process-related indexes. Food Chem. 2022, 372, 131337. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fang, Y.; Huang, S.; Shao, J.; Liu, J.; Huang, G. Profiles of sturgeon protein prepared by two methods and the correlation between protein fractions and functional properties. Int. J. Food Sci. Technol. 2022, 57, 5450–5460. [Google Scholar] [CrossRef]
- Shao, J.; Chi, Y.; Chu, J.; Huang, S.; Ye, S.; Sun, J.; Wang, J.; Huang, G.; Fang, Y. Separation of Protein from the Meat of Sturgeon by a Subcritical Dimethyl Ether Method. J. Aquat. Food Prod. Technol. 2023, 32, 655–666. [Google Scholar] [CrossRef]
- Kanda, H.; Katsube, T.; Hoshino, R.; Kishino, M.; Wahyudiono; Goto, M. Ethanol-free antisolvent crystallization of glycine by liquefied dimethyl ether. Heliyon 2020, 6, e05258. [Google Scholar] [CrossRef]
- Kanda, H.; Katsube, T.; Wahyudiono; Goto, M. Preparation of Liposomes from Soy Lecithin Using Liquefied Dimethyl Ether. Foods 2021, 10, 1789. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Kanda, H.; Ando, D.; Hoshino, R.; Yamamoto, T.; Wahyudiono; Suzuki, S.; Shinohara, S.; Goto, M. Surfactant-Free Decellularization of Porcine Aortic Tissue by Subcritical Dimethyl Ether. ACS Omega 2021, 6, 13417–13425. [Google Scholar] [CrossRef]
- Kanda, H.; Ando, D.; Oya, K.; Wahyudiono; Goto, M. Surfactant-free preparation of an ostrich carotid artery scaffold using liquefied dimethyl ether and DNase. Arab. J. Chem. 2021, 14, 103280. [Google Scholar] [CrossRef]
- Kanda, H.; Oya, K.; Wahyudiono; Goto, M. Surfactant-Free Decellularization of Porcine Auricular Cartilage Using Liquefied Dimethyl Ether and DNase. Materials 2023, 16, 3172. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Oya, K.; Irisawa, T.; Wahyudiono; Goto, M. Tensile strength of ostrich carotid artery decellularized with liquefied dimethyl ether and DNase: An effort in addressing religious and cultural concerns. Arab. J. Chem. 2023, 16, 104578. [Google Scholar] [CrossRef]
- Bianchelli, S.; Martire, M.L.; Pola, L.; Gambi, C.; Fanelli, E.; Danovaro, R.; Corinaldesi, C. Impact of hypersaline brines on benthic meio- and macrofaunal assemblages: A comparison from two desalination plants of the Mediterranean Sea. Desalination 2022, 532, 115756. [Google Scholar] [CrossRef]
