Abstract
The health and balance of the gut microbiota are known to be linked to diet composition and source, with fermented products and dietary proteins potentially providing an exceptional advantage for the gut. The purpose of this study was to evaluate the effect of protein hydrolysis, using a probiotic beverage enriched with either cricket protein (CP) or cricket protein hydrolysates (CP.Hs), on the composition of the gut microbiota of rats. Taxonomic characterization of the gut microbiota in fecal samples was carried out after a 14-day nutritional study to identify modifications induced by a CP- and CP.H-enriched fermented probiotic product. The results showed no significant differences (p > 0.05) in the diversity and richness of the gut microbiota among the groups fed with casein (positive control), CP-enriched, and fermented CP.H-enriched probiotic beverages; however, the overall composition of the microbiota was altered, with significant modifications in the relative abundance of several bacterial families and genera. In addition, fermented CP.H-enriched probiotic beverages could be related to the decrease in the number of potential pathogens such as Enterococcaceae. The association of gut microbiota with the nutritional parameters was determined and the results showed that digestibility and the protein efficiency ratio (PER) were highly associated with the abundance of several taxa.
1. Introduction
Intestinal microbiota is very important for health, playing a key role in proper digestion, the functioning of the immune system, and other aspects of health [1]. On the other hand, an imbalanced gut microbiota can contribute to the development of various health conditions such as elevated circulating blood lipids, obesity, and high blood sugar [2]. Several factors can affect the balance of gut microbial communities, including health status, age, and, most importantly, diet [3].
With this in mind, probiotic-fortified food products are in high demand, as they have the potential to alter the composition of gut microbiota while resulting in beneficial health effects [4,5]. They have been shown to lead to reduction of gut inflammation [6], prevention of antibiotic-associated diarrhea (AAD), and improvement of symptoms associated with irritable bowel syndrome (IBS) [7].
Dietary protein has significant effects on the gut microbiota in a source-dependent manner, with increased prevalence of pathogenic bacteria being associated with higher levels of undigested protein [8,9,10], though several open questions regarding the interaction between the gut microbiota and dietary protein remain, not the least of which is the impact of protein digestibility [11]. Further, the absorption of protein could affect postprandial energy metabolism [8]. This effect is conducted by the gut microbiota by converting the dietary proteins into other metabolically active compounds such as short-chain fatty acids, branched chain fatty acids, and different nitrogen-containing compounds [8,12].
In recent years, insects have come to be considered one of the most interesting protein sources, given their amino-acid composition, levels of other nutrients, and low environmental impact compared to traditional farm animals [13]. A growing body of research suggests that the consumption of insects could have a positive impact on human health, stimulating the growth of the intestinal microbiota, improving immune function, and decreasing inflammatory factors [14,15]. On the other hand, insects have been shown to be rich in antimicrobial peptides [16]. Insects are also a source of fiber, namely chitin, which can lead to poor digestibility and reduce essential amino-acid (EAA) bioavailability [17]. Therefore, the partial or total elimination of chitin could be a good solution for improving the nutritional aspect of insect proteins [18]. Some physical processes, such as irradiation and ultrasound, and biological treatments, such as enzymatic hydrolysis using protease enzymes and fermentation by lactic acid bacteria, have been successfully applied to greatly improve the digestibility and the nutritional and functional properties of insect proteins and to decrease their allergenicity [19,20,21,22,23].
This work is a continuation of a previous 14-day in vivo rat study, in which the nutritional quality of a probiotic beverage enriched with either cricket protein in its native (CP) or hydrolysed (CP.H) form was investigated in order to determine the effect of hydrolysis pre-treatment on nutritional parameters such as the protein efficiency ratio (PER), the net protein efficiency ratio (NPR), true digestibility (TD), and apparent digestibility (AD) [24]. Cricket protein was utilized because its amino acid composition and scores meet the requirements of the World Health Organization (WHO), with an EAA score of 44.5% (>36% required) [24]. The results of the study indicated that cricket proteins benefit from a complete amino-acid profile rich in growth-including methionine and cysteine [24]. Moreover, nutritional improvements of NPR, PER, and AD (similar to that of casein) were observed in protein hydrolysates compared to whole proteins. Here, we evaluate the effect of the above-described 14-day protocol on the composition of the gut microbiota and the association of bacterial taxa with diet nutritional parameters.
2. Materials and Methods
2.1. Cricket Protein Hydrolysates Preparation
The fermented and the non-fermented beverages used in this study were produced by Bio-K Plus International Inc., a Kerry company (Laval, QC, Canada). Organic cricket flour (60% protein content) was produced by Nexxus Foods (Montreal, QC, Canada). The cricket protein hydrolysates were prepared in two steps, according to a previous study carried out in our laboratory [25]: the first step consisted of pretreating a cricket protein suspension (40% w/v) by ultrasound (US) using a QSonica Q500 sonicator (model FB-505; Fisher Scientific, Ottawa, ON, Canada). The parameters of the sonicator were programmed as follows: power of 500 W, frequency of 20 kHz, amplitude of 60%, and treatment duration of 15 min in pulsed mode. Then, the cricket suspension was submitted to an enzymatic hydrolysis using Alcalase® 2.4 L FG (Novozymes A/S, Bagsvaerd, Denmark), according to the following conditions: enzyme/substrate (E:S) ratio of 1:10 (w/w), reaction time of 180 min, hydrolysis temperature of 55 °C, and pH 8.0. Then, the suspension was heated to 95 °C for 10 min for enzyme inactivation. Afterwards, the suspension was cooled down at room temperature and centrifuged at 13,000× g for 20 min. The supernatant was lyophilized (Labconco Freezone® 2.5 L, model 7670521, Fisher Scientific) to form protein hydrolysates (CP.Hs) used for the enrichment of fermented beverages.
2.2. Preparation of Beverages
A commercial Bio-K+® blueberry fermented beverage, in powdered form, was used for enrichment. The powder was weighed and hydrated with filtered water before being enriched with cricket powder having a total protein content of 13% and the proteins were pre-treated with selected processes, as described previously [24,25,26]. Other beverages non-enriched (with 3% of protein) and non-fermented were produced and used as controls for comparison. The beverage was then pasteurized at 90 ± 2 °C for 60 s, then cooled to 37 °C. Individual probiotic bacteria (Lactobacillus acidophilus CL1285, Lacticaseibacillus casei LBC80R, and Lacticaseibacillus rhamnosus CLR2) were kept frozen at −80 °C in MRS broth containing glycerol (20% w/v). The starter culture was prepared by thawing each individual strain, then inoculating the bacteria in increasing volumes of MRS broth. These cultures were then placed at 37 °C for 24 h three consecutive times. Finally, bacteria were mixed, and this represented the starter culture used to ferment the protein-enriched beverage. The beverage was inoculated with Bio-K+™ starter culture at 108 CFU/mL, packaged in bottles containing 98 g of product, aluminum-sealed, incubated at 37 ± 1 °C for 14 ± 2 h, and cooled to 4 °C. Another beverage was prepared using the same protocol, but without addition of the probiotic bacteria. This preparation served as control. The beverages were then freeze-dried and milled to pass through a 20-mesh sieve prior to preparation of the diets. The total weight of the freeze-dried products was 1.1 kg each, with a 49.5% protein content.
2.3. Preparation of Experimental Diets and Animal-Study Design
Briefly, four diets (in pellet form) were produced and evaluated: CP-based and CP.H-based supplemented probiotic beverage diets, casein-containing diets, and protein-free diets. The formulations were prepared according to an official method of analysis (AOAC 960.48) [27]. The percentage of proteins was adjusted to 10% for all the formulations except the protein-free diet, which served as a negative control. Casein was used as a reference protein (positive control). The lyophilized fermented beverages’ CP and CP.H were used as protein sources. Soybean oil, vitamins, minerals, cellulose, sucrose, and starch were also added to the formulations and their amounts were calculated to obtain equal calorie counts for all diets [24]. The dose of probiotics used to ferment the beverages was a standard dose of the combination of 3 probiotic strains that allowed for an adequate fermentation profile and appropriate organoleptic properties of the fermented beverages (from the manufacturer’s protocol of Bio-K+, a Kerry company).
For the animal study, the experiment was conducted according to prior approval by the National Experimental Biology Laboratory (LNBE) and the Institutional Animal Care Committee (CIPA) of the INRS–Armand–Frappier Health and Biotechnology Research Centre, in accordance with the principles of the Canadian Council on Animal Care (CCAC), by using the CIPA no. 1809-04 protocol [24]. Male Wistar rats, 20–23 days old, were distributed in 4 groups of 7 rats and housed in separate cages. Cycles of 12 hours of light–dark and a temperature of 20 ± 0.5 °C were fixed throughout the experiment period. For the first 5 days (the acclimation phase), the rats received a standard diet. In the subsequent 14 days, the rats were fed ad libitum with the protein-free (n = 4), casein (n = 5), CP (n = 5), or CP.H (n = 5) experimental diets. The survival rate of the experimental animals was 100%.
