The Role of Camellia Shell Substrates in Modulating the Nutritional Characteristics of Pleurotus pulmonarius
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of P. pulmonarius Strain and Growing Substrates
2.2. Cultivation of P. pulmonarius
2.3. Determination of Proximate Composition
2.4. Hydrolyzed Amino Acid Assay
2.5. Antioxidant Capacity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Camellia Shell Substrate on Yield and Biological Efficiency (BE)
3.2. Camellia Shell Substrates Affect Moisture Content and Aqueous Extract Content
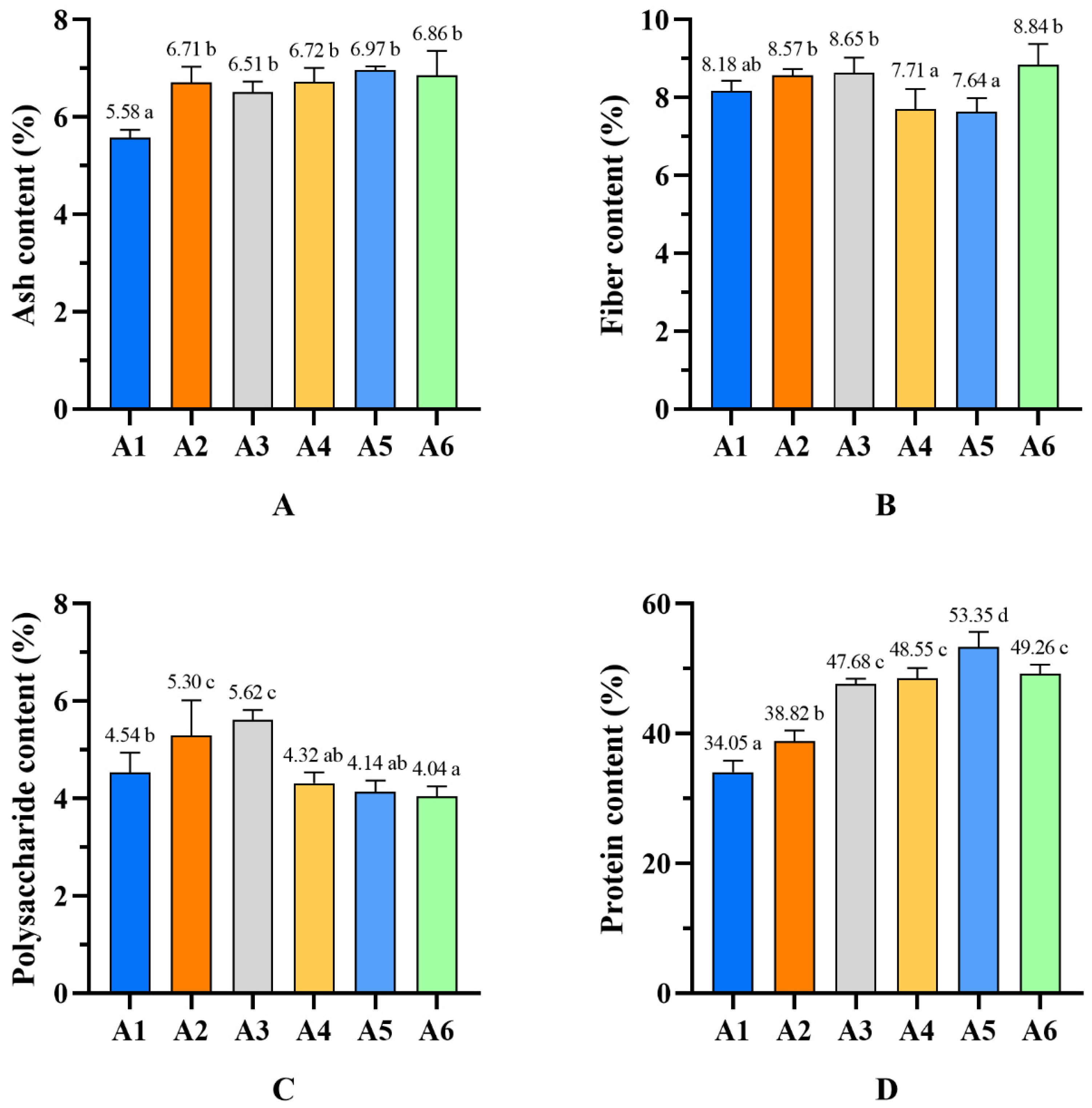
3.3. Camellia Shell Substrates Improve the Contents of Proximate Composition
3.4. Camellia Shell Substrates Enhance the Antioxidant Activities of Mushroom Aqueous Extracts
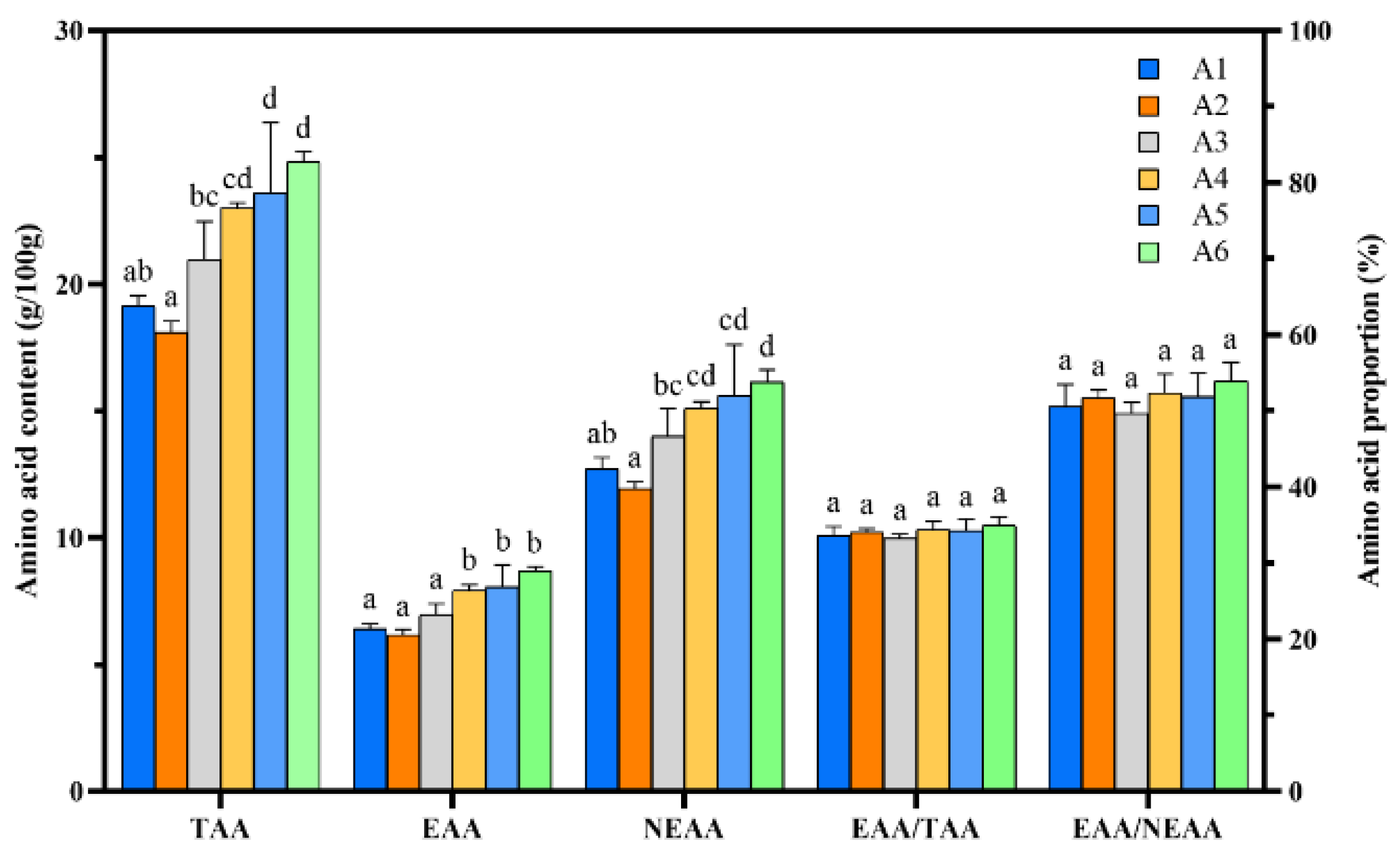
3.5. Camellia Shell Substitution Affects the Amino Acid Content in Fruit Bodies of P. pulmonarius
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Gu, Y.; Zhou, J.; Yuan, P.; Jiang, N.; Wu, Z.; Tan, X. Whole-Genome Identification and Analysis of Multiple Gene Families Reveal Candidate Genes for Theasaponin Biosynthesis in Camellia oleifera. Int. J. Mol. Sci. 2022, 23, 6393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The three-year action plan for accelerating the development of Camellia oleifera industry was issued. Land Green. 2023, 1, 42. [Google Scholar]
- Zhang, L.; He, Y.; Zhu, Y.; Liu, Y.; Wang, X. Camellia oleifera shell as an alternative feedstock for furfural production using a high surface acidity solid acid catalyst. Bioresour. Technol. 2018, 249, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Tan, X.; Cai, Y.; He, M.; Xiang, Z.; Ye, H.; Ma, J. Characterizations and application potentials of the hemicelluloses in waste oil-tea camellia fruit shells from Southern China. Ind. Crop. Prod. 2022, 178, 114551. [Google Scholar] [CrossRef]
- Zhang, J.; Ying, Y.; Li, X.; Yao, X. Evaluation of Three Kinds of Nutshell with Respect to Utilization as Culture Media. Bioresources 2018, 13, 7508–7518. [Google Scholar] [CrossRef]
