Cardiac Hypertrophy in Pregnant Rats, Descendants of Fructose-Fed Mothers, an Effect That Worsens with Fructose Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. DNA Extraction and Quantification
2.3. Lipid Extraction and Triglycerides Determination
2.4. Lactate and Uric Acid Quantification
2.5. Protein Carbonyls Determination
2.6. RNA Extraction and Gene Expression Determination by qPCR
2.7. Statistical Analysis
3. Results
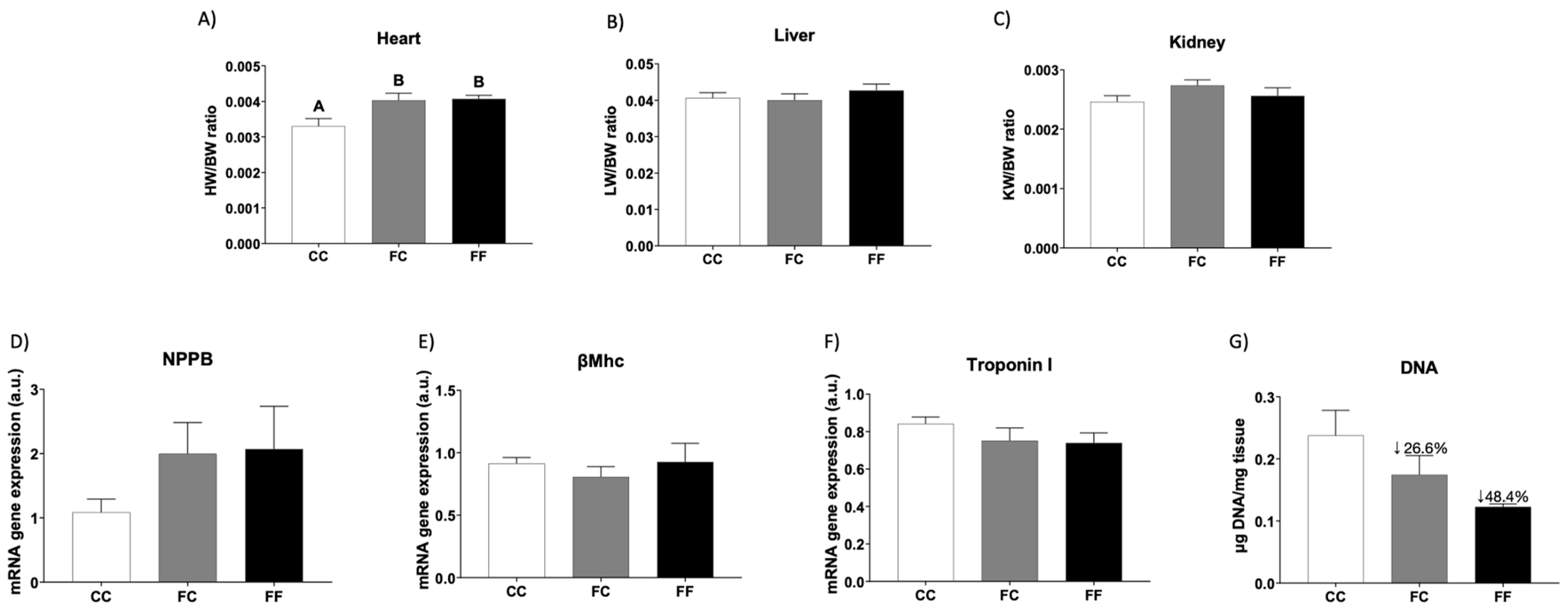
3.1. Descendants of Fructose-Fed Mothers Subjected or Not to Liquid Fructose Showed Maternal Cardiac Hypertrophy
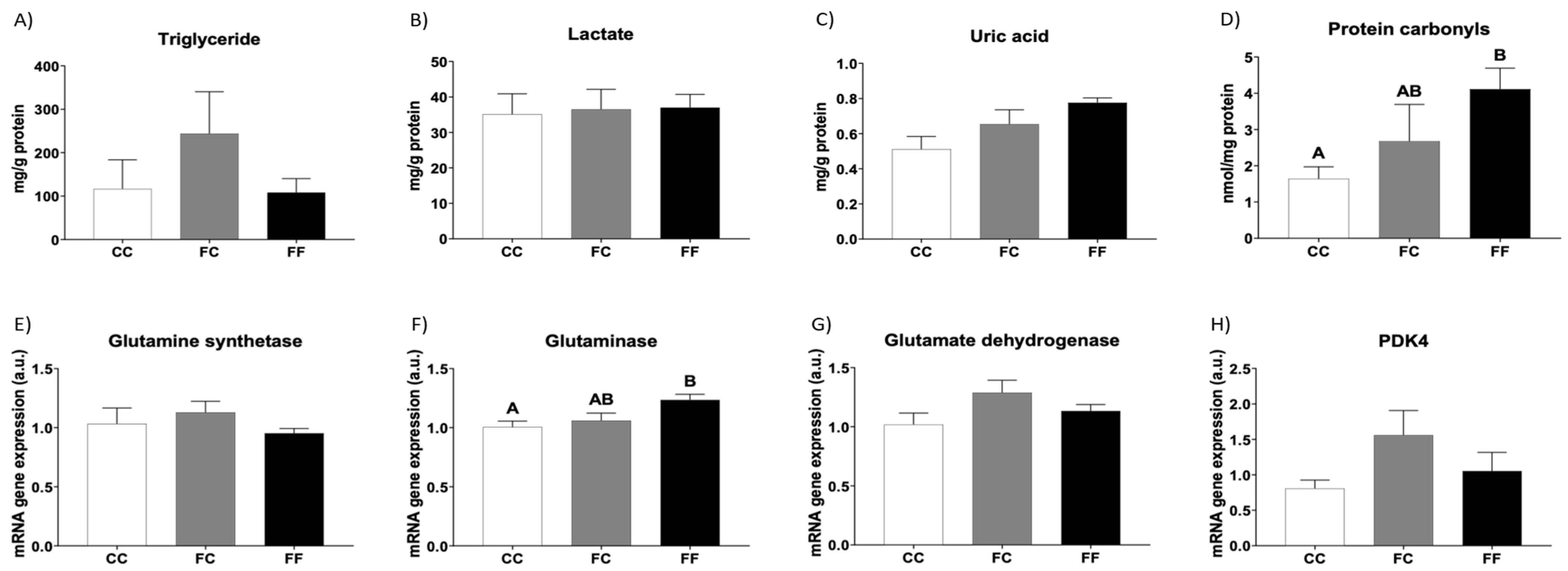
3.2. Metabolic and Oxidative Stress Parameters Related to Cardiac Hypertrophy in Descendants of Fructose-Fed Mothers Subjected or Not to Liquid Fructose
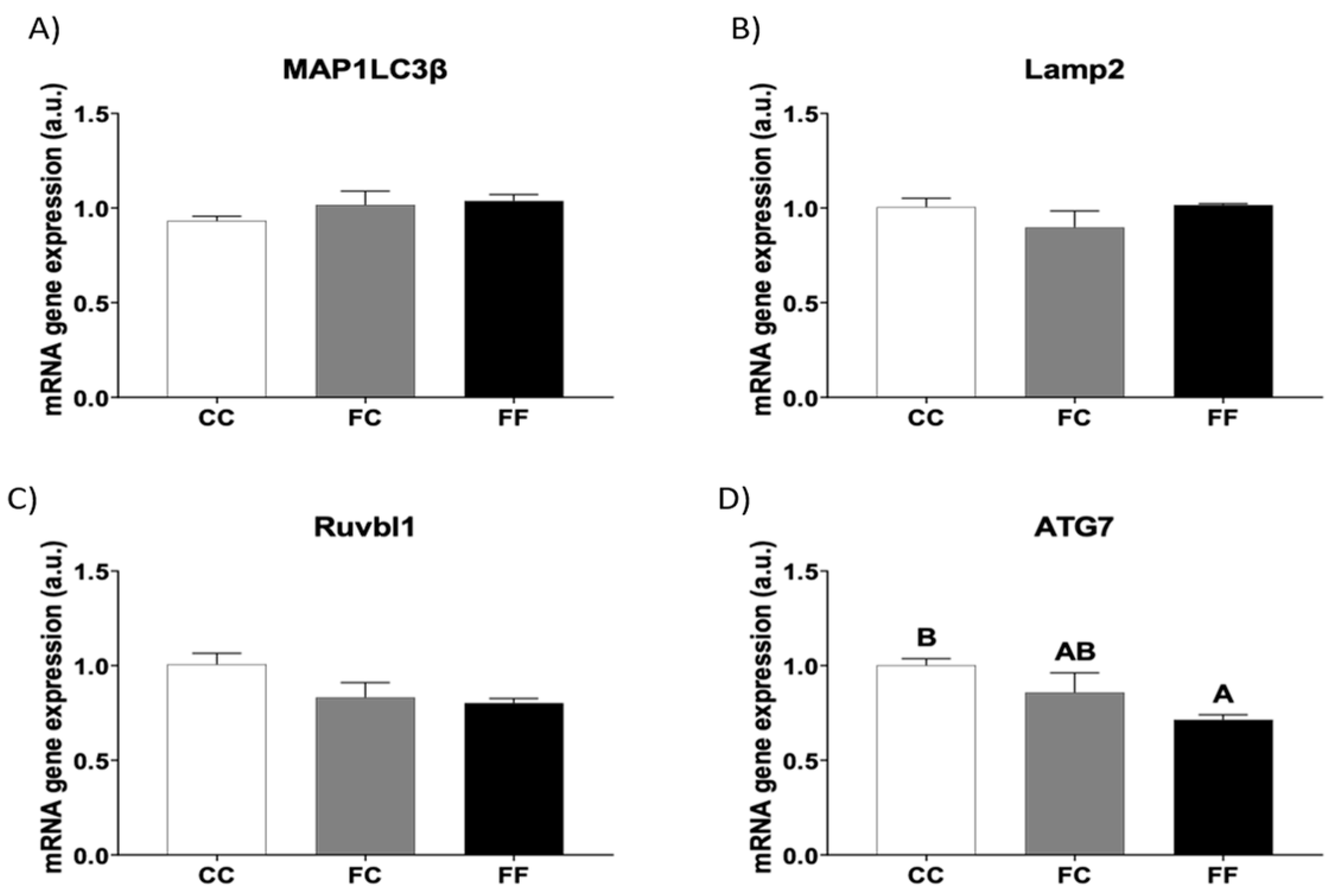
3.3. Fructose Consumption during Pregnancy Affects the Expression of Cardiac Autophagy-Related Genes of Their Descendants
3.4. Descendants of Fructose-Fed Mothers Subjected or Not to Liquid Fructose Present a Stimulated Cardiac Gene Expression of HIF1α Target Genes
3.5. Descendants of Fructose-Fed Mothers Subjected or Not to Liquid Fructose Present Altered Expression of Cardiac Genes Related to Osmolality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Y.; Li, W.; Yin, J. The intestinal-hepatic axis: A comprehensive review on fructose metabolism and its association with mortality and chronic metabolic diseases. Crit. Rev. Food Sci. Nutr. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and Sugar: A Major Mediator of Nonalcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Jung, S.; Bae, H.; Song, W.; Jang, C. Dietary Fructose and Fructose-Induced Pathologies. Annu. Rev. Nutr. 2022, 42, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, Y.; Chang, S.; Zhang, R.; Shi, C. High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am. J. Transl. Res. 2016, 8, 4869–4880. [Google Scholar] [PubMed]
- Oldfield, C.J.; Duhamel, T.A.; Dhalla, N.S. Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can. J. Physiol. Pharmacol. 2020, 98, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Mirtschink, P.; Krek, W. Hypoxia-driven glycolytic and fructolytic metabolic programs: Pivotal to hypertrophic heart disease. Biochim. Biophys. Acta 2016, 1863, 1822–1828. [Google Scholar] [CrossRef]