In terms of study limitations, this work was carried out in a rodent model; thus, it assessed effects on a rodent microbiota, which is different from that of humans. Thus, the results may not all translate to humans. Furthermore, the duration of the study was only 14 days, and a longer protocol may have resulted in different observations with respect to the adaptation of bacteria to the diets. Also, we did not vary the protein concentration, which could also reduce the impact on the results. Finally, the experimental design was limited to the effect of fermented, probiotic, cricket protein-enriched beverages compared to a non-fermented casein-based diet (without probiotic), used as a positive control for the in vivo test.
2.4. rDNA Sequencing and Metagenomic Analysis
At the end of 14 days of experiment, feces were collected from the colons of the rats and stored in a sterile plastic tube. DNA from fecal samples was extracted using the Qiagen DNeasy PowerSoil kit. The DNA concentrations of the extracts were measured fluorometrically with the Quant-iT PicoGreen dsDNA kit (Thermo Fisher Scientific, Waltham, MA, USA), and the DNA samples were stored at −20 °C until 16S rDNA library preparation was completed according to the Illumina “Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System” protocol. Briefly, 15 ng of DNA was used as the template, and the V3–V4 region of the 16S rRNA gene was amplified by PCR using the following primers: 16S Amplicon PCR forward primer = 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′, 16S Amplicon PCR reverse primer = 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′ followed by a second PCR reaction to introduce indices (Nextera XT Index; Illumina, San Diego, CA, USA). The 16S metagenomic libraries were eluted in 30 μL of nuclease-free water and 1 μL was qualified with a Bioanalyser DNA 1000 chip (Agilent Technologies, Santa Clara, CA, USA) to verify the amplicon size (expected size ∼600 bp) and then quantified with a Qubit (Thermo Fisher Scientific, Waltham, MA, USA). The libraries were then normalized and pooled to 2 nM, denatured, diluted to a final concentration of 10 pM, and supplemented with 5% PhiX control (Illumina). Sequencing (2 × 300 bp paired-end) was performed using the MiSeq reagent kit V3 (600 cycles) on an Illumina MiSeq system (Illumina, San Diego, CA, USA). Sequencing reads were generated in less than 65 h. Image analysis and base calling were carried out directly on the MiSeq. The preprocessing of obtained sequences and bacterial taxa assignation was performed according to the Dada2 pipeline (version 1.10.1) using the Ribosomal Database Project (RDP release 11) reference database [28]. Analyses were then conducted on sequence counts normalized by cumulative-sum scaling (CSS) (MetagenomeSeq R package) [29]. CSS divides sequence counts by the median sequence counts of each sample [30]. CSS-normalized counts are thus expressed in relation to the entire bacterial composition of each sample and are viewed as being more appropriate than total-sum scaling [31].
In order to determine if the nutritional parameters [24] related to different diets (see above) after feeding the rats for 14 days correlated with alterations in microbial composition, we performed a Spearman’s rank-order correlation.
2.5. Statistical Analysis
The gut microbiota characteristics, the potential relationship between the diet groups, and the microbial community structure were examined via PCA and PCoA, performed using Bray–Curtis dissimilarity indices of beta-diversity and permutational multivariate analysis of variance (PERMANOVA) (VeganR package) [32]. Two-way ANOVA (taxa abundance ~ Genotype*Diet), followed by the Tukey HSD post hoc test, was conducted to evaluate the influence of the diet group and the protein type on gut microbial taxa. The results were considered statistically significant at p < 0.05 or FDR-adjusted p < 0.1. Analyses were performed with R software, version 3.4.3. [29].
3. Results and Discussion
3.1. Gut Microbiota: Diet Effect
3.1.1. Diet Effect on Gut Microbiota: Comparison of PCA and PCoA Methods
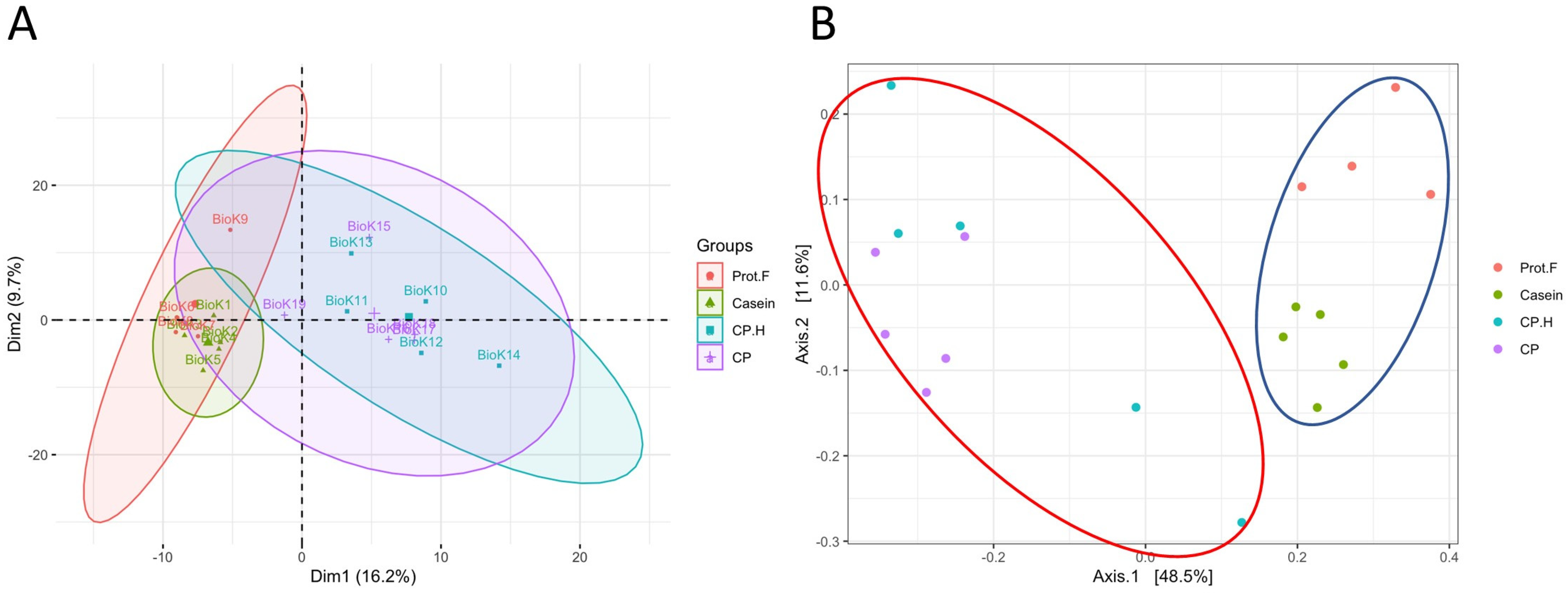
We analyzed whether protein-free, casein-, CP-based, and CP.H-based fermented beverage diets modified the gut microbiota of rats after 14 days of ad libitum feeding. Given the rapidity at which the gut microbiota responds to dietary interventions [33], we hypothesized that our protocol would result in demonstrable differences between the experimental groups. Principle component analysis (PCA) suggested that the casein-based diet did not significantly alter the gut microbiota compared to the protein free-based diet, as both groups clustered together, while the fermented CP-based and CP.H-based diets clustered mostly independently from the control diets (Figure 1A). Subsequent principal coordinate analysis (PCoA) followed by permutational multivariate analysis of variance (PERMANOVA) confirmed that the fecal microbiota of rats fed the protein free-based and casein-based diets were not significantly different from one another (p > 0.05); however, the fermented CP.H and CP diets were statistically different from the casein-based diet (p = 0.024 and p = 0.024, respectively) and the protein-free diet (p = 0.049 and p = 0.024, respectively) (Figure 1B).
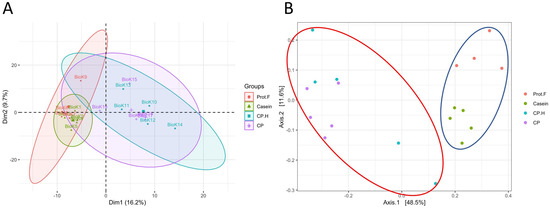
Figure 1.
Comparison between the gut microbiota of rats according to diet and protein-type feeding. (A) Principal component analysis (PCA), (B) principal coordinate analysis (PCoA) of fecal microbiota from feed with different diets. Permutational multivariate analysis of variance (PERMANOVA) confirmed that the microbiota from rats fed with cricket protein-containing fermented beverage-based diets (red ellipse) were different from those fed control diets (blue ellipse). Each point represents one biological sample. Prot.F: protein free-based control diet; casein: casein-based control diet; CP.H: cricket hydrolysates-enriched fermented beverage diet; CP: whole-cricket protein-enriched fermented beverage diet.
