The Influence of Different Hydrothermal Processes Used in the Preparation of Brussels Sprouts on the Availability of Glucosinolates to Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Methods
2.2.1. Dry Matter
2.2.2. In Vitro Digestion
2.2.3. Determination of Glucosinolates
2.2.4. Determination of Indoles and Isothiocyanates
2.2.5. Bioaccessibility
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Stability of Glucosinolates and Its Metabolites during Thermal Processing
4.2. Bioaccessibility of Phytochemicals after In Vitro Digestion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, E.-S.; Bornhorst, G.M.; Oteiza, P.I.; Mitchell, A.E. Assessing the fate and bioavailability of glucosinolates in kale (Brassica oleracea) using simulated human digestion and caco-2 cell uptake models. J. Agric. Food Chem. 2019, 67, 9492–9500. [Google Scholar] [CrossRef] [PubMed]
- Kuljarachanan, T.; Fu, N.; Chiewchan, N.; Devahastin, S.; Chen, X.D. Evolution of important glucosinolates in three common Brassica vegetables during their processing into vegetable powder and in vitro gastric digestion. Food Funct. 2020, 11, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Vancoillie, F.; Verkempinck, S.H.E.; Sluys, L.; De Mazière, S.; Van Poucke, C.; Hendrickx, M.E.; Van Loey, A.M.; Grauwet, T. Stability and bioaccessibility of micronutrients and phytochemicals present in processed leek and Brussels sprouts during static in vitro digestion. Food Chem. 2024, 445, 138644. [Google Scholar] [CrossRef] [PubMed]
- Pilipczuk, T.; Dawidowska, N.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. Simultaneous determination of indolic compounds in plant extracts by solid-phase extraction and high-performance liquid chromatography with UV and fluorescence detection. Food Anal. Method. 2015, 8, 2169–2177. [Google Scholar] [CrossRef]
- Pilipczuk, T.; Kusznierewicz, B.; Chmiel, T.; Przychodzeń, W.; Bartoszek, A. Simultaneous determination of individual isothiocyanates in plant samples by HPLC-DAD-MS following SPE and derivatization with N-acetyl-l-cysteine. Food Chem. 2017, 214, 587–596. [Google Scholar] [CrossRef]
- Doniec, J.; Florkiewicz, A.; Duliński, R.; Filipiak-Florkiewicz, A. Impact of Hydrothermal Treatments on Nutritional Value and Mineral Bioaccessibility of Brussels Sprouts (Brassica oleracea var. gemmifera). Molecules 2022, 27, 1861. [Google Scholar] [CrossRef]
- Doniec, J.; Pierzchalska, M.; Mickowska, B.; Grabacka, M. The in vitro digestates from Brussels sprouts processed with various hydrothermal treatments affect the intestinal epithelial cell differentiation, mitochondrial polarization and glutathione level. Agric. Food Sci. 2023, 32, 154–165. [Google Scholar] [CrossRef]
- Martínez-Castro, J.; De Haro-Bailón, A.; Obregón-Cano, S.; García Magdaleno, I.M.; Moreno Ortega, A.; Cámara-Martos, F. Bioaccessibility of glucosinolates, isothiocyanates and inorganic micronutrients in cruciferous vegetables through INFOGEST static in vitro digestion model. Food Res. Int. 2023, 166, 112598. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Drabińska, N.; Jeleń, H.H. Thermal processing-induced changes in volatilome and metabolome of Brussels sprouts: Focus on glucosinolate metabolism. Eur. Food Res. Technol. 2023, 249, 2165–2174. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Bączkowicz, M.; Pietrzyk, S. Jakość sensoryczna warzyw kapustnych gotowanych metodą sous-vide oraz tradycyjnymi technikami obróbki hydrotermicznej. Żywność Nauka Technol. Jakość 2018, 25, 150–171. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Socha, R.; Filipiak-Florkiewicz, A.; Topolska, K. Sous-vide technique as an alternative to traditional cooking methods in the context of antioxidant properties of Brassica vegetables. J. Sci. Food Agric. 2019, 99, 173–182. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 712:2012; Cereals and Cereal Products—Determination of Moisture Content—Reference Method. Polish Committee for Standardization: Warsaw, Poland, 2012.