- Annandale, M.; Daniels, L.J.; Li, X.; Neale, J.P.H.; Chau, A.H.L.; Ambalawanar, H.A.; James, S.L.; Koutsifeli, P.; Delbridge, L.M.D.; Mellor, K.M. Fructose Metabolism and Cardiac Metabolic Stress. Front. Pharmacol. 2021, 12, 695486. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Madlala, H.P.; Maarman, G.J.; Ojuka, E. Uric acid and transforming growth factor in fructose-induced production of reactive oxygen species in skeletal muscle. Nutr. Rev. 2024, 74, 259. [Google Scholar] [CrossRef]
- Mirtschink, P.; Jang, C.; Arany, Z.; Krek, W. Fructose metabolism, cardiometabolic risk, and the epidemic of coronary artery disease. Eur. Heart J. 2018, 39, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Mirtschink, P.; Krishnan, J.; Grimm, F.; Sarre, A.; Hörl, M.; Kayikci, M.; Fankhauser, N.; Christinat, Y.; Cortijo, C.; Feehan, O.; et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 2015, 522, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Olaniyi, K.S.; Olatunji, L.A. Preventive effects of l-glutamine on gestational fructose-induced cardiac hypertrophy: Involvement of pyruvate dehydrogenase kinase-4. Appl. Physiol. Nutr. Metab. 2019, 44, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Wu, K.L.H.; Lee, W.C.; Train, Y.L.; Chan, J.Y. Maternal Fructose Intake Exacerbates Cardiac Remodeling in Offspring with Ventricular Pressure Overload. Nutrients 2021, 13, 3267. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Yu, H.; Chan, J.Y.H.; Wu, K.L.; Lee, W.C.; Train, Y.L. The Impact of Gut Microbiome on Maternal Fructose Intake-Induced Developmental Programming of Adult Disease. Nutrients 2022, 14, 1031. [Google Scholar] [CrossRef]
- Aguila, M.B.; Ornellas, F.; Mandarim-de-Lacerda, C.A. Nutritional research and fetal programming: Parental nutrition influences the structure and function of the organs. Int. J. Morphol. 2021, 39, 327. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.J.; Lopez-Frias, M.; Diaz-Castro, J. Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review. Nutrients 2020, 12, 3900. [Google Scholar] [CrossRef]