3.1.2. Diet Effect on Microbial Diversity: Comparison of Shannon Alpha-Diversity Index and Firmicutes-to-Bacteroidetes Ratio
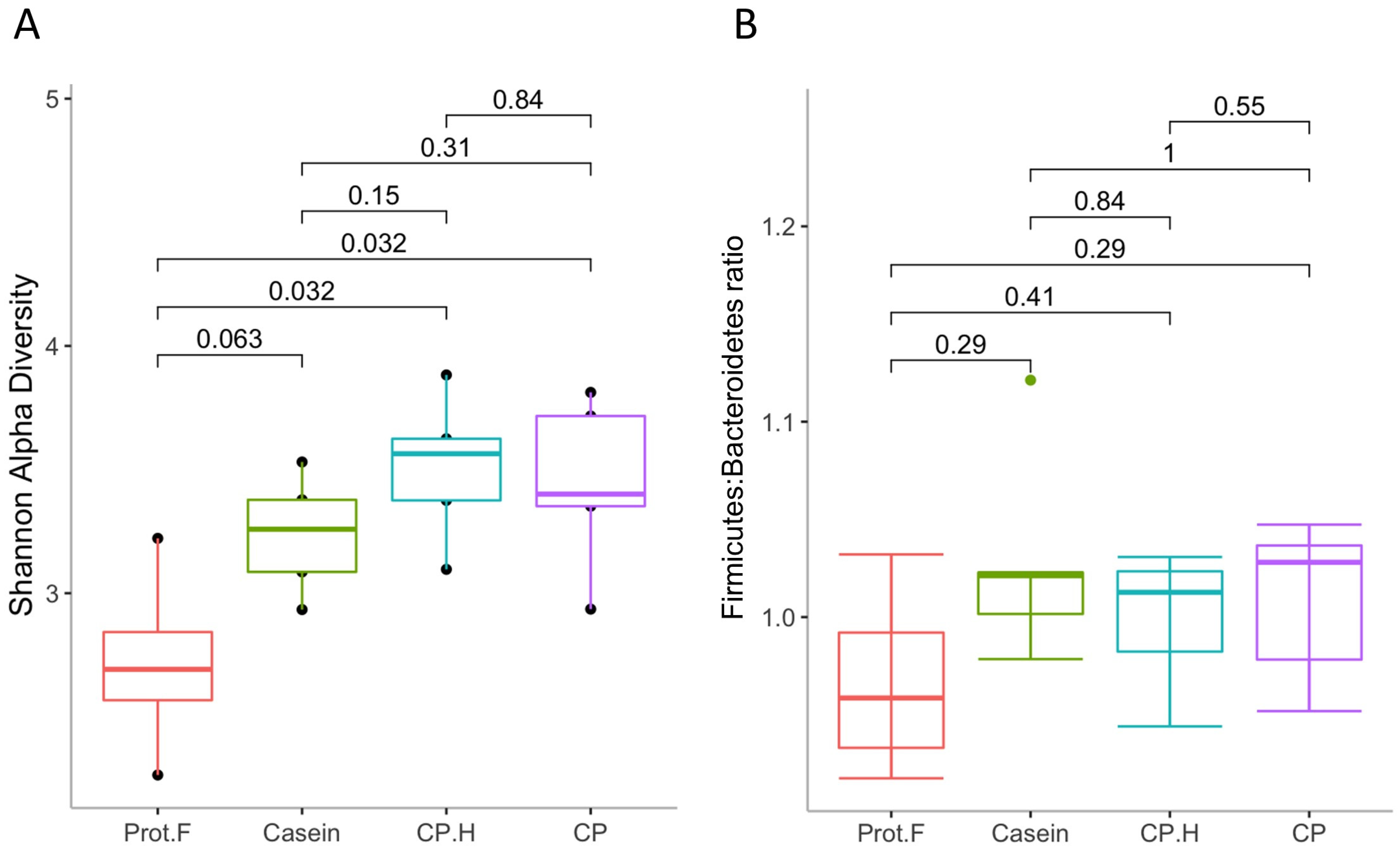
The effect of the diet group on microbial diversity within the gastrointestinal tract was determined using the Shannon alpha-diversity index (Figure 2A). Supplementation with casein showed a trend for increased alpha diversity compared to the protein-free diet (p = 0.063); however, both the CP.H-based and CP-based diets had significantly increased alpha diversity at the end of the protocol. No difference in alpha diversity was observed between casein-, CP.H-based, and C.P-based diets, indicating that the alpha diversity of gut microbiota increased due to the diverse nutrients, such as the amino acids provided by protein-based diets that serve to provide a variation of substrates [34,35]. These data contrast with the general observation that increased protein consumption is associated with decreased bacterial diversity [36,37]; however, none of these studies compared the effects to a protein-free diet, as we have here. None of the diets showed any differences in the Firmicutes-to-Bacteroidetes ratio (F:B ratio) (Figure 2B). However, we noted a non-statistically significant trend for a decreased F:B ratio in the group fed the protein free-based diet compared to groups fed the protein-based diets (casein, fermented CP.H, and fermented CP). While the F:B ratio has been thought to correlate with obesity and other diseases [38,39], recent evidence has cast doubt on this with respect to obesity [40,41]; the protein-based diet used in this study did not cause obesity, as the rats in our study were all of normal weight for their age [24]. However, while the animals fed with protein did not show obese characteristics, animals fed the protein free-based diet showed a loss of weight after 14 days (from 103.3 ± 3.1 g to 78.8 ± 1.9 g). The results of this research are in a good agreement with those of Stull et al. (2018) [16] in humans and Jarett et al. (2019) [42] in dogs, in which cricket consumption did not alter alpha and beta diversity when compared to other proteins used in the studies. Increased alpha diversity is generally associated with weight loss [43]; however, again, we point out that our control diet reflects an extreme, in that it is protein-free and that most studies assessing alpha diversity and weight loss focus on experimental protocols that result in overweight/obesity or participants who are overweight/obese.
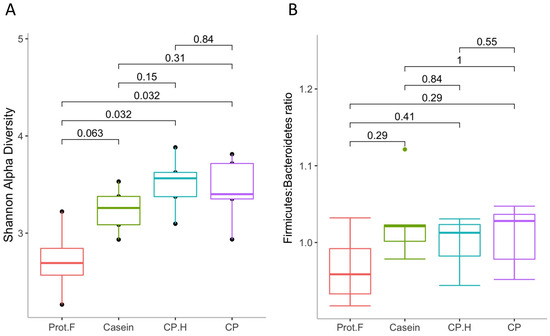
Figure 2.
(A) Shannon alpha-diversity index, evaluating gut microbiota richness and evenness and (B) Firmicutes-o-Bacteroidetes ratio. Prot.F: protein free-based control diet; casein: casein-based control diet; CP.H: cricket hydrolysates-enriched fermented beverage; CP: whole-cricket protein-enriched fermented beverage.