- Żyła, K.; Ledoux, D.R.; Garcia, A.; Veum, T.L. An in vitro procedure for studying enzymic dephosphorylation of phytate in maize-soya bean feeds for turkey poults. Br. J. Nutr. 1995, 74, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Starzyńska-Janiszewska, A.; Dulinski, R.; Stodolak, B.; Mickowska, B.; Wikiera, A. Prolonged tempe-type fermentation in order to improve bioactive potential and nutritional parameters of quinoa seeds. J. Cereal Sci. 2016, 71, 116–121. [Google Scholar] [CrossRef]
- ISO 9167-1:1992; Rapeseed. Determination of Glucosinolates Content. Part 1: Method Using High-Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 1992.
- Kusznierewicz, B.; Iori, R.; Piekarska, A.; Namieśnik, J.; Bartoszek, A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during liquid chromatography–diode array–electrospray ionisation mass spectrometry analysis: Method verification for sprouts of different Brassicaceae species extracts. J. Chrom. A 2013, 1278, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Seljåsen, R.; Kusznierewicz, B.; Bartoszek, A.; Mølmann, J.; Vågen, I.M. Effects of post-harvest elicitor treatments with ultrasound, UV and photosynthetic active radiation on polyphenols, glucosinolates and antioxidant activity in a waste fraction of white cabbage (Brassica oleracea var. capitata). Molecules 2022, 27, 5256. [Google Scholar] [CrossRef]
- Zhang, Y.; Wade, K.L.; Prestera, T.; Talalay, P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1, 2-benzenedithiol. Anal. Biochem. 1996, 239, 160–167. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a natural chemical arsenal: More to tell than the myrosinase story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Martos, F.; Obregón-Cano, S.; Mesa-Plata, O.; Cartea-González, M.E.; De Haro-Bailón, A. Quantification and in vitro bioaccessibility of glucosinolates and trace elements in Brassicaceae leafy vegetables. Food Chem. 2021, 339, 127860. [Google Scholar] [CrossRef] [PubMed]