- Davenport, D.M.; Deshmukh, A.; Werber, J.R.; Elimelech, M. High-Pressure Reverse Osmosis for Energy-Efficient Hypersaline Brine Desalination: Current Status, Design Considerations, and Research Needs. Environ. Sci. Technol. Lett. 2018, 5, 467–475. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. J. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, A.V.; Bartholomew, T.V.; Mauter, M.S. Cost optimization of multi-stage gap membrane distillation. J. Membr. Sci. 2021, 627, 119228. [Google Scholar] [CrossRef]
- Desai, D.; Beh, E.S.; Sahu, S.; Vedharathinam, V.; van Overmeere, Q.; de Lannoy, C.F.; Jose, A.P.; Völkel, A.R.; Rivest, J.B. Electrochemical Desalination of Seawater and Hypersaline Brines with Coupled Electricity Storage. ACS Energy Lett. 2018, 3, 375–379. [Google Scholar] [CrossRef]
- Boo, C.; Winton, R.K.; Conway, K.M.; Yip, N.Y. Membrane-less and Non-Evaporative Desalination of Hypersaline Brines by Temperature Swing Solvent Extraction. Environ. Sci. Technol. Lett. 2019, 6, 359–364. [Google Scholar] [CrossRef]
- Foo, Z.H.; Stetson, C.; Dach, E.; Deshmukh, A.; Lee, H.; Menon, A.K.; Prasher, R.; Yip, N.Y.; Lienhard, J.H.; Wilson, A.D. Solvent-driven aqueous separations for hypersaline brine concentration and resource recovery. Trends Chem. 2022, 4, 1078–1093. [Google Scholar] [CrossRef]
- Deshmukh, A.; Foo, Z.H.; Stetson, C.; Lee, H.; Orme, C.J.; Wilson, A.D.; Lienhard, J.H. Thermodynamics of solvent-driven water extraction from hypersaline brines using dimethyl ether. J. Chem. Eng. 2022, 434, 134391. [Google Scholar] [CrossRef]
- Wibowo, C.; Ng, K.M. Unified approach for synthesizing crystallization-based separation processes. AIChE J 2000, 46, 1400–1421. [Google Scholar] [CrossRef]
- Demirel, H.S.; Svärd, M.; Uysal, D.; Doğan, Ö.M.; Uysal, B.Z.; Forsberg, K. Antisolvent crystallization of battery grade nickel sulphate hydrate in the processing of lateritic ores. Sep. Purif. Technol. 2022, 286, 120473. [Google Scholar] [CrossRef]
- McNally, J.S.; Foo, Z.H.; Deshmukh, A.; Orme, C.J.; Lienhard, J.H.; Wilson, A.D. Solute displacement in the aqueous phase of water–NaCl–organic ternary mixtures relevant to solvent-driven water treatment. RSC Adv. 2020, 10, 29516–29527. [Google Scholar] [CrossRef] [PubMed]
- Stetson, C.; Prodius, D.; Lee, H.; Orme, C.; White, B.; Rollins, H.; Ginosar, D.; Nlebedim, I.C.; Wilson, A.D. Solvent-driven fractional crystallization for atom-efficient separation of metal salts from permanent magnet leachates. Nat. Commun. 2022, 13, 3789. [Google Scholar] [CrossRef]