3.1.3. Diet Effect on the Relative Abundance of Gut Microbiota at Phylum and Family Levels
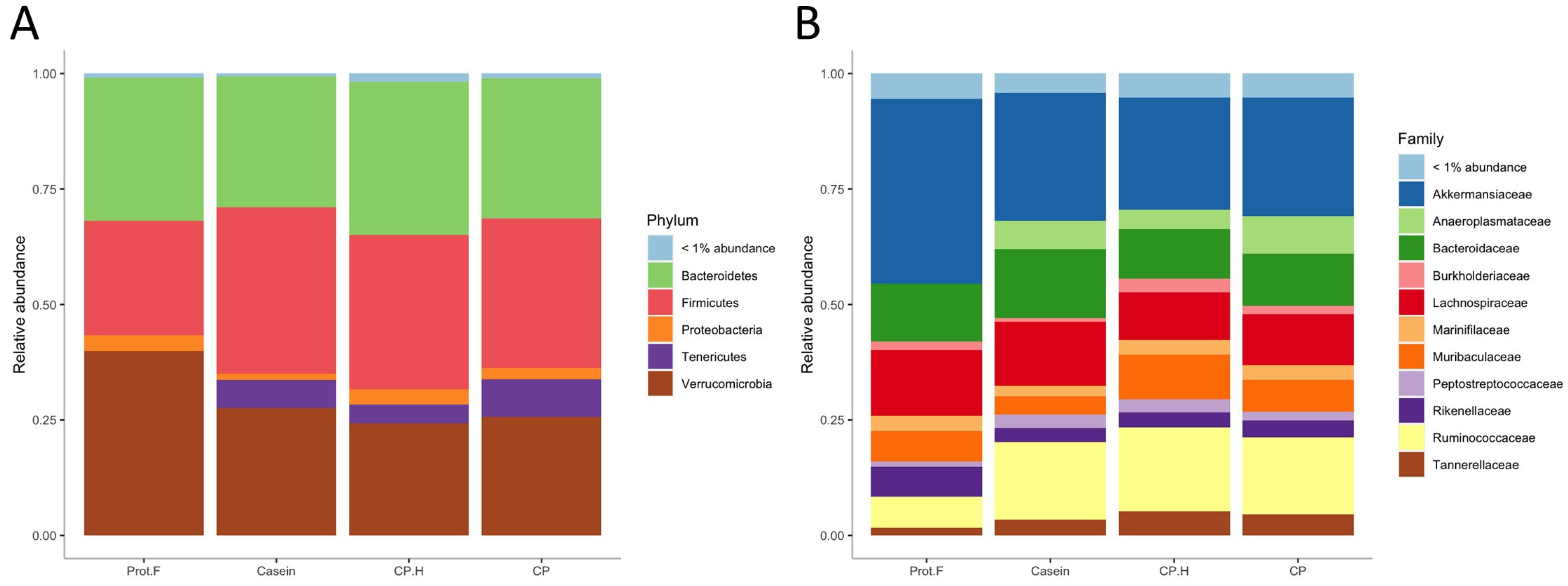
The relative abundance of fecal bacteria from rats fed the different diets is presented in Figure 3. At the phylum level (Figure 3A), the most predominant phyla for the four groups were Verrucomicrobia, Bacteroidetes, Firmicutes, and Proteobacteria. Further, an additional phylum (Tenericutes) was significantly increased in all protein-containing groups, compared to the protein-free group (casein vs. protein-free; p = 6.7 × 10−8, CP.H vs. protein-free; p = 7.5 × 10−7, CP vs. protein-free; p = 1.9 × 10−7). It may be that members of the Tenericutes phylum are beneficial for the integrity of the intestine. It has also been found to be decreased within inflammatory bowel disease patients [44].
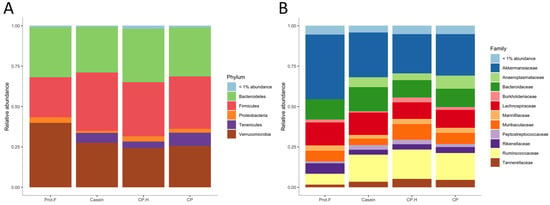
Figure 3.
(A) Relative abundance of gut microbiota at the phylum, (B) family level. Prot.F: protein free-based diet; casein: casein-based control diet; CP.H: cricket hydrolysates-enriched fermented beverage; CP: whole-cricket powder-enriched fermented beverage.
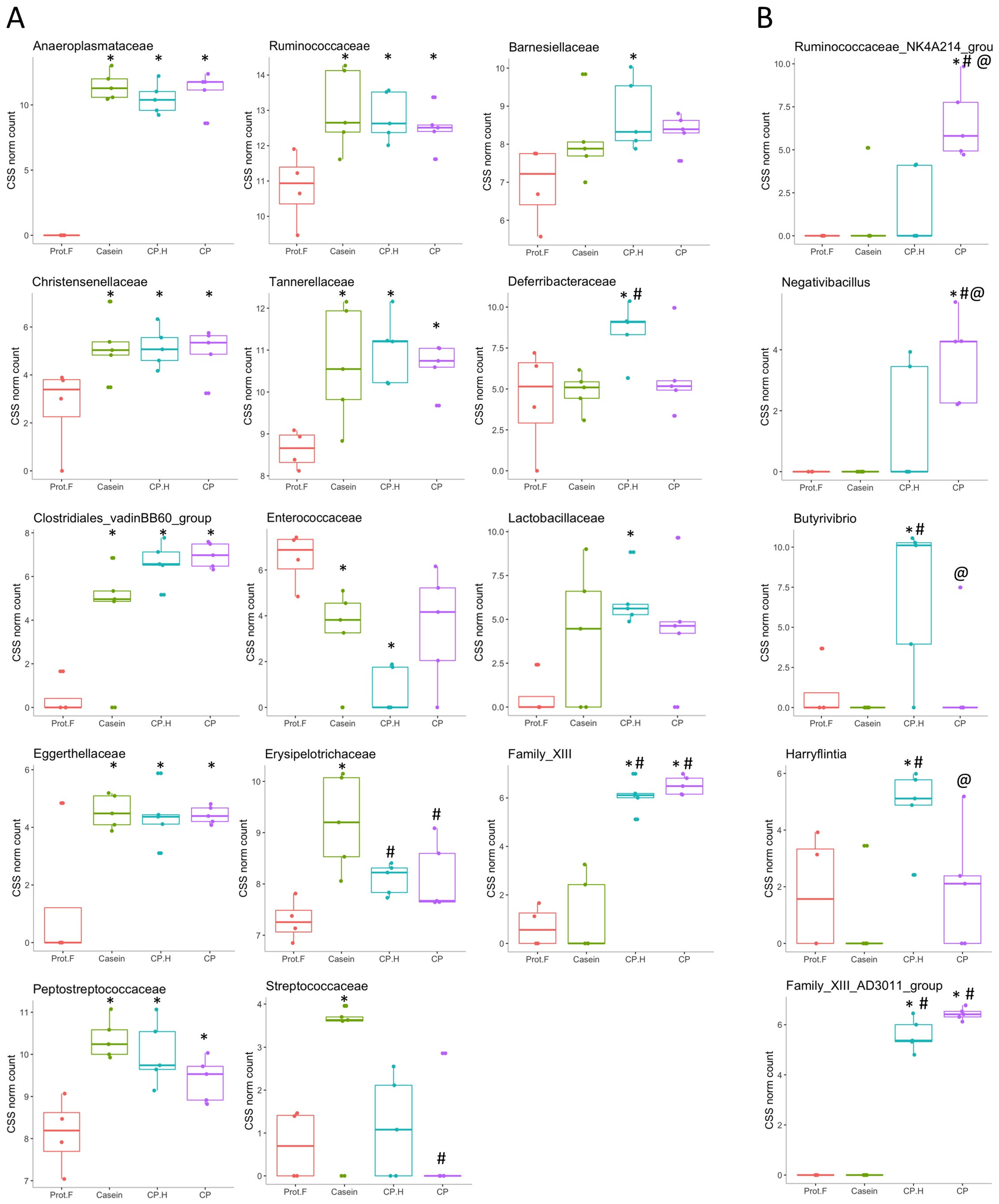
At the family level (Figure 3B and Figure 4A), significant differences in abundance of several taxa were observed between groups fed with protein-based diets, independently of the protein type, and the group fed the protein free-based diet. Indeed, Anaeroplasmataceae (which belongs to the Tenericutes phylum) was only detected in the gut of animals fed with protein when compared to the group fed without protein, while Christensenellaceae, Clostridiales_vadinBB60_group, Eggerthellaceae, Peptostreptococcaceae, Ruminococcaceae, and Tannerellaceae were all increased in response to protein-supplemented diets (Figure 4A and Supplementary Table S1). Indeed, the increase in Tenericutes appears to be driven by the increase in the families of Anaeroplasmataceae and Clostridiales_vadinBB60_group. Interestingly, increased abundance of Tenericutes/Anaeroplasmataceae has been associated with positive energy balance [45], which is in line with our data, given that protein-fed rats were heavier than those on the protein-free diet. Based on these results, casein, fermented CP.H, and fermented CP could be defined as dietary components contributing to the maintenance of energy and supporting the growth in rodents. Furthermore, Yu et al. (2020) [46] reported that a duck egg-white diet led to a significant increase in the abundance of Proteobacteria and Peptostreptococcaceae in rats, supporting our data that the abundance of this family is increased with protein. In addition, Peptostreptococcaceae was suggested to maintain intestinal homeostasis in humans [46], suggesting that dietary protein can potentially contribute to intestinal health by supporting the abundance of this family.