- Ciska, E.; Drabińska, N.; Honke, J.; Narwojsz, A. Boiled Brussels sprouts: A rich source of glucosinolates and the corresponding nitriles. J. Funct. Foods 2015, 19, 91–99. [Google Scholar] [CrossRef]
- Williams, D.E. Indoles derived from glucobrassicin: Cancer chemoprevention by Indole-3-carbinol and 3, 3’-diindolylmethane. Front. Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Ciska, E.; Filipiak-Florkiewicz, A.; Topolska, K. Comparison of Sous-vide methods and traditional hydrothermal treatment on GLS content in Brassica vegetables. Eur. Food Res. Technol. 2017, 243, 1507–1517. [Google Scholar] [CrossRef][Green Version]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 16, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Verkerk, R.; Dekker, M. Isothiocyanates from Brassica Vegetables—Effects of processing, cooking, mastication, and digestion. Mol. Nutr. Food Res. 2018, 62, 1701069. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Y.; Pang, X.; Tian, S.; Hu, Q.; Li, X.; Liu, J.; Wang, J.; Lu, Y. The effect of processing and cooking on glucoraphanin and sulforaphane in Brassica vegetables. Food Chem. 2021, 360, 130007. [Google Scholar] [CrossRef]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef]
- Fernández-León, A.M.; Fernández-León, M.F.; González-Gómez, D.; Ayuso, M.C.; Bernalte, M.J. Quantification and bioaccessibility of intact glucosinolates in broccoli “Parthenon” and Savoy cabbage “Dama”. J. Food Compos. Anal. 2017, 61, 40–46. [Google Scholar] [CrossRef]
- Cuomo, V.; Luciano, F.B.; Meca, G.; Ritieni, A.; Mañes, J. Bioaccessibility of glucoraphanin from broccoli using an in vitro gastrointestinal digestion model. CyTA J. Food 2015, 13, 361–365. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, M.C.; Medina, S.; Gil-Izquierdo, A.; Martínez-Ballesta, M.C.; Moreno, D.A. Broccoli isothiocyanate content and in vitro availability according to variety and origin. Maced. J. Chem. Chem. Eng. 2013, 32, 251–264. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated in vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef]
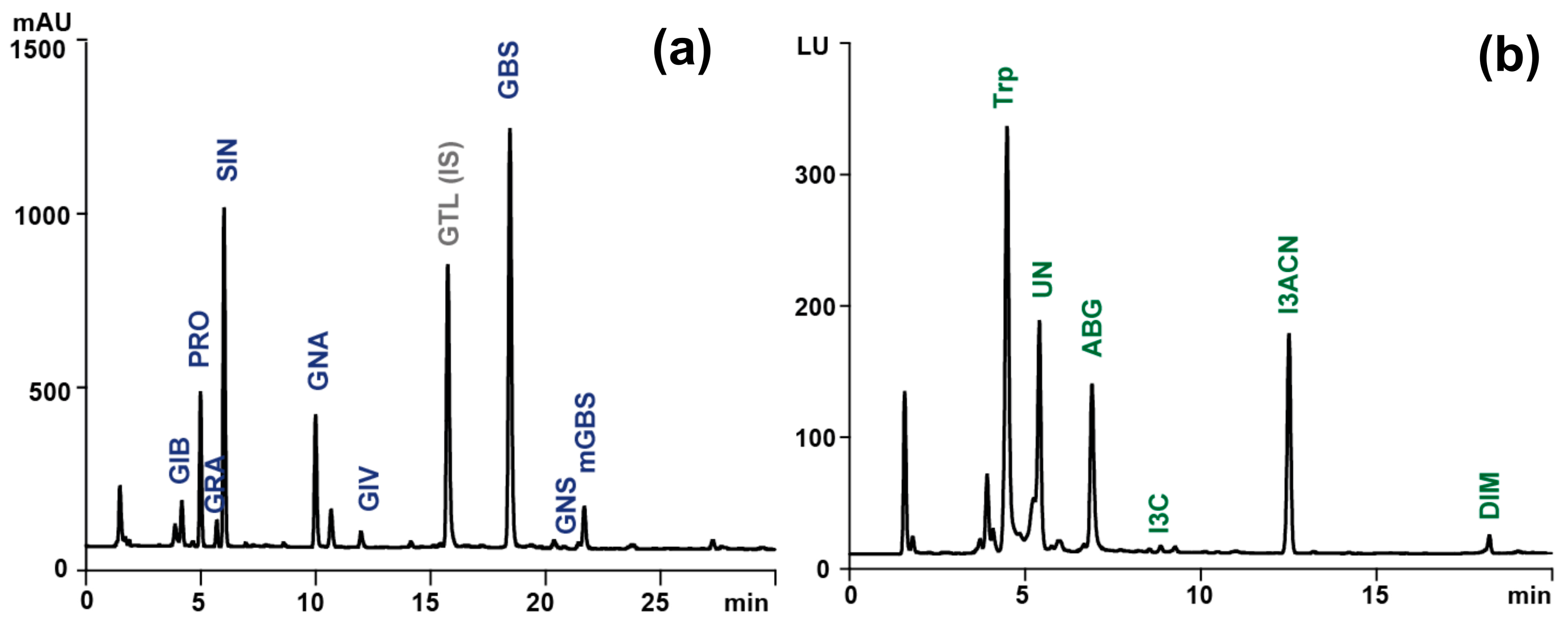
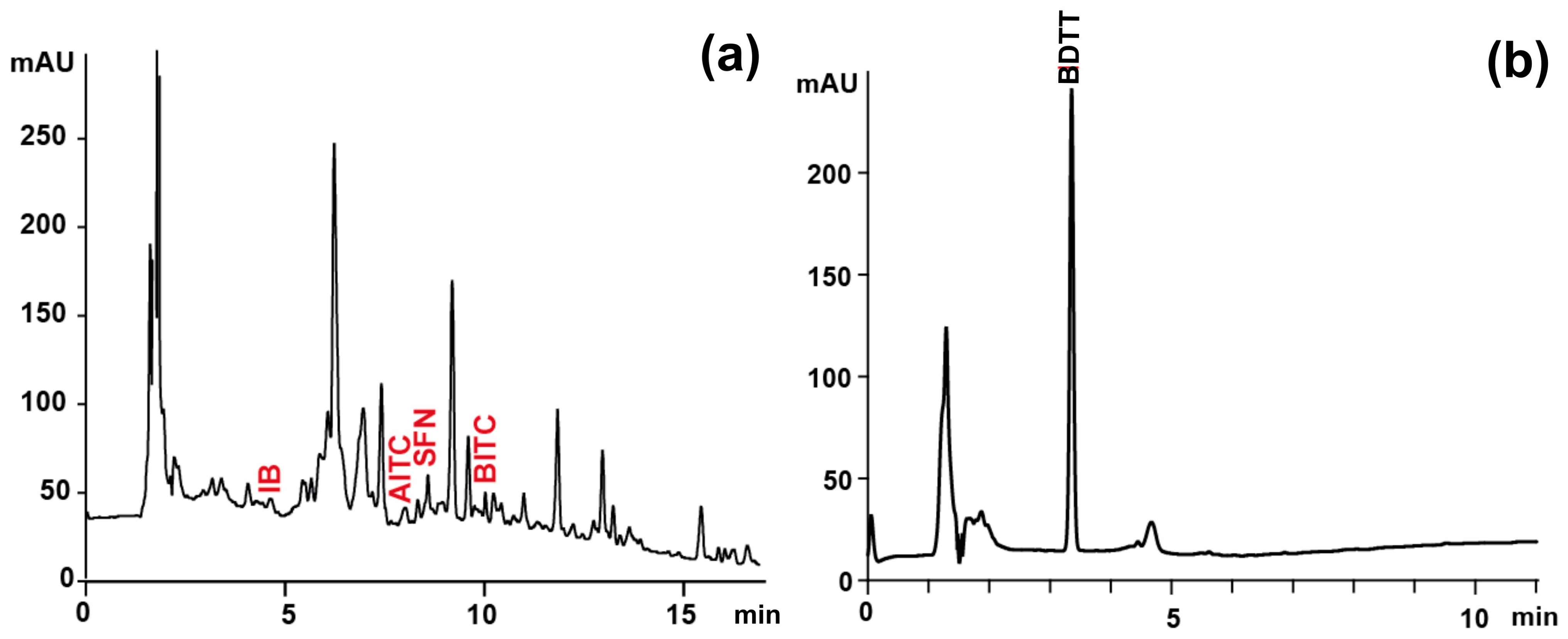
