Raman Spectroscopic Analysis of Steviol Glycosides: Spectral Database and Quality Control Algorithms
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Their Preparation Procedures
2.2. Raman Spectroscopy
2.3. Evaluation of Sweetness Quality
3. Experimental Results
3.1. Raman Spectroscopic Library of Elementary Compounds
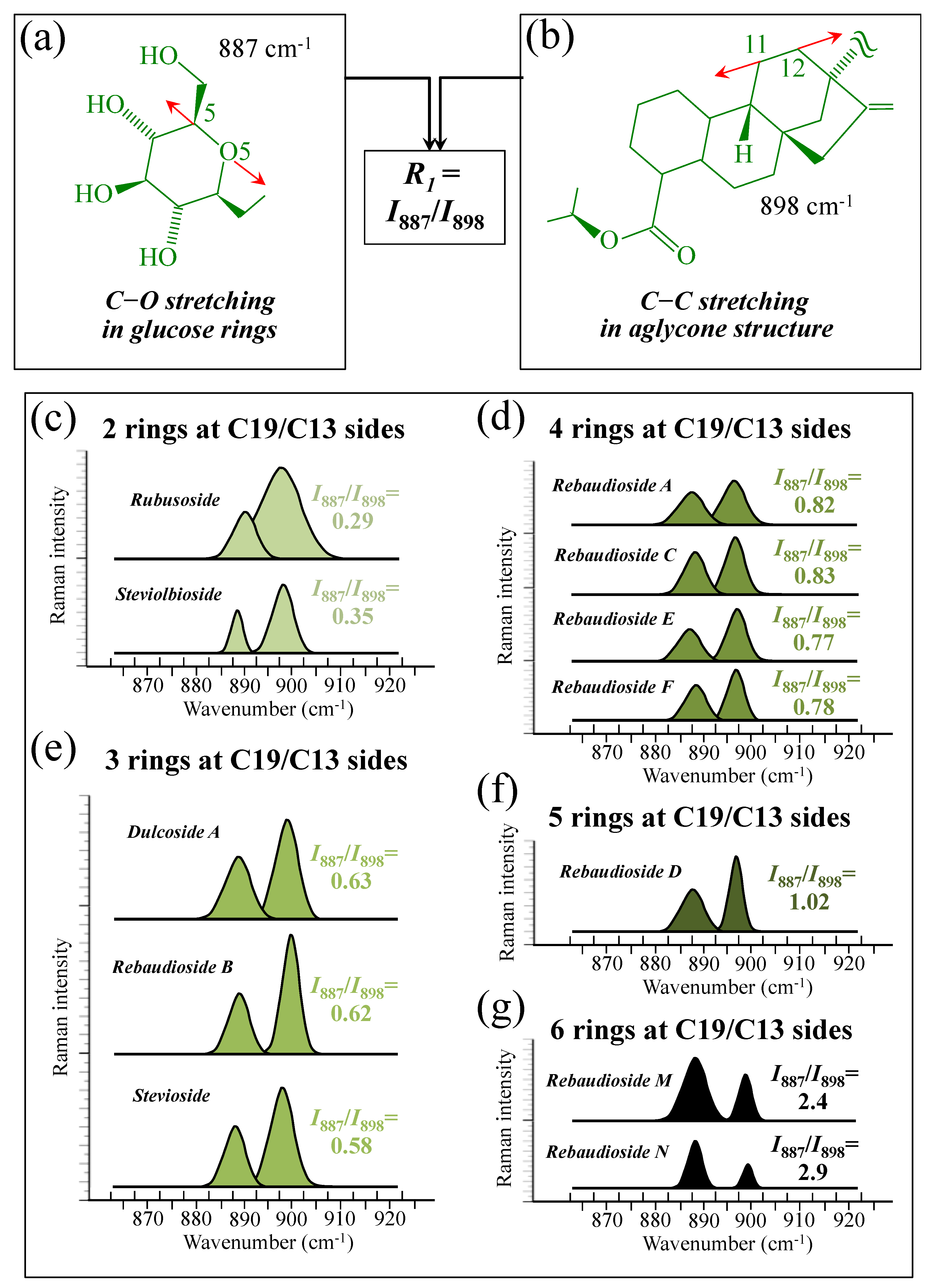
3.2. Raman Parameters for Structural Assessments of Steviol Diterpenes
3.3. Evaluation of Commercially Available Stevia Products
4. Discussion
4.1. The Molecular Origin of Sweetness and Bitter Retrotaste
- (i)
- Fewer glucosyl groups on C19 result in shorter time for initial stimulation and longer perception of bitterness.