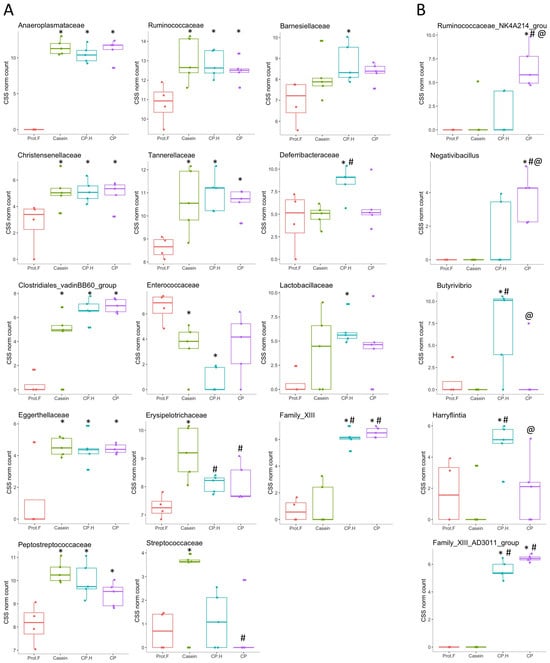
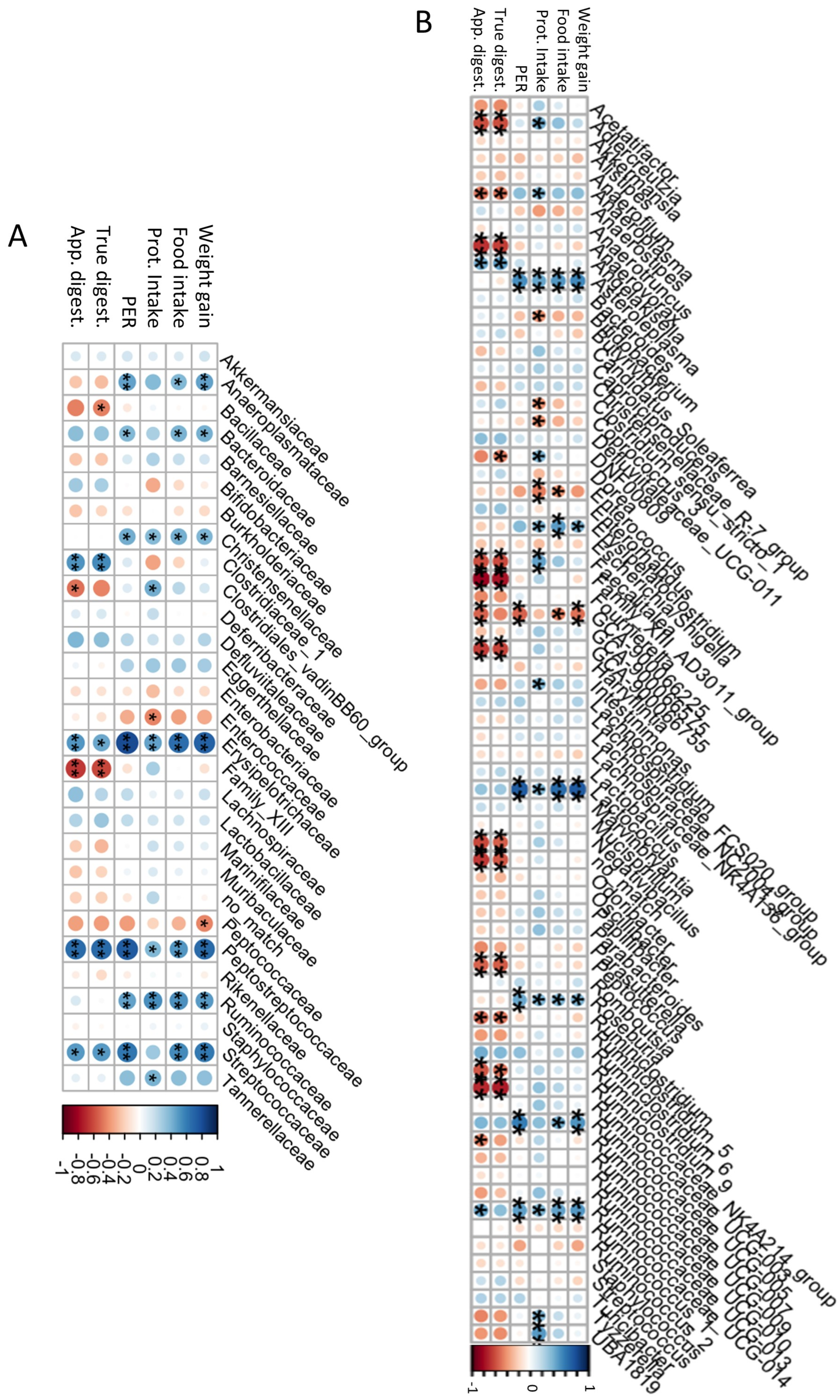
Figure 4.
Assessment of differences in the family level (A) and in the genus level (B) of the gut microbiota of rats in receipt of different diets. * p < 0.1 denotes statistically different groups compared to the Prot-F group; # p < 0.1 denotes statistically different groups compared to the casein group. @ p < 0.1 denote statistically different groups compared to: cricket hydrolysates-enriched fermented beverage group. Prot.F: protein free-based control diet; Casein: casein-based control diet; CP.H: cricket hydrolysates-enriched fermented beverage; CP: whole-cricket powder-enriched fermented beverage.
Previous studies [42,47] showed that the abundance of Ruminococcaceae (with genes encoding for chitin-digesting enzymes) in the gut is thought to have roles in the fermentation of fibers. Thus, the increase of this family in the cricket-protein diets suggests that cricket chitin may be a source of fermentable and indigestible fiber, and that whole-cricket protein may favor the colonization of gut bacteria that have the capacity to degrade chitin. However, our results do not distinguish if chitin or protein led to that increase in Ruminococcaceae. Further targeted studies should be conducted to clarify this observation (Figure 3B). In addition, Nicholson et al. (2012) [48] showed that gut bacteria can utilize indigestible carbohydrates to produce short-chain fatty acids for colonocytes. Furthermore, short-chain fatty acids have been shown to be correlated with healthier metabolic states (e.g., better glucose homeostasis and lipid metabolism) and reduced colon-cancer risk [49]. These results confirmed that the fermented CP-based and CP.H-based beverages did not disrupt the healthy microbiota; on the contrary, they could be potential health-promoting ingredients.
In contrast to the number of families that were observed to be increased similarly by all protein-supplemented diets, only Enterococcaceae was found to be decreased in a statistically significant manner, and then only by casein-fed and fermented CP.H-fed rats (though fermented CP-fed rats showed a tendency to have decreased levels as well) (Figure 4A). This family is one of the first to colonize the gut microbiota in response to exclusive breastfeeding and its abundance decreases gradually with the introduction of other foods [50]. Therefore, it is somewhat counterintuitive that we observed a decrease in response to protein feeding here; however, this is a rather broad family that includes many potential pathogens, many of which are of concern in the development of antibiotic resistance [51]. We were unable to detect changes in the abundance of genera belonging to this family; therefore, we cannot confirm the possibility that some potential pathogenic bacteria were decreased in response to our diets.
Our analysis also identified a subset of bacterial families whose abundances were specifically increased by individual protein-supplemented diets (Figure 4A). Erysipelotrichaceae and Streptococcaceae were increased by casein alone, with the former having higher abundances than both the fermented CP.H-supplemented diet and the CP-supplemented diet, while the latter had a significantly higher abundance than that of the CP-supplemented diet. Streptococcaceae/Lactococcus has been reported to be associated with inflammation and metabolic syndrome [52]. Other studies [53,54] found that the decrease in Streptococcaceae/Lactococcus might play an important role in the prevention of metabolic syndrome. In our study, the family of Streptococcaceae showed significantly lower levels in rats fed with CP compared to rats fed with casein, while Lactococcus abundance was lower for both fermented CP.H-based and CP-based diets compared to casein diets (p = 0.0028 and 0.0011, respectively; Supplementary Table S2).
In contrast, Deferribacteraceae, Barnesiellaceae, and Lactobacillaceae were only increased by CP.H [though trends for increases were observed in the CP-fed rats for the latter two as well). Similarly, Ijaz et al. (2020) [55] observed that mice fed with a pork-protein diet showed an increase in Deferribacteraceae and Lactobacillaceae. In contrast, Family_XIII was strongly increased with the cricket protein-based diets, independently of the protein form (hydrolysates or whole protein), but it was not altered by the casein diet. Family_XIII and Clostridiales (Figure 4A) may have roles in the metabolism of protein in the intestinal tract and are associated with the butyrate kinase butyrate–synthesis pathway that produces butyrate from protein [56,57]. Chai et al. (2019) [58] found that several genera within these families are potential butyrate producers, many of which are believed to have potential clinical applications, especially for the treatment of inflammatory bowel disease and Clostridioides difficile (C. difficile) infection [59].