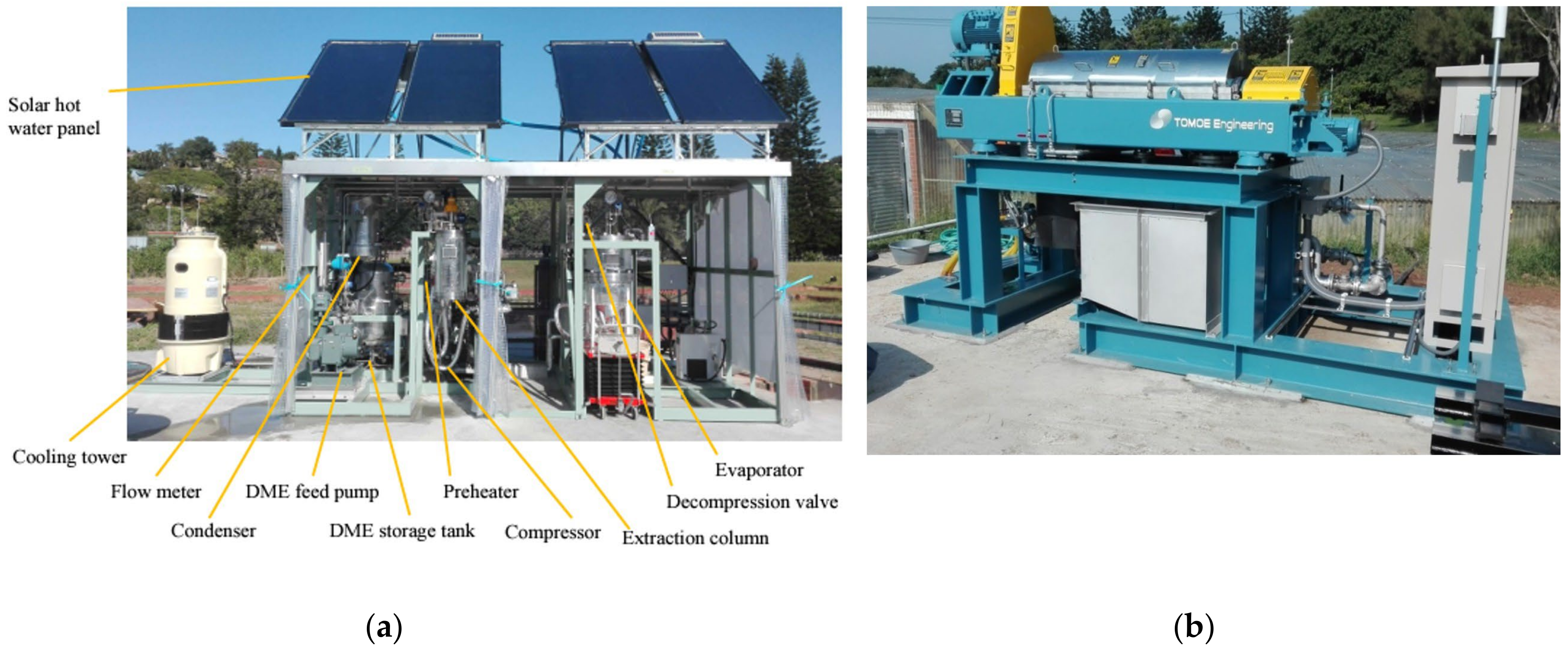


| Product Name | Specification | Solvents and Applications | Reference |
|---|---|---|---|
| Dexso Butanex 345/600 mm Extractor | A 125 or 50 cm extraction tube, for up to 40 g or 100 g (1.4 or 3.6 ounces) Centerpiece with magnetic tripod, easy assembly Magnetic feet, safe stand O-ring seal, safe extraction Emptying unit, easy emptying Reusable stainless-steel screen, low maintenance Temperature: room temperature | Supports DME and butane. Ideal for small amounts of plant material and trimming. | [141] |
| Pilot Extraction Plants | Volume extractor: 3 L Volume extract/solvent tank: 2 × 20 L Design temperature: −10/+50 °C | Supports DME, propane, and butane. Solvents can be reused/recycled. | [142] |
| Subcritical extraction equipment | Extraction tank volume: 2.5 L Production capacity: 20 L Temperature: <40 °C Using pressure: 0.8 MPa | Supports DME, butane, hexane, and ethanol. Ideal for plant oil extraction. | [143] |
| Mini solvent extraction unit for lab | Volume of extraction pot: 5–200 L Separation tank: 5–200 L Temperature: room temperature Buffer tank: 5–200 L Condenser: 1–10 m2 Solvent pot 19–159 L Gauge tank: 1–145 L Heater: 6 kw | Supports DME. Apply to precious vegetable oils, essential oils, animal oils, microalgae oils, natural dyes, vegetable proteins, and general-purpose spices. Solvents can be reused/recycled. | [144] |
| No. | Compounds | δd | δp | δh | RED | Reference |
|---|---|---|---|---|---|---|
| (MPa1/2) | (MPa1/2) | (MPa1/2) | ||||
| Soluble | ||||||
| 1 | Natural rubber | 16.4 | 3.1 | 4.1 | 0.87 | [218] |
| 2 | Nitrile rubber | 20.4 | 12.4 | 4.1 | 0.88 | [218] |
| 3 | Styrene-butadiene rubber | 18 | 2.9 | 2.3 | 0.86 | [219] |
| 4 | Ethylene-propylene rubber | 17.2 | 2 | 2.6 | 0.94 | [220] |
| 5 | Hydrogenated nitrile rubber | 18.4 | 6 | 4.5 | 0.53 | [221] |
| 6 | Fluoro rubber | 16.1 | 9.3 | 6.6 | 0.78 | [222] |
| 7 | Resveratrol | 20.9 | 6.7 | 13.1 | 0.55 | [204] |
| 8 | Phenanthrene | 20.8 | 2.6 | 5.4 | 0.66 | [223] |
| 9 | Pyrene | 22.5 | 1.6 | 4 | 1.02 | [223] |
| 10 | Lecithin | 16.1 | 6.4 | 9.1 | 0.66 | [224] |
| 11 | Camphor | 17.3 | 10 | 4.9 | 0.73 | SMILES |
| 12 | Ferulic acid | 19.3 | 8.4 | 15.8 | 0.74 | [225] |
| 13 | Caffeine | 19.5 | 10.1 | 13 | 0.58 | [226] |