| Compound Name | RT [min] | Molecular Formula | Precursor Ion (m/z) | Diagnostic Ions (m/z) | ||
|---|---|---|---|---|---|---|
| Theoretical | Experimental | Δm (ppm) | ||||
| Glucosinolates | [MDS-GLS-H]− | [MDS-GLS-H-162]− | ||||
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (1E)-4-methylsulfinyl-N-sulfooxybutanimidothioate (glucoiberin; GIB) | 3.9 | C11H21NO10S3 | 342.068123 | 342.068329 | 0.60 | 180.016 [C5H10NO2S2]− |
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (3R)-3-hydroxy-N-sulfooxypent-4-enimidothioate (Progoitrin; PRO) | 4.2 | C11H19NO10S2 | 308.080401 | 308.080780 | 1.23 | 146.027 [C5H8NO2S]− |
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (1E)-5-methylsulfinyl-N-sulfooxypentanimidothioate (glucoraphanin; GRA) | 5.7 | C12H23NO10S3 | 356.083772 | 356.084106 | 0.94 | 194.030 [C6H12NO2S2]− |
| [(E)-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylbut-3-enylideneamino] sulfate (Sinigrin; SIN) | 5.9 | C10H17NO9S2 | 278.069836 | 278.070099 | 0.95 | 116.016 [C4H6NOS]− |
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (1E)-N-sulfooxypent-4-enimidothioate (gluconapin; GNA) | 9.9 | C11H19NO9S2 | 292.085486 | 292.085937 | 1.54 | 130.032 [C5H8NOS]− |
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (1E)-4-methylsulfanyl-N-sulfooxybutanimidothioate (glucoiberverin; GIV) | 11.9 | C11H21NO9S3 | 326.073208 | 326.073547 | 1.04 | 164.020 [C5H10NOS2]− |
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] 2-(1H-indol-3-yl)-N-sulfooxyethanimidothioate (glucobrassicin; GBS) | 18.4 | C16H20N2O9S2 | 367.096385 | 367.096558 | 0.47 | 205.043 [C10H9N2OS]− |
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] 3-phenyl-N-sulfooxypropanimidothioate (gluconasturtiin; GNS) | 21.4 | C15H21NO9S2 | 342.101136 | 342.101471 | 0.98 | 180.048 [C9H10NOS]− |
| [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] 2-(4-methoxy-1H-indol-3-yl)-N-sulfooxyethanimidothioate (4-methoxyglucobrassicin; metGBS) | 21.7 | C17H22N2O10S2 | 397.106950 | 397.106735 | 0.54 | 235.054 [C11H11N2O2S]− |
| Isothiocyanates | [MNAC-ITC+H]+ | [MNAC-ITC+H-NAC]+ | ||||