When we went on to examine the effects of the different diets at the genus level, several interesting observations were made. Anaeroplasma, Roseburia, Romboutsia, and Ruminiclostridium_9 were all significantly increased in all the protein-containing diets, compared to the protein-free control diet (Supplementary Table S2). These bacteria are associated with the fermentation of fiber into metabolites such as short-chain fatty acids, including acetate, propionate, and butyrate, which act as an anti-inflammatory constitutives [60]. In contrast, other genera showed protein-specific alterations in relative abundance. Rats fed with casein showed strong trends for increased abundance of UCG-014 and USG-005 (belonging to Ruminococcaceae), Asteroleplasma, and Lactococcus (though only the latter showed a statistically significant higher level, compared to every other diet), while Ruminococcus_2 was decreased (Supplementary Table S2). Conversely, CP and/or CP.H specifically increased the abundances of Parabacteroides, Tyzzerella, Intestinimonas, and Caproiciproducen, associated with short-chain fatty-acid production [61], in comparison to the protein-free diet, and the abundances of Anaerovorax, the Ruminococcaceae genera UCG-007, UCG-009, UCG-013, and UBA1819 in comparison to the casein diet (Supplementary Table S2). This suggests that these latter genera are specifically responsive to the fermented protein diets and not just to protein-rich diets in general. The anti-inflammatory effect of Parabacteroides goldsteinii (belonging to Parabacteroides) has been investigated by Lai et al. (2022) [62], who reported that the bacteria could decrease the production of pro-inflammatory cytokines while increasing intestinal integrity. Interestingly, we identified several genera that were specifically increased by either CP (GCA-900066755, Faecalitalea, Ruminococcaceae_NK4A214_group, and Negativibacillus, with the latter two being significantly increased in CP vs. CP.H.; Figure 4B) or CP.H (GCA-900066575, Candidatus_Soleaferrea, Butyrivibrio, and Harryflintia, with the latter two being significantly increased in CP.H vs. CP (Figure 4B). These latter genera, therefore, appear to be very sensitive to whether the cricket protein is hydrolyzed or not.
Family_XIII_AD3011_group was greatly increased in the fermented CP.H and CP groups compared to either control, where it was not detected, and thus it appears to be the driving force for the increased abundance of Family_XIII, mentioned above. Similarly, the Lactobacillus genus abundance mirrored that observed for Lactobacillaceae, being higher only in rats fed with CP.H compared to rats fed the protein-free diet, (p = 0.056; Supplementary data, Table S2), while the protein-free group had the lowest abundance of this genus. Lactobacillus spp. play key roles in the maintenance of metabolic balance [63] and in the reduction of the antigen load from gut microbiota to the host, resulting in an anti-inflammatory response [64]. In addition, they are important species within commercial probiotics [65,66,67]; thus, their increased abundance may confer a beneficial effect on animals fed the CP.H diet. The presence of Lactobacillus spp. in high abundance for rats fed with CP.H could be explained by the survival of Lactobacillus spp. from the fermented beverage during digestion. Indeed, the presence of proteins in the form of hydrolysates can be a more assimilable substrate for bacteria, which can influence the viability of these bacteria, potentially by increasing their ability to resist stressful gastrointestinal conditions. The CP-based diet did not have such an effect on the Lactobacillus spp. abundance, despite the presence of these bacteria in the fermented beverage. Thus, our results suggest that cricket-protein hydrolysates may be more beneficial for the protection and proliferation of Lactobacillus genera, compared to the whole-cricket protein.
3.2. Correlation between Bacterial Taxa and Nutritional Parameters
The nutrition parameters related to growth, food, and protein intake, PER, TD, and AD, respectively, of the different diets (CP, CP.H, casein, and protein free-based diets) were evaluated in the previous study of [24]. Briefly, the results pointed out that the incorporation of CP.H, in addition to in vivo digestibility enhancement, increased the PER and the net protein ratio (NPR) significantly (p ≤ 0.05), compared to the incorporation of CP, from 1.7 to 2.0 and from 0.4 to 1.0, respectively. The AD of CP.H was 94%, which was close to that of the casein group (96%) and significantly (p ≤ 0.05) higher than that of the CP group (85%).
The association of gut microbiota with the nutritional parameters was determined and the results are shown in Figure 5. At the family level (Figure 5A), Anaeroplasmataceae, Bacteroidaceae, Christensenellaceae, and Ruminococcaceae were positively correlated with weight gain (rho = 0.47, 0.42, 0.40, and 0.50, respectively), food intake (rho = 0.39, 0.41, 0.42, and 0.53, respectively), protein intake (rho = 0.38, 0.27, 0.38, and 0.54, respectively), and PER (rho = 0.47, 0.39, 0.43, and 0.47, respectively). The high abundance of these families reflects an increase in proteolytic bacteria with that of protein intake, and casein-based, CP-based, and CP.H-based diets did not produce differences in their abundances. Erysipelotrichaceae, Peptostreptococcaceae, and Streptococcaceae were positively correlated with all the nutritional parameters, as well as with digestibility (rho = 0.45, 0.68, and 0.50, respectively), with the latter having a statistically insignificant correlation with protein intake. Clostridiales_vadinBB60_group showed a positive correlation with protein intake (rho = 0.43) and a negative correlation with apparent digestibility (rho = −0.43), while Enterococcaceae was also negatively correlated with protein intake (rho = −0.43). Conversely, Family_XIII, which was particularly changed by the fermented CP.H-based and CP-based diets, showed a decrease when the digestibility increased (rho = −0.60). Similar negative correlations were observed for Bacillaceae and Clostridiales_vadinBB60_group with digestibility (rho = −0.44 and 0.43, respectively). Other bacteria did not show any correlation with nutritional parameters. Pozuelo et al. (2015) [68] observed a high proportion of Erysipelotrichaceae in healthy and lean individuals, related to the availability of butyrate. This suggests that a high-protein diet increases the abundance of Erysipelotrichaceae (high abundance observed for CP-based, CP.H-bsed, and casein-based diets), which may increase the availability of butyrate.
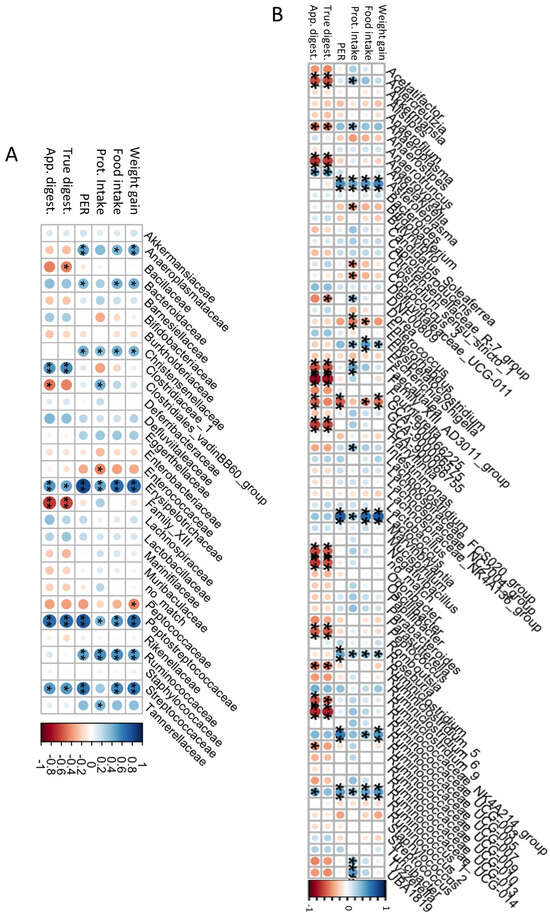
Figure 5.
Associations among bacterial families (A), genera (B), and the nutritional quality. Prot: Intake: protein intake; PER: protein efficiency ratio; true digest: true digestibility; app. digest: apparent digestibility; * p < 0.1, ** p < 0.05.
A study in humans [69] showed an anti-inflammatory property of butyrate, which could be implicated in the regulation of immune responses. In addition, butyrate has been shown to be effective in suppressing cancer and treating mucosal inflammation in both human and animal models [70,71]. Furthermore, Van der Wielen et al. (2000) [72] reported that the increase in butyric acid could be related to the decrease in the amount of Enterobacteriaceae. The positive correlation between Ruminococcaceae and the nutritional parameters suggests the importance of a protein-enriched diet for the protection of the intestinal microbiota. On the other hand, no correlation was observed between Lactobacillaceae abundance and the nutritional parameters mentioned above, despite the presence of these bacteria in the fermented CP.H-based and CP-based diets. These results support the specificity of the effects of a protein-based diet on specific bacterial taxa, although the influence of the included probiotics within the fermented beverage on the metabolic activities of the gut microbiota should be taken into account and are beyond the reach of this study.