- Williams, S.J.; Campbell, M.E.; McMillen, I.C.; Davidge, S.T. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. American journal of physiology. Regul. Integr. Comp. Physiol. 2005, 288, R360–R367. [Google Scholar] [CrossRef]
- Fauste, E.; Panadero, M.I.; Donis, C.; Otero, P.; Bocos, C. Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses. Nutrients 2021, 13, 3667. [Google Scholar] [CrossRef]
- Fauste, E.; Donis, C.; Pérez-Armas, M.; Rodríguez, L.; Rodrigo, S.; Álvarez, J.J.; Otero, P.; Panadero, M.I.; Bocos, C. Maternal fructose boosts the effects of a Western-type diet increasing SARS-CoV-2 cell entry factors in male offspring. J. Funct. Foods 2023, 100, 105366. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.P.; Andresen, C.J.; Rudel, L.L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 1993, 26, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. In Anonymous Methods in Enzymology; Elsevier Science & Technology: San Diego, CA, USA, 1990; Volume 186, pp. 464–478. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ku, H.J.; Kim, J.K.; Park, J.W.; Lee, J.H. Amelioration of High Fructose-Induced Cardiac Hypertrophy by Naringin. Sci. Rep. 2018, 8, 9464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Wang, Z.W.; Higa, M.; Newgard, C.B.; Unger, R.H. Reversing adipocyte differentiation: Implications for treatment of obesity. Proc. Natl. Acad. Sci. USA 1999, 96, 2391–2395. [Google Scholar] [CrossRef]
- Mellor, K.M.; Bell, J.R.; Young, M.J.; Ritchie, R.H.; Delbridge, L.M. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J. Mol. Cell. Cardiol. 2011, 50, 1035–1043. [Google Scholar] [CrossRef]
- Baena, M.; Sangüesa, G.; Hutter, N.; Sánchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose supplementation impairs rat liver autophagy through mTORC activation without inducing endoplasmic reticulum stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 107–116. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Li, D.; Ye, J.M. Role of the mTOR-autophagy-ER stress pathway in high fructose-induced metabolic-associated fatty liver disease. Acta Pharmacol. Sin. 2022, 43, 10–14. [Google Scholar] [CrossRef]