| 1-isothiocyanato-3-methylsulfinylpropane (Iberin; IB) | 4.6 | C5H9NOS2 | 327.050699 | 327.049974 | 2.22 | 164.017 [C5H10NOS2]+ |
| 3-isothiocyanatoprop-1-ene (Allyl-ITC; AITC) | 7.2 | C4H5NS | 263.052412 | 263.051697 | 2.72 | 100.022 [C4H6NS]+ |
| 1-isothiocyanato-4-methylsulfinylbutane (sulforaphane; SFN) | 7.7 | C6H11NOS2 | 341.066349 | 341.065643 | 2.07 | 178.035 [C6H12NOS2]+ |
| 4-isothiocyanatobut-1-ene (3-butenyl-ITC; BITC) | 8.9 | C5H7NS | 277.068062 | 277.0674438 | 2.23 | 114.037 [C5H8NS]+ |
| Indoles | [M+H]+ | |||||
| (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid (tryptophan; Trp) | 4.5 | C11H12N2O2 | 205.097703 | 205.097107 | 2.91 | 118.065 [C8H8N]+, 146.060 [C9H8NO]+ |
| Unknown (UN) | 5.4 | C14H15NO4 | 262.107934 | 262.107330 | 2.30 | 130.065 [C9H8N]+, 118.065 [C8H8N]+ |
| (3S,3aR,6aS)-3,6,6a-trihydroxy-6-(1H-indol-3-ylmethyl)-3,3a-dihydro-2H-furo [3,2-b] furan-5-one (ascorbigen; ABG) | 6.9 | C15H15NO6 | 306.097764 | 306.096924 | 2.74 | 130.065 [C9H8N]+ |
| 1H-indol-3-ylmethanol (indole-3-carbinol; I3C) | 8.8 | C9H9NO | 148.076239 | 148.0765378 | 2.02 | 130.065 [C9H8N]+, 118.065 [C8H8N]+ |
| 2-(1H-indol-3-yl) acetonitrile (3-Indoleacetonitrile; I3ACN) | 12.5 | C10H8N2 | 157.076573 | - | - | - |
| 3-(1H-indol-3-ylmethyl)-1H-indole (3,3’-diindolylmethane; DIM) | 18.2 | C17H14N2 | 247.123523 | - | - | - |
| Content [µmol/g d.w.] (Mean ± Standard Deviation, n = 3) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Raw (R) | Sous Vide (SV) | Steam (S) | Boiled (B) | Sous Vide-Digested (SV-D) | Steam-Digested (S-D) | Boiled-Digested (B-D) |
| Glucosinolates | |||||||
| GIB * | 0.736 ab ± 0.016 | 0.674 a ± 0.036 | 0.774 b ± 0.025 | 0.861 c ± 0.032 | 0.183 d ± 0.012 | 0.162 d ± 0.002 | 0.080 e ± 0.010 |
| PRO * | 2.162 ab ± 0.193 | 1.639 c ± 0.221 | 1.925 ac ± 0.154 | 2.542 b ± 0.293 | 0.088 d ± 0.027 | 0.259 d ± 0.012 | 0.278 d ± 0.023 |
| GRA ** | 0.295 a ± 0.018 | 0.244 a ± 0.03 | 0.352 a ± 0.062 | 0.368 a ± 0.045 | <LOD | <LOD | <LOD |
| SIN * | 4.779 a ± 0.336 | 4.196 a ± 0.451 | 4.495 a ± 0.187 | 4.966 a ± 0.443 | 0.398 b ± 0.025 | 0.587 b ± 0.019 | 1.070 b ± 0.008 |