At the genus level (Figure 5B), we detected a dichotomy between genera with respect to their correlations with various parameters: while Asteroleplasma, Erysipelatoclostridium, Lactococcus, Roseburia, and the Ruminococcaceae genera UCG-005 and UCG-014 were all positively correlated with weight gain, food intake, protein intake, and PER, but not with digestibility, Aldecreutzia, Anaerovorax, Faecalitalea, Family_XII_AD3011_group, GCA900066755, Negativibacillus, Peptococcus, Ruminoclostridium_9, and Ruminococcaceae_NK4A214_group were all negatively correlated with digestibility intake (rho = −0.58, −0.25, −0.59, −0.75, −0.43, −0.53, −0.54, −0.50, and −0.73, respectively). Bifidobacterium was negatively correlated with protein intake (rho = −0.39). According to Stull et al. (2018) [15], a cricket-based diet could increase Bifidobacterium in humans. However, in our study, the abundance did not differ among diets. Similar observations were observed by Jarett et al. (2019) [42], who found that a cricket-based diet did not affect this genus in the gut microbiota of dogs. The presence of chitin, characterized by a structure similar to that of cellulose [73], could explain the high abundance of Ruminococcaceae_NK4A214_group in rats fed with the CP-based diet, as well as the negative correlation with the digestibility, as the CP showed low digestibility compared with casein and CP.H. Erysipelatoclostridium, considered to be a potential opportunistic pathogen [74,75], was observed to be lower for animals fed with fermented CP.H and CP, compared to those fed with a casein-diet or a protein free-based diet (see above). These results confirm that whole-cricket protein or cricket-protein hydrolysates may maintain a more balanced composition and reduce the pathogens within the gut bacteria. Other potential pathogens, such as those belonging to Enterococcaceae, showed a decrease when the weight gain, food intake, protein intake, and digestibility increased (rho = −0.38, −0.39, −0.45, and −0.35, respectively), indicating the importance of a diet rich in protein for the protection of the gut microbiota against pathogens.
4. Conclusions
This metagenomic study examined the impact of CP (whole-cricket protein)-enriched and CP.H (cricket protein hydrolysates)-enriched probiotic beverages on the gut microbiota of rats. The results of PCA and PCoA analyses showed that the global gut microbiota profile was modified by the diets containing fermented CP and CP.H proteins. While alpha-diversity was similarly increased by the fermented beverage diets and several bacterial taxa were identified as being similarly altered, independent of the proteins added (casein, fermented CP, or fermented CP.H), we did identify taxa that were either similarly or differentially affected by fermented CP-enriched and CP.H-enriched diets in comparison to the casein-enriched diet. These alterations likely resulted in the significant differences in overall gut microbiota architectures induced by the fermented CP-enriched and CP.H-enriched diets compared to the control diets. Moreover, the fermented CP.H-enriched probiotic beverage could be related to the decrease of the number of potential pathogenic members of Enterococcaceae. Correlation analysis highlighted the contribution of protein-enriched, fermented diets in general between nutritional parameters and the bacterial families Anaeroplasmataceae, Christensenellaceae, Peptostreptococcaceae, and Ruminococcaceae, all of which were similarly increased in these diets. At the genus level, Asteroleplasma, Erysipelatoclostridium, Lactococcus, and Roseburia, were all positively correlated with weight gain, food intake, protein intake, and PER, though these taxa were not altered by the protein-enriched fermented diets. Hence, these results suggest that protein-enriched, fermented diets alter the microbiome, in correlation with improved nutritional parameters, and that CP or CP.H may be viable alternative protein sources of probiotic product supplementation. It must be stated, however, that the above-mentioned effects cannot be separated from the general effects of the fermented probiotic beverage through this set of experiments. Therefore, further studies should be carried out to acquire a broader understanding of the impact of cricket protein-enrichment of fermented probiotic formulations on producing functional foods with effects on the gut microbiota and the ability to support digestive health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13020204/s1, Table S1: Assessment of differences in the family level of the gut microbiota of rats in receipt of different diets: p-value results; Table S2: Assessment of differences in the genera level of the gut microbiota of rats in receipt of different diets: p-value results.
Author Contributions
Conceptualization, M.M. and M.L.; methodology, C.D., M.M. and M.L.; software, C.D., T.V. and C.S.; validation, M.M., S.S., C.S. and M.L.; formal analysis, C.D., S.L., T.V., C.S. and V.D.M.; investigation, C.D., M.M., S.S., C.S. and M.L.; resources, M.M. and M.L.; data curation, C.D. and C.S.; writing—original draft preparation, C.D.; writing—review and editing, M.M., B.R.A.U., S.S., E.S., Z.A., V.D.M., C.S. and M.L.; visualization, M.M., S.S., C.S. and M.L.; supervision, M.M. and M.L.; project administration, M.L.; funding acquisition, V.D.M. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Economy and Innovation (MEI) of Quebec (PSR-SIIRI-984), the Natural Sciences and Engineering Research Council of Canada (NSERC, no: CRDPJ/505365-2016), the Ministry of Agriculture, Fisheries and Food (MAPAQ; government of Quebec) via the PPIA12 Research Chair, Bio-K+, a Kerry company (Laval, QC, Canada), the International Atomic Energy Agency (IAEA), the Canada Excellence Research Chair on the Microbiome-Endocannabinoidome Axis in Metabolic Health (CERC-MEND; Vincenzo Di Marzo), which in turn is funded by the Canada First/Apogée program of the Tri-Agency of the Canadian Federal Government. Chaima Dridi was a fellowship recipient of the Armand–Frappier Foundation.
Institutional Review Board Statement
The animal study protocol was approved by the National Experimental Biology Laboratory (LNBE) and the Institutional Animal Care Committee (CIPA) of the INRS–Armand–Frappier Health and Biotechnology Research Centre, in accordance with the principles of the Canadian Council on Animal Care (CCAC), by using the CIPA no. 1809-04 protocol.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge Bio-K+, a Kerry Company, for supplying Bio-K+ beverages and cricket powder. The authors also thank Julie Auclair and Myriam Coutu for their technical support in the extraction of DNA and the quantification of probiotics, and Fettah Rezzouk, Meriem Haddi for the preparation of the probiotic beverages. Cristoforo Silvestri is associated with and supported by the Canada Excellence Research Chair on the Microbiome–Endocannabinoidome Axis in Metabolic Health, held by Vincenzo Di Marzo, and supported by the Canadian Federal Tri-Agency. Tommaso Venneri was partly supported by a travel grant from the Joint International Unit between the National Research Council (CNR) of Italy and Laval University on Chemical and Biomolecular Research on the Microbiome and its Impact on Metabolic Health and Nutrition (UMI-MicroMeNu), which in turn is partly supported by the Sentinelle Nord–Apogée (Canada First) program funded by the Federal Tri-Agency.