- Äijälä, M.; Malo, E.; Ukkola, O.; Bloigu, R.; Lehenkari, P.; Autio-Harmainen, H.; Santaniemi, M.; Kesäniemi, Y.A. Long-term fructose feeding changes the expression of leptin receptors and autophagy genes in the adipose tissue and liver of male rats: A possible link to elevated triglycerides. Genes Nutr. 2013, 8, 623–635. [Google Scholar] [CrossRef]
- Suwannakul, N.; Armartmuntree, N.; Thanan, R.; Midorikawa, K.; Kon, T.; Oikawa, S.; Kobayashi, H.; Ma, N.; Kawanishi, S.; Murata, M. Targeting fructose metabolism by glucose transporter 5 regulation in human cholangiocarcinoma. Genes Dis. 2022, 9, 1727–1741. [Google Scholar] [CrossRef]
- Kanbay, M.; Altıntas, A.; Yavuz, F.; Copur, S.; Sanchez-Lozada, L.G.; Lanaspa, M.A.; Johnson, R.J. Responses to Hypoxia: How Fructose Metabolism and Hypoxia-Inducible Factor-1a Pathways Converge in Health and Disease. Curr. Nutr. Rep. 2023, 12, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, T.; Schönenberger, M.J.; Walter, K.M.; Charles, K.N.; Faust, P.L.; Kovacs, W.J. Peroxisome-Deficiency and HIF-2α Signaling Are Negative Regulators of Ketohexokinase Expression. Front. Cell Dev. Biol. 2020, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Strange, K. Cellular volume homeostasis. Adv. Physiol. Educ. 2004, 28, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.B.; Ferraris, J.D. Intracellular Organic Osmolytes: Function and Regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.K.; Dahl, S.C.; Handler, J.S.; Kwon, H.M. Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. American journal of physiology. Ren. Physiol. 2000, 278, F1006–F1012. [Google Scholar] [CrossRef]
- Rodríguez, L.; Panadero, M.I.; Roglans, N.; Otero, P.; Rodrigo, S.; Álvarez-Millán, J.J.; Laguna, J.C.; Bocos, C. Fructose only in pregnancy provokes hyperinsulinemia, hypoadiponectinemia, and impaired insulin signaling in adult male, but not female, progeny. Eur. J. Nutr. 2016, 55, 665–674. [Google Scholar] [CrossRef]
- Rodríguez, L.; Panadero, M.I.; Rodrigo, S.; Roglans, N.; Otero, P.; Álvarez-Millán, J.J.; Laguna, J.C.; Bocos, C. Liquid fructose in pregnancy exacerbates fructose-induced dyslipidemia in adult female offspring. J. Nutr. Biochem. 2016, 32, 115–122. [Google Scholar] [CrossRef]