- (ii)
- The more the glucosyl groups on C13 the faster the increase and the stronger the intensity of sweetness.
- (iii)
- A lower ratio between the number of glucosyl groups on C13 to that on C19 leads to a faster sweetness peak perception, although this parameter did not affect the bitter taste.
- (iv)
- Higher numbers and larger sizes of substitutions at the C19 position of steviol glycosides increase desorption and lead to a quicker decay of sweetness.
- (v)
- Rubusoside and Stevioside compounds, which contain fewer glucosyl groups, undergo lower desorption and thus longer bitter aftertaste.
- (vi)
- The addition of glucosyl groups tends to concurrently generate stronger sweetness and less bitterness, but only when the number of substituents on C13 is close to that on C19.
4.2. Raman Algorithms to Assess Sweetness/Bitterness of Commercial Stevia
4.3. The Role of Raman Spectroscopy in Quality Control of Stevia Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandle, J.E.; Starratt, A.N.; Gijen, M. Stevia rebaudiana: Its agricultural, biological and chemical properties. Can. J. Plant Sci. 1998, 78, 527–536. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Upreti, M.; Prakash, I. Diterpene glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552–3562. [Google Scholar] [CrossRef] [PubMed]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021, 20, 1412–1430. [Google Scholar]
- Ashwell, M. Stevia, nature’s zero-calorie sustainable sweetener: A new player in the fight against obesity. Nutr. Today 2015, 50, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Paucar, A.M. Steviol glycosides from Stevia rebaudiana: An updated overview of their sweetening activity, pharmacological properties, and safety aspects. Molecules 2023, 28, 1258. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-Leon, A.M.; Sierra-Perez, M.; Estruch, R.; Casas, R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Carakostas, M.; Prakash, I.; Kinghorn, A.D.; Wu, C.D.; Soejarto, D.D. Steviol glycosides. In Alternative Sweeteners, 4th ed.; O’Brien Nabors, L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 159–180. [Google Scholar]
- Muenprasitivej, N.; Tao, R.; Nardone, S.J.; Cho, S. The effect of steviol glycosides on sensory properties and acceptability of ice cream. Foods 2022, 11, 1745. [Google Scholar] [CrossRef]
- Allen, A.L.; McGeary, J.E.; Hayes, J.E. Rebaudioside A and Rebaudioside D bitterness do not covery with Acesulfame K bitterness or polymorphism in TAS2R9 and TAS2R31. Chemosens. Percept. 2013, 6, 109–117. [Google Scholar] [CrossRef]
- Hellfritsch, C.; Brockhoff, A.; Staehler, F.; Meyerhof, W.; Hofmann, T. Human psychometric and taste receptor responses to steviol glycosides. J. Agric. Food Chem. 2012, 60, 6782–6793. [Google Scholar] [CrossRef]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Wölwer-Rieck, U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: A review. J. Agric. Food Chem. 2012, 60, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Chaturvedula, V.; Markosyan, A. Isolation, characterization and sensory evaluation of a hexa-D-glucopyranosyl diterpene from Stevia rebaudiana. Nat. Prod. Commun. 