| GNA * | 2.795 a ± 0.152 | 1.799 b ± 0.196 | 2.235 c ± 0.060 | 2.493 ac ± 0.170 | 0.110 d ± 0.023 | 0.520 e ± 0.021 | 0.162 de ± 0.007 |
| GIV ** | 0.304 ab ± 0.014 | 0.257 a ± 0.021 | 0.381 b ± 0.038 | 0.335 ab ± 0.056 | <LOD | <LOD | <LOD |
| GBS * | 3.753 a ± 0.059 | 4.074 a ± 0.781 | 4.792 ab ± 0.407 | 5.519 b ± 0.398 | 0.352 c ± 0.010 | 0.638 c ± 0.007 | 0.100 c ± 0.037 |
| GNS ** | 0.119 ab ± 0.015 | 0.076 a ± 0.011 | 0.191 b ± 0.046 | 0.107 a ± 0.031 | <LOD | <LOD | <LOD |
| metGBS ** | 0.105 a ± 0.037 | 0.299 b ± 0.031 | 0.444 c ± 0.052 | 0.460 c ± 0.057 | 0.120 a ± 0.012 | 0.153 a ± 0.021 | <LOD |
| Total * | 15.049 ab ± 0.425 | 13.259 a ± 1.670 | 15.589 bc ± 0.446 | 17.651 c ± 1.176 | 1.251 d ± 0.023 | 2.319 d ± 0.035 | 1.689 d ± 0.032 |
| Isothiocyanates | |||||||
| IB | 0.298 ± 0.036 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| AITC | 0.248 ± 0.007 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| SFN | 0.035 ± 0.001 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BITC | 0.332 ± 0.025 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Total | 0.914 ± 0.019 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Isothiocyanates by Zhang method | |||||||
| Total ITC ** | 0.899 a ± 0.061 | 0.216 abc ± 0.087 | 0.093 abc ± 0.012 | 0.216 abc ± 0.057 | 0.078 abc ± 0.008 | 0.065 bc ± 0.003 | 0.061 bc ± 0.005 |
| Indoles | |||||||
| Trp ** | 0.044 a ± 0.003 | 0.033 a ± 0.002 | 0.038 a ± 0.006 | 0.036 a ± 0.009 | 0.035 a ± 0.007 | 0.042 a ± 0.014 | 0.034 a ± 0.001 |
| UN ** | 0.021 a ± 0.001 | 0.009 ab ± 0.002 | 0.004 ab ± 0.001 | 0.007 ab ± 0.001 | 0.006 ab ± 0.001 | 0.003 ab ± 0.001 | 0.002 b ± 0.001 |
| ABG ** | 0.018 ± 0.001 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| I3C | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| I3ACN ** | 0.025 a ± 0.002 | 0.085 ab ± 0.003 | 0.063 ab ± 0.002 | 0.071 ab ± 0.005 | 0.093 b ± 0.003 | 0.067 ab ± 0.011 | 0.071 ab ± 0.014 |