Conflicts of Interest
Author Mathieu Millette is a paid employee of Bio-K+, a Kerry company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- McNulty, N.P.; Yatsunenko, T.; Hsiao, A.; Faith, J.J.; Muegge, B.D.; Goodman, A.L.; Henrissat, B.; Oozeer, R.; Cools-Portier, S.; Gobert, G.; et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci. Transl. Med. 2011, 3, 106ra106. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Cooney, J.C. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 175285. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.M.; Cryan, J.F.; Shanahan, F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota modulation and implications for host health: Dietary strategies to influence the gut–brain axis. Innov. Food Sci. Emerg. Technol. 2014, 22, 239–247. [Google Scholar] [CrossRef]
- Madsen, L.; Myrmel, L.S.; Fjære, E.; Liaset, B.; Kristiansen, K. Links between dietary protein sources, the gut microbiota, and obesity. Front. Physiol. 2017, 8, 1047. [Google Scholar] [CrossRef]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.D.A. Effect of dietary protein and processing on gut microbiota—A systematic review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2018, 20, 145–154. [Google Scholar] [CrossRef]
- Bartlett, A.; Kleiner, M. Dietary protein and the intestinal microbiota: An understudied relationship. iScience 2022, 25, 105313. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. IJMS 2016, 17, 603. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, S.; Gordya, N.; Suborova, T. Insect antimicrobial peptide complexes prevent resistance development in bacteria. PLoS ONE 2015, 10, e0130788. [Google Scholar] [CrossRef] [PubMed]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In Vitro Crude protein digestibility of Tenebrio Molitor and Hermetia Illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, L.; Zabodalova, L.; Baranenko, D. On the potential of lupin protein concentrate made by enzymatic hydrolysis of carbohydrates in dairy-like applications. Agron. Res. 2014, 12, 727–736. [Google Scholar]
- Sinha, R.; Radha, C.; Prakash, J.; Kaul, P. Whey protein hydrolysate: Functional properties, nutritional quality and utilization in beverage formulation. Food Chem. 2007, 101, 1484–1491. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Protein solubility, digestibility and fractionation after germination of Sorghum varieties. PLoS ONE 2012, 7, e31154. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, M.; Fortier, N.; Guénette, S.; L’Ecuyer, A.; Savoie, M.; Franco, M.; Lachaîne, J.; Weiss, K. Effect of a fermented milk combining Lactobacillus acidophilus CL1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: A randomized, double-blind, placebo-controlled trial. Can. J. Gastroenterol. 2007, 21, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Körzendörfer, A.; Schäfer, J.; Hinrichs, J.; Nöbel, S. Power ultrasound as a tool to improve the processability of protein-enriched fermented milk gels for Greek yogurt manufacture. J. Dairy Sci. 2019, 102, 7826–7837. [Google Scholar] [CrossRef] [PubMed]
- Dridi, C.; Millette, M.; Aguilar Uscanga, B.R.; Salmieri, S.; Allahdad, Z.; Lacroix, M. Evaluation of the nutritional quality and in vivo digestibility of probiotic beverages enriched with cricket proteins. Food Bioprocess Technol. 2023, 16, 1992–2000. [Google Scholar] [CrossRef]
- Dridi, C.; Millette, M.; Aguilar, B.; Manus, J.; Salmieri, S.; Lacroix, M. Effect of physical and enzymatic pre-treatment on the nutritional and functional properties of fermented beverages enriched with cricket proteins. Foods 2021, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Manus, J.; Millette, M.; Dridi, C.; Salmieri, S.; Aguilar Uscanga, B.R.; Lacroix, M. Protein quality of a probiotic beverage enriched with pea and rice protein. J. Food Sci. 2021, 86, 3698–3706. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. AOAC: Methods 942.15, 960.48. In Official methods of analysis of the Association of Official Analytical Chemists International, 17th ed; AOAC International: Gaithersburg, ML, USA, 2000; Volume 1, p. 771. [Google Scholar]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Antibacterial and antiproliferative peptides in synbiotic yogurt—Release and stability during refrigerated storage. J. Dairy Sci. 2016, 99, 4233–4242. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B.D. The R Project in statistical computing. MSOR Connect. 2001, 1, 23–25. [Google Scholar] [CrossRef]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vazquez-Baeza, Y.; Brimingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Bourdeau-Julien, I.; Castonguay-Paradis, S.; Rochefort, G.; Perron, J.; Lamarche, B.; Flamand, N.; di Marzo, V.; Veilleux, A.; Raymond, F. The diet rapidly and differentially affects the gut microbiota and host lipid mediators in a healthy population. Microbiome 2023, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, R.; Pérez de León, A.A.; Dowd, S.E.; Guerrero, F.D.; Bendele, K.G.; Scoles, G.A. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; McNulty, N.P.; Rey, F.E.; Gordon, J.I. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science 2011, 333, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.J.; Chew, Y.V.; Colakoglu, F.; Cliff, J.B.; Klaassens, E.; Read, M.N.; Solon-Biet, S.M.; McMahon, A.C.; Cogger, V.C.; Ruohonen, K.; et al. Diet-Microbiome Interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 2017, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the gut microbiome in obesity and diabetes mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Backhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Li, Q.; Lauber, C.L.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S.S. Effects of the dietary protein and carbohydrate ratio on gut microbiomes in dogs of different body conditions. mBio 2017, 8, e01703–e01716. [Google Scholar] [CrossRef]
- Jarett, J.K.; Carlson, A.; Rossoni Serao, M.; Strickland, J.; Serfilippi, L.; Ganz, H.H. Diets with and without edible cricket support a similar level of diversity in the gut microbiome of dogs. PeerJ 2019, 7, e7661. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut Microbes 2022, 14, 2020068. [Google Scholar] [CrossRef]
- Xu, X.; Ocansey, D.K.W.; Hang, S.; Wang, B.; Amoah, S.; Yi, C.; Zhang, X.; Liu, L.; Mao, F. The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. 2022, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.; Ingerslev, H.C.; Sturek, M.; Alloosh, M.; Cirera, S.; Christoffersen, B.Ø.; Moesgaard, S.G.; Larsen, N.; Boye, M. Characterisation of gut microbiota in Ossabaw and Göttingen Minipigs as models of obesity and metabolic syndrome. PLoS ONE 2013, 8, e56612. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qiu, N.; Meng, Y.; Keast, R. A comparative study of the modulation of the gut microbiota in rats by dietary intervention with different sources of egg-white proteins. J. Sci. Food Agric. 2020, 100, 3622–3629. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Bahl, M.I.; Michaelsen, K.F.; Licht, T.R. First foods and gut microbes. Front. Microbiol. 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.S.; Clewell, D.B.; Ike, Y.; Shankar, N. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014; pp. 91–139. [Google Scholar]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Lu, F.; Wang, X.; Liao, X.; Hu, X.; Zhang, Y. Beneficial effects of a chlorophyll-rich spinach extract supplementation on prevention of obesity and modulation of gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2019, 60, 103436. [Google Scholar] [CrossRef]
- Schots, P.C.; Jansen, K.M.; Mrazek, J.; Pedersen, A.M.; Olsen, R.L.; Larsen, T.S. Obesity-induced alterations in the gut microbiome in female mice fed a high-fat diet are antagonized by dietary supplementation with a novel, wax ester–rich, marine oil. Nutr. Res. 2020, 83, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Ahmad, M.I.; Hussain, M.; Khan, I.A.; Zhao, D.; Li, C. Meat protein in high-fat diet induces adipogensis and dyslipidemia by altering gut microbiota and endocannabinoid dysregulation in the adipose tissue of mice. J. Agric. Food Chem. 2020, 68, 3933–3946. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Lansade, L.; Bars-Cortina, D.; Dhorne-Pollet, S.; Foury, A.; Moisan, M.P.; Ruet, A. Gut microbiota resilience in horse athletes following holidays out to pasture. Sci. Rep. 2021, 11, 5007. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.J.; Lu, Z.M.; Zhang, X.J.; Ma, J.; Xu, P.X.; Qian, W.; Xiao, C.; Wang, S.T.; Shen, C.H.; Shi, J.S.; et al. Zooming in on butyrate-producing Clostridial consortia in the fermented grains of baijiu via gene sequence-guided microbial isolation. Front. Microbiol. 2019, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, X.Y.; Cao, Y.J.; Zheng, T.T.; Cheng, W.J.; Chen, L.J.; Lv, X.C.; Ni, L.; Rao, P.F.; Liang, P. The beneficial effects of Lactobacillus brevis FZU0713-fermented Laminaria japonica on lipid metabolism and intestinal microbiota in hyperlipidemic rats fed with a high-fat diet. Food Funct. 2021, 12, 7145–7160. [Google Scholar] [CrossRef]
- Lai, H.C.; Lin, T.L.; Chen, T.W.; Kuo, Y.L.; Chang, C.J.; Wu, T.R.; Shu, C.C.; Tsai, Y.H.; Swift, S.; Lu, C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 2022, 71, 309–321. [Google Scholar] [CrossRef]
- Drissi, F.; Raoult, D.; Merhej, V. Metabolic role of lactobacilli in weight modification in humans and animals. Microb. Pathog. 2017, 106, 182–194. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Frappier, M.; Millette, M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): Characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin. Infect. Dis. 2015, 60, S135–S143. [Google Scholar] [CrossRef] [PubMed]
- Frappier, M.; Auclair, J.; Bouasker, S.; Gunaratnam, S.; Diarra, C.; Millette, M. Screening and characterization of some Lactobacillaceae for detection of cholesterol-lowering activities. Probiotics Antimicro. Prot. 2022, 14, 873–883. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Ship, N.; Auclair, J.; Millette, M. Primary prevention of Clostridium difficile infections with a specific probiotic combining Lactobacillus acidophilus, L. casei, and L. rhamnosus strains: Assessing the evidence. J. Hosp. Infect. 2018, 99, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with irritable bowel syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef] [PubMed]
- Säemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stockl, J.; Horl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Khempaka, S.; Chitsatchapong, C.; Molee, W. Effect of chitin and protein constituents in shrimp head meal on growth performance, nutrient digestibility, intestinal microbial populations, volatile fatty acids, and ammonia production in broilers. J. Appl. Poultry Res. 2011, 20, 1–11. [Google Scholar] [CrossRef]
- McIntyre, A.; Gibson, P.R.; Young, G.P. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 1993, 34, 386–391. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.J.J.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.P.; van Knapen, F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef]
- Takegawa, A.; Murakami, M.A.; Kaneko, Y.; Kadokawa, J.I. Preparation of chitin/cellulose composite gels and films with ionic liquids. Carbohydr. Polym. 2010, 79, 85–90. [Google Scholar] [CrossRef]
- Auch, A.F. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genom. Sci. 2010, 2, 117–134. [Google Scholar] [CrossRef]
- Gouret, P.; Thompson, J.D.; Pontarotti, P. PhyloPattern: Regular expressions to identify complex patterns in phylogenetic trees. BMC Bioinform. 2009, 10, 298. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).