2013, 8, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Bunders, C.; Devkota, K.P.; Charan, R.D.; Ramirez, C.; Priedemann, C.; Markosyan, A. Isolation and characterization of a novel Rebaudioside M isomer from a bioconversion reaction of Rebaudioside A and NMR comparison studies of Rebaudioside M isolated from Stevia rebaudiana Bertoni and Stevia rebaudiana Morita. Biomolecules 2014, 4, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Woelwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia leaf to Stevia Sweetener: Exploring its science, benefits, and future potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; Markosyan, A.; Bunders, C. Development of next generation stevia sweetener: Rebaudioside M. Foods 2014, 3, 162–175. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Jia, X.; Zhu, L.; Yin, H. Comparative transcriptomic of Stevia rebaudiana provides insight into Rebaudioside D and Rebaudioside M biosynthesis. Plant Physiol. Biochem. 2021, 167, 541–549. [Google Scholar] [CrossRef]
- Montoro, P.; Molfetta, I.; Maldini, M.; Ceccarini, L.; Piacente, S.; Pizza, C.; Macchia, M. Determination of six steviol glycosides of Stevia rebaudiana (Bertoni) from different geographical origin by LC-ESI-MS/MS. Food Chem. 2013, 141, 745–753. [Google Scholar] [CrossRef]
- Phungsiangdee, Y.; Chaotong, P.; Karnpanit, W.; Tanaviyutpakdee, P. Validation of UHPLC-ESI-MS/MS method for determining steviol glycoside and its derivatives in foods and beverages. Foods 2023, 12, 3941. [Google Scholar] [CrossRef]
- Shah, R.; De Jager, L.S.; Begley, T.H. Simultaneous determination of steviol and steviol glycosides by liquid chromatography-mass spectrometry. Food Addit. Contam. Part A 2012, 29, 1861–1871. [Google Scholar] [CrossRef]
- Pieri, V.; Belancic, A.; Morales, S.; Stuppner, H. Identification and quantification of major steviol glycosides in Stevia rebaudiana purified extracts by 1H NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 4378–4384. [Google Scholar] [CrossRef]
- Tada, A.; Takahashi, K.; Ishizuki, K.; Sugimoto, N.; Suematsu, T.; Arifuku, K.; Tahara, M.; Akiyama, T.; Ito, Y.; Yamazaki, T.; et al. Absolute quantitation of Stevioside and Rebaudioside A in commercial standards by quantitative NMR. Chem. Pharmac. Bull. 2013, 61, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mauri, P.; Catalano, G.; Gardana, C.; Pietta, P. Analysis of Stevia glycosides by capillary electrophoresis. Electrophoresis 1996, 17, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.F.Y. Separation and determination of stevia sweeteners by capillary electrophoresis and high performance liquid chromatography. J. Liq. Chromatogr. 1995, 18, 1703–1719. [Google Scholar] [CrossRef]
- Kurek, J.M.; Krol, E.; Krejpcio, Z. Steviol glycosides supplementation affects lipid metabolism in high-fat fed STZ-induced diabetic rats. Nutrients 2021, 13, 112. [Google Scholar] [CrossRef]
- McCullagh, M.; Douce, D.; Van Hoeck, E.; Goscinny, S. Exploring the complexity of steviol glycosides analysis ion mobility mass spectrometry. Anal. Chem. 2018, 90, 4585–4595. [Google Scholar] [CrossRef]