- Huang, W.; Tao, X.; Ye, C. Analysis and evaluation of nutritional components of Lentinula edodes cultivated in camellia shell and pecan shell. Edible Fungi 2020, 42, 63–64. [Google Scholar]
- Zhang, J.; Lan, X.; Deng, B.; Guo, Y.; Li, X. Study on the cultivation technology of edible fungi of white wine lees and camellia shell. Contemp. Farm Mach. 2022, 4, 76–78. [Google Scholar]
- Zhang, W.; Liu, S.; Su, G.; Ma, L. Using camellia shell instead of cottonseed shell and bran to cultivate Hericium erinaceus. Acta Edulis Fungi 2016, 23, 24–28. [Google Scholar]
- Liu, L.; Zhou, Y.; Chen, H.; Weng, B.; Lin, D.; Liu, P.; Jiang, Z. Research progress of Pleurotus geesteranus. Microbiol. China 2020, 47, 3650–3657. [Google Scholar]
- Zhang, W.; Liu, S.; Su, G.; Ma, L. Camellia oleifera Seed Shell: An Effective Substrate for Producing Flammulina velutipes Fruit Bodies with Improved Nutritional Value. Int. J. Agric. Biol. 2019, 21, 989–996. [Google Scholar]
- Zhang, J.; Li, X.; Ying, Y.; Yao, X. Effects of the Camellia oleifera Shell Substrate on the Yield and Nutritional Composition of Pleurotus geesteranus. Agric. Sci. 2019, 10, 1298–1311. [Google Scholar]
- Li, X.; Chen, G.; Li, X.; Yao, F. Three Pleurotus mushroom species cultivated in a mixed Phragmites australis substrate differ in nutrient utilization capacity. J. Food Compos. Anal. 2023, 115, 104924. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, L.; Kang, S.; Ren, F.; Yang, L.; Zhang, Q.; Jia, Q. Comprehensive Evaluation of the Nutritional Properties of Different Germplasms of Polygonatum cyrtonema Hua. Foods 2024, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Xin, G.; Gong, X.; Wang, Y.; Wang, L.; Sun, B. Umami taste and its association with energy status in harvested Pleurotus geesteranus stored at different temperatures. Food Chem. 2019, 279, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yin, Z.; Liu, X.; Ma, C.; Wang, J.; Zhang, Y.; Kang, W. A glucomannogalactan from Pleurotus geesteranus: Structural characterization, chain conformation and immunological effect. Carbohydr. Polym. 2022, 287, 119346. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cui, W.; Meng, F.; Xia, Q.; Li, X.; Hou, M.; Jia, L.; Zhang, J. Glucopyranose from Pleurotus geesteranus prevent alcoholic liver diseases by regulating Nrf2/HO-1-TLR4/NF-kappa B signalling pathways and gut microbiota. Food Funct. 2022, 13, 2441–2455. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Z.; Chen, X.; Lei, J.; Weng, B.; Huang, Q. Use of biogas fluid-soaked water hyacinth for cultivating Pleurotus geesteranus. Bioresour. Technol. 2010, 101, 2397–2400. [Google Scholar] [CrossRef]
- Guan, Q.; Gong, M.; Lin, T.; Xu, C. Effect of bamboo waste replacing cottonseed husk on cultivation of Pleurotus geesteranus. In Proceedings of the 3rd International Conference on Agricultural and Food Science (ICAFS), Kuala Lumpur, Malaysia, 8–11 December 2019. [Google Scholar]
- Wang, Q.; Li, H.; Chen, T.; Han, J. Yield, polysaccharides content and antioxidant properties of Pleurotus abalonus and Pleurotus geesteranus produced on asparagus straw as substrate. Sci. Hortic. 2012, 134, 222–226. [Google Scholar] [CrossRef]
- GB 5009.3-2016; The National Standard for Food Safety—The Determination of Moisture in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB/T 8305-2013; The National Standard for Food Safety—The Determination of water extracts content. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2013.
- GB 5009.4-2016; The National Standard for Food Safety—The Determination of Ash in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.10-2003; The National Standard for Food Safety—The Determination of Crude Fiber in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2003.
- SN/T 4260-2015; Determination of Crude Polysaccharides in Plant Source Foods for Export—Phehol-furic Acid Colormetry. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2015.
- Horwitz, W.; Allee, G.; Latimer, G.; Kenneth, H. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; p. 59. [Google Scholar]
- GB 5009.124-2016; The National Standard for Food Safety—The Determination of Amino Acid in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Xue, C.; Jiang, J.; Deng, H.; Pan, L.; Song, W.; Yang, X.; Guan, Y. Analysis of nutritional quality and protein nutritional value of Lentinula edodes cultivated with camellia shell substrate combined with rich zinc and selenium. J. Dalian Minzu Univ. 2022, 24, 389–395. [Google Scholar]