- Laukka, T.; Mariani, C.J.; Ihantola, T.; Cao, J.Z.; Hokkanen, J.; Kaelin, W.G.; Godley, L.A.; Koivunen, P. Fumarate and Succinate Regulate Expression of Hypoxia-inducible Genes via TET Enzymes. J. Biol. Chem. 2016, 291, 4256–4265. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in Cardiomyocytes and Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef]
- Chaanine, A.H.; Gordon, R.E.; Kohlbrenner, E.; Benard, L.; Jeong, D.; Hajjar, R.J. Potential role of BNIP3 in cardiac remodeling, myocardial stiffness, and endoplasmic reticulum: Mitochondrial calcium homeostasis in diastolic and systolic heart failure. Circ. Heart Fail. 2013, 6, 572–583. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kita, S.; Iyoda, T.; Yamada, T.; Iwamoto, T. New Molecular Mechanisms for Cardiovascular Disease: Cardiac Hypertrophy and Cell-Volume Regulation. J. Pharmacol. Sci. 2011, 116, 343–349. [Google Scholar] [CrossRef]


| CC | FC | FF | |
|---|---|---|---|
| Body weight (conceptus free) (g) | 286.2 ± 3.6 B | 261.5 ± 6.9 A | 268.9 ± 1.7 AB |
| Liver weight (g) | 12.0 ± 0.5 | 10.5 ± 0.4 | 11.3 ± 0.5 |
| Kidney weight (g) | 0.68 ± 0.04 | 0.72 ± 0.03 | 0.68 ± 0.04 |
| Heart weight (g) | 0.91 ± 0.08 | 1.05 ± 0.03 | 1.10 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donis, C.; Fauste, E.; Pérez-Armas, M.; Otero, P.; Panadero, M.I.; Bocos, C. Cardiac Hypertrophy in Pregnant Rats, Descendants of Fructose-Fed Mothers, an Effect That Worsens with Fructose Supplementation. Foods 2024, 13, 2944. https://doi.org/10.3390/foods13182944
Donis C, Fauste E, Pérez-Armas M, Otero P, Panadero MI, Bocos C. Cardiac Hypertrophy in Pregnant Rats, Descendants of Fructose-Fed Mothers, an Effect That Worsens with Fructose Supplementation. Foods. 2024; 13(18):2944. https://doi.org/10.3390/foods13182944
Chicago/Turabian StyleDonis, Cristina, Elena Fauste, Madelín Pérez-Armas, Paola Otero, María I. Panadero, and Carlos Bocos. 2024. "Cardiac Hypertrophy in Pregnant Rats, Descendants of Fructose-Fed Mothers, an Effect That Worsens with Fructose Supplementation" Foods 13, no. 18: 2944. https://doi.org/10.3390/foods13182944
APA StyleDonis, C., Fauste, E., Pérez-Armas, M., Otero, P., Panadero, M. I., & Bocos, C. (2024). Cardiac Hypertrophy in Pregnant Rats, Descendants of Fructose-Fed Mothers, an Effect That Worsens with Fructose Supplementation. Foods, 13(18), 2944. https://doi.org/10.3390/foods13182944