- Martono, Y.; Rohman, A. Quantitative analysis of stevioside and rebaudioside A in Stevia rebaudiana leaves using infrared spectroscopy and multivariate calibration. Int. J. Appl. Pharmac. 2019, 11, 38–42. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P. IR spectral analysis of diterpene glycosides isolated from Stevia rebaudiana. Food Nutr. Sci. 2012, 3, 1467–1471. [Google Scholar]
- Ahmed, M.A.; Jayakumar, S.; Rajesh, G.M.; Rajan, N.D.; Gayathri, G.S.; Sylaya, P. Spectroscopic investigations of Stevia rebaudiana—Medicinal plant. Int. J. Multidiscipl. Educ. Res. 2021, 10, 110–113. [Google Scholar]
- Vargas-Jentzsch, P.; Torrico-Vallejos, S.; Mendieta-Brito, S.; Ramos, L.A.; Ciobota, V. Detection of counterfeit stevia products using a handheld Raman spectrometer. Vib. Spectrosc. 2016, 83, 126–131. [Google Scholar] [CrossRef]
- Kubota, A.; Kubo, I. Bitterness and chemical structure. Nature 1969, 223, 97–99. [Google Scholar] [CrossRef]
- Mennella, J.A.; Pepino, M.Y.; Reed, D.R. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 2005, 115, e216–e222. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhong, F.; Xia, Y. Dynamic characteristics of sweetness and bitterness and their correlation with chemical structures for six steviol glycosides. Food Res. Int. 2022, 151, 110848. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yasmin, M.N.; Chen, Y.; Liu, Y.; Guan, S.; Wang, Z.; Hua, X. Computational simulations on the taste mechanism of steviol glycosides based on their interactions with receptor proteins. Int. J. Biol. Macromol. 2024, 255, 128110. [Google Scholar] [CrossRef] [PubMed]
- Noya-Leal, F.; van der Wielen, N.; Behrens, S.; Rouschop, S.; van Arkel, J.; Jongsma, M.; Witkamp, R.; Mes, J.J.; Bastiaan-Net, S.; Meijerink, J. Rebaudioside A from Stevia rebaudiana stimulates GLP-1 release by enteroendocrine cells via bitter taste signalling pathways. Food Funct. 2023, 14, 6914–6928. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, W.; Gonzales-Nilo, F.; Agosin, E. Docking and molecular dynamics of steviol glycoside—Human bitter receptor interactions. J. Agric. Food Chem. 2016, 64, 7585–7596. [Google Scholar] [CrossRef]
- Acevedo, W.; Ramirez-Sarmiento, C.A.; Agosin, E. Identifying the interactions between natural, non-caloric sweeteners and the human sweet receptor by molecular docking. Food Chem. 2018, 264, 164–171. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Singh, R.; Singh, P.; Purohit, R.; Kumar, S. Elimination of bitter-off taste of stevioside through structure modification and computational interventions. J. Theor. Biol. 2020, 486, 110094. [Google Scholar] [CrossRef]
- Birch, G.G.; Mylvaganam, A.R. Evidence for the proximity of sweet and bitter receptor sites. Nature 1976, 260, 632–634. [Google Scholar] [CrossRef]
- Jaitak, V. Interaction model of steviol glycosides from Stevia rebaudiana (Bertoni) with sweet taste receptors: A computational approach. Phytochemistry 2015, 116, 12–20. [Google Scholar]