- Leong, Y.; Yang, F.; Chang, J. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Meng, L.; Wang, X.; Zhao, W.; Shi, X.; Wang, W.; Li, Z.; Wang, L. The yield, nutritional value, umami components and mineral contents of the first-flush and second-flush Pleurotus pulmonarius mushrooms grown on three forestry wastes. Food Chem. 2022, 397, 133714. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Gao, Y.; Jiang, Z.; Zhao, Z.; Zeng, W.; Xie, M.; Liu, S.; Liu, R.; Chao, Y.; et al. Valorization of Camellia oleifera oil processing byproducts to value-added chemicals and biobased materials: A critical review. Green Energy Environ. 2024, 9, 28–53. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Zhang, F.; Jiang, J. Xylo-oligosaccharides and lignin production from Camellia oleifera shell by malic acid hydrolysis at mild conditions. Bioresour. Technol. 2021, 341, 125897. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Yin, D.; Liu, Y.; Gao, Z.; Cao, Y.; Chen, T.; Huang, Z.; Jia, Q.; Wang, D. Succession of endophytic fungi and rhizosphere soil fungi and their correlation with secondary metabolites in Fagopyrum dibotrys. Front. Microbiol. 2023, 14, 1220431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, Y.; Zhang, L.; Lu, M.; Yang, L.; Wang, D.; Jia, Q. The accumulation of active ingredients of Polygonatum cyrtonema Hua is associated with soil characteristics and bacterial community. Front. Microbiol. 2024, 15, 1347204. [Google Scholar] [CrossRef]
- Tepsongkroh, B.; Jangchud, K.; Trakoontivakorn, G. Antioxidant properties and selected phenolic acids of five different tray-dried and freeze-dried mushrooms using methanol and hot water extraction. J. Food Meas. Charact. 2019, 13, 3097–3105. [Google Scholar] [CrossRef]
- Chen, G.; Qin, Y.; Lu, Y.; Wei, J.; Chen, D. Effects of Different Culture Materials on Growth and Main Nutritional Components of Pleurotus pulmonarius. Edible Fungi 2018, 40, 33–34. [Google Scholar]