| DIM | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Total * | 0.109 a ± 0.007 | 0.128 a ± 0.008 | 0.106 a ± 0.007 | 0.115 a ± 0.005 | 0.134 a ± 0.010 | 0.114 a ± 0.026 | 0.108 a ± 0.015 |
| Bioaccessibility [%] | |||
|---|---|---|---|
| Compound | Sous-Vide Digested (SV-D) | Steam-Digested (S-D) | Boiled-Digested (B-D) |
| Glucosinolates | |||
| GIB | 30 | 41 | 41 |
| PRO | 25 | 35 | 41 |
| GRA | 27 | 46 | 43 |
| SIN | 29 | 37 | 36 |
| GNA | 21 | 31 | 31 |
| GIV | 28 | 49 | 38 |
| GBS | 36 | 50 | 51 |
| GNS | 21 | 63 | 31 |
| metGBS | 94 | 164 | 152 |
| Total | 29 | 40 | 41 |
| Isothiocyanates by Zhang method | |||
| Total ITC | 8 | 4 | 8 |
| Indoles | |||
| Trp | 25 | 34 | 28 |
| UN | 14 | 8 | 11 |
| ABG | 2 | 1 | 3 |
| I3C | 0 | 0 | 0 |
| I3ACN | 112 | 99 | 98 |
| DIM | 0 | 0 | 0 |
| Total | 38 | 38 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska-Rociek, A.; Doniec, J.; Kusznierewicz, B.; Dera, T.; Filipiak-Florkiewicz, A.; Florkiewicz, A. The Influence of Different Hydrothermal Processes Used in the Preparation of Brussels Sprouts on the Availability of Glucosinolates to Humans. Foods 2024, 13, 2988. https://doi.org/10.3390/foods13182988
Sadowska-Rociek A, Doniec J, Kusznierewicz B, Dera T, Filipiak-Florkiewicz A, Florkiewicz A. The Influence of Different Hydrothermal Processes Used in the Preparation of Brussels Sprouts on the Availability of Glucosinolates to Humans. Foods. 2024; 13(18):2988. https://doi.org/10.3390/foods13182988
Chicago/Turabian StyleSadowska-Rociek, Anna, Joanna Doniec, Barbara Kusznierewicz, Tomasz Dera, Agnieszka Filipiak-Florkiewicz, and Adam Florkiewicz. 2024. "The Influence of Different Hydrothermal Processes Used in the Preparation of Brussels Sprouts on the Availability of Glucosinolates to Humans" Foods 13, no. 18: 2988. https://doi.org/10.3390/foods13182988
APA StyleSadowska-Rociek, A., Doniec, J., Kusznierewicz, B., Dera, T., Filipiak-Florkiewicz, A., & Florkiewicz, A. (2024). The Influence of Different Hydrothermal Processes Used in the Preparation of Brussels Sprouts on the Availability of Glucosinolates to Humans. Foods, 13(18), 2988. https://doi.org/10.3390/foods13182988