- Ahmed, J.; Preissner, S.; Dunkel, M.; Worth, C.L.; Eckert, A.; Preissner, R. SuperSweet—A resource on natural and artificial sweetening agents. Nucleic Acids Res. 2010, 39 (Suppl. 1), D377–D382. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, B.; Wang, H.; Zhao, L.; Chen, Z. Pungency evaluation of hydroxyl-sanshool compounds after dissolution in taste carriers per time-related characteristics. Chem. Senses 2017, 42, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Cho, S. Consumer-based sensory characterization of steviol glycosides (Rebaudioside A, D, and M). Foods 2020, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Prakash, I.; DuBois, G.E.; Clos, J.F.; Wilkens, K.L.; Fosdick, L.E. Development of Rebiana, a natural, non-caloric sweetener. Food Chem. Toxicol. 2008, 46, S75–S82. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-W.; Kim, S.-B.; Chung, S.-J. Effect of concentration range on the accuracy of measuring sweetness potencies of sweeteners. Food Qual. Prefer. 2020, 79, 103753. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Sweeteners determination in table top formulations using FT-Raman spectrometry and chemometric analysis. Anal. Chim. Acta 2004, 521, 149–155. [Google Scholar] [CrossRef]
- Silveira, L., Jr.; Moreira, L.M.; Conceição, V.G.B.; Casalechi, H.L.; Muñoz, I.S.; Fernandes da Silva, F.; Silva, M.A.S.R.; de Souza, R.A.; Pacheco, M.T.T. Determination of sucrose concentration in lemon-type soft drinks by dispersive Raman spectroscopy. Spectroscopy 2009, 23, 217–226. [Google Scholar] [CrossRef]
- Mazurek, S.; Szostak, R. Quantification of aspartame in commercial sweeteners by FT-Raman spectroscopy. Food Chem. 2011, 125, 1051–1057. [Google Scholar] [CrossRef]
- Özbalci, B.; Boyaci, I.H.; Topcu, A.; Kadılar, C.; Tamer, U. Rapid analysis of sugars in honey by processing Raman spectrum using chemometric methods and artificial neural networks. Food Chem. 2013, 136, 1444–1452. [Google Scholar] [CrossRef]
- Buyukgoz, G.G.; Bozkurt, A.G.; Akgul, N.B.; Tamer, U.; Boyaci, I.H. Spectroscopic detection of aspartame in soft drinks by surface-enhanced Raman spectroscopy. Eur. Food Res. Technol. 2015, 240, 567–575. [Google Scholar] [CrossRef]











| Commercial Name | Chemical Names | Chemical Formula | Molecular Weight | Rings on C19 Side | Rings on C13 Side |
|---|---|---|---|---|---|
| Dulcoside A | (4α)-13-{[2-O-(6-Deoxy-α-L -mannopyranosyl)-β-D -glucopyranosyl]oxy}-kaur-16-en -18-oic acid-β-D-glucopyranosyl ester | C38H60O17 | 788.87 | 1 | 2 |
| Steviolbioside | (4α)-13-[(2-O-β-D-glucopyranosyl-β -D-glucopyranosyl)oxyl]-kaur-16-en -18-oic acid-β-D-glucopyranosyl ester; Steviosin | C38H60O18 | 804.87 | 1 | 2 |
| Steviolbioside | (4α)-13-[(2-O-β-D-glucopyranosyl-β -D-glucopyranosyl)oxyl]-kaur-16-en -18-oic acid | C32H50O13 | 642.73 | 0 | 2 |
| Rubusoside | (4α)-13-(β-D-glucopyranosyl)-kaur -16-en-18-oic acid, β-D -glucopyranosyl ester | C32H50O13 | 642.73 | 1 | 1 |
| Rebaudioside A | (4α)-13-[(2-O-β-D-glucopyranosyl -(1→2)-O-[β-D-glucopyranosyl -(1→3)]-β-D-glucopyranosyl)oxy] -kaur-16-en-18-oic acid-β-D -glucopyranosyl ester; Stevioside α3; Rebiana | C44H70O23 | 967.01 | 1 | 3 |
| Rebaudioside B | (4α)-13-[(O-β-D-glucopyranosyl -(1→2)-O-[β-D-glucopyranosyl -(1→3)]-β-D-glucopyranosyl)oxy] -kaur-16-en-18-oic acid; Stevioside A4 | C38H60O18 | 804.87 | 0 | 3 |