| 14 | Curcumin | 18.8 | 7.7 | 11.1 | 0.27 | [227] |
| 15 | γ-oryzanol | 18.6 | 6.5 | 3.3 | 0.64 | [228] |
| 16 | Phytosterol | 17.1 | 1.9 | 3 | 0.92 | SMILES |
| 17 | Policosanol | 15.9 | 1.7 | 4.4 | 1.00 | [229] |
| 18 | Triterpenoid | 18 | 9.2 | 12.8 | 0.55 | SMILES |
| 19 | trans-Resveratrol | 20.6 | 7.3 | 15.9 | 0.78 | [230] |
| 20 | β-carotene | 17.1 | 2.4 | 5.5 | 0.73 | [231] |
| 21 | Policosanol | 16.1 | 2.4 | 5 | 0.90 | [229] |
| 22 | Oleic acid | 16 | 2.8 | 6.2 | 0.84 | [232] |
| 23 | Linoleic acid | 18.1 | 2.9 | 7.2 | 0.48 | [233] |
| 24 | Lutein | 15.2 | 1.8 | 8.5 | 0.98 | [234] |
| 25 | Xanthone | 20.6 | 8.4 | 5.2 | 0.57 | SMILES |
| 26 | Fucoxanthin | 18.2 | 4.1 | 5.1 | 0.54 | [235] |
| 27 | Astaxanthin | 22.2 | 4.6 | 8.9 | 0.66 | [236] |
| 28 | Capsaicin | 18.3 | 15.4 | 8.9 | 0.99 | SMILES |
| 29 | Butyl rubber | 17.3 | 1.4 | 2.6 | 0.96 | [218] |
| Insoluble | ||||||
| 30 | Chloroprene rubber | 17.2 | 2.4 | 1.2 | 1.04 | SMILES |
| 31 | Polytetrafluoroethylene | 16.2 | 1.8 | 3.4 | 1.01 | [237] |
| 32 | Low density polyethylene | 16.2 | 2.1 | 2.4 | 1.06 | [238] |
| 33 | Polyvinyl alcohol | 17 | 9 | 18 | 1.09 | Solubility experiments |
| 34 | Polyvinylpyrrolidone | 18.1 | 10 | 18 | 1.04 | Solubility experiments |
| 35 | Chitosan | 22.8 | 17.1 | 26.6 | 2.31 | Solubility experiments |
| 36 | Chitin | 23.3 | 15 | 22.5 | 1.90 | [239] |
| 37 | Polyacrylamide | 19.5 | 19.7 | 16.4 | 1.62 | Solubility experiments |
| 38 | Polyglutamic acid sodium salt | 19.3 | 12.1 | 16.5 | 0.99 | Solubility experiments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhu, L.; Mei, L.; Kanda, H. Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review. Foods 2024, 13, 352. https://doi.org/10.3390/foods13020352
Wang T, Zhu L, Mei L, Kanda H. Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review. Foods. 2024; 13(2):352. https://doi.org/10.3390/foods13020352
Chicago/Turabian StyleWang, Tao, Li Zhu, Li Mei, and Hideki Kanda. 2024. "Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review" Foods 13, no. 2: 352. https://doi.org/10.3390/foods13020352
APA StyleWang, T., Zhu, L., Mei, L., & Kanda, H. (2024). Extraction and Separation of Natural Products from Microalgae and Other Natural Sources Using Liquefied Dimethyl Ether, a Green Solvent: A Review. Foods, 13(2), 352. https://doi.org/10.3390/foods13020352