| Rebaudioside C | (4α)-13-[(O-6-Deoxy-α-L -mannopyranosyl-(1→2)-O-[β-D -glucopyranosyl-(1→3)]-β-D -glucopyranosyl)oxy]-kaur-16-en -18-oic acid-β-D-glucopyranosyl ester; Dulcoside B | C44H70O22 | 951.01 | 1 | 3 |
| Rebaudioside D | (4α)-13-[(O-β-D-glucopyranosyl -(1→2)-O-[β-D-glucopyranosyl -(1→3)]-β-D-glucopyranosyl)oxy] -kaur-16-en-18-oic acid 2-O-β-D -glucopyranosyl ester | C50H80O28 | 1129.15 | 2 | 3 |
| Rebaudioside E | (4α)-13-[(2-O-β-D-glucopyranosyl-β -D-glucopyranosyl)oxyl]-kaur-16-en -18-oic acid-2-O-β-D-glucopyranosyl -β-D-glucopyranosyl ester | C44H70O23 | 967.01 | 2 | 2 |
| Rebaudioside F | (4α)-13-[(O-β-D-glucopyranosyl -(1→3)-O-[β-D-xlyopyranosyl -(1→2)]-β-D-glucopyranosyl)oxy] -kaur-16-en-18-oic acid -β-D -glucopyranosyl ester | C43H68O22 | 936.99 | 1 | 3 |
| Rebaudioside M | (4α)-O-β-D-glucopyranosyl-(1→2)-O -[β-D -glucopyranosyl-(1→3)]-β-D -glucopyranosyl ester 13-[(O-β-D -glucopyranosyl-(1→2)-O-[β-D -glucopyranosyl-(1→3)]-β-D -glucopyranosyloxyl-kaur–16-en -18-oic acid; Rebaudioside X | C56H90O33 | 1291.29 | 3 | 3 |
| Rebaudioside N | 13-[(O-β-D-glucopyranosyl-(1→2)-O -[β-D-glucopyranosyl-(1→3)]-β-D -glucopyranosyl)oxy]-kaur-16 en -18-oic acid (4α)-O-6-deoxy-α-L -mannopyranosyl-(1→2)-O-[β-D -glucopyranosyl-(1→3)]-β-D -glucopyranosyl ester | C56H90O32 | 1275.29 | 3 | 3 |
| Product Name | Rings on C19 | Total Rings 1 * | Total Rings 2 * | C13/C19 Ring Ratio | Taste Characteristics |
|---|---|---|---|---|---|
| Morita 1 | 1.15 | 3.31 | 3.31 | 1.88 | Highest sweetness Fastest sweet perception Shortest bitter perception |
| Morita 2 | 0.58 | 2.33 | 2.33 | 3.02 | Lowest sweetness Slowest sweet perception Longest bitter perception |
| China 1 | 0.69 | 2.78 | 2.78 | 3.03 | Intermediate sweetness Slow sweet perception Long bitter perception |
| China 2 | 0.63 | 2.29 | 2.30 | 2.63 | Lowest sweetness Slowest sweet perception Longest bitter perception |
| Fermented | 0.61 | 2.30 | 2.31 | 2.77 | Lowest sweetness Slowest sweet perception Longest bitter perception |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzotti, G.; Zhu, W.; Aoki, T.; Miyamoto, A.; Fujita, I.; Nakagawa, M.; Kobayashi, T. Raman Spectroscopic Analysis of Steviol Glycosides: Spectral Database and Quality Control Algorithms. Foods 2024, 13, 3068. https://doi.org/10.3390/foods13193068
Pezzotti G, Zhu W, Aoki T, Miyamoto A, Fujita I, Nakagawa M, Kobayashi T. Raman Spectroscopic Analysis of Steviol Glycosides: Spectral Database and Quality Control Algorithms. Foods. 2024; 13(19):3068. https://doi.org/10.3390/foods13193068
Chicago/Turabian StylePezzotti, Giuseppe, Wenliang Zhu, Takashi Aoki, Akihiro Miyamoto, Isao Fujita, Manabu Nakagawa, and Takuya Kobayashi. 2024. "Raman Spectroscopic Analysis of Steviol Glycosides: Spectral Database and Quality Control Algorithms" Foods 13, no. 19: 3068. https://doi.org/10.3390/foods13193068
APA StylePezzotti, G., Zhu, W., Aoki, T., Miyamoto, A., Fujita, I., Nakagawa, M., & Kobayashi, T. (2024). Raman Spectroscopic Analysis of Steviol Glycosides: Spectral Database and Quality Control Algorithms. Foods, 13(19), 3068. https://doi.org/10.3390/foods13193068