- Wani, B.A.; Bodha, R.H.; Wani, A.H. Nutritional and medicinal importance of mushrooms. J. Med. Plants Res. 2010, 4, 2598–2604. [Google Scholar]
- Salami, A.O.; Bankole, F.A.; Olawole, O.I. Effect of different substrates on the growth and protein content of oyster mushroom (Pleurotus florida). Int. J. Biol. Chem. Sci. 2016, 10, 475. [Google Scholar] [CrossRef]
- Altas, S.; Kizil, G.; Kizil, M.; Ketani, A.; Haris, P.I. Protective effect of Diyarbakir watermelon juice on carbon tetrachloride-induced toxicity in rats. Food Chem. Toxicol. 2011, 49, 2433–2438. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, J.; Song, X.; Zhang, J.; Wang, X.; Jing, H.; Ren, Z.; Li, S.; Zhang, C.; Jia, L. Antioxidative, anti-inflammation and lung-protective effects of mycelia selenium polysaccharides from Oudemansiella radicata. Int. J. Biol. Macromol. 2017, 104, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Chen, T.; Ding, Y.; Yu, D.; Zhang, J.; Zhang, R.; Zhang, Y.; Wang, X.; Xu, T.; Li, J. Effects of Rhizopus-arrhizus-31-Assisted Pretreatment on the Extraction and Bioactivity of Total Flavonoids from Hibiscus manihot L. Molecules 2024, 29, 1046. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, P.; Wang, T.; Yan, L.; Lin, M.; Chen, C. Synergistic effects of surfactant-assisted biodegradation of wheat straw and production of polysaccharides by Inonotus obliquus under submerged fermentation. Bioresour. Technol. 2019, 278, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, W.; Cui, W.; Jia, L.; Zhang, J. A polysaccharide of PFP-1 from Pleurotus geesteranus attenuates alcoholic liver diseases via Nrf2 and NF-κB signaling pathways. Food Funct. 2021, 12, 4591–4605. [Google Scholar] [CrossRef]
- He, D.; Li, F.; Zeng, W.; Huang, C.; Huang, X. Primary analysis of components of fresh mushroom from Pleurotus geesteranus cultivated with mulberry branch as raw material. Edible Fungi China 2015, 34, 25–27. [Google Scholar]
- Yang, B.; Li, Q.; Cheng, K.; Fang, J.; Mustafa, G.; Pan, J.; Xing, B.; Lv, Q.; Zhang, L.; Cheng, K. Proteomics and metabolomics reveal the mechanism underlying differential antioxidant activity among the organs of two base plants of Shiliang tea (Chimonanthus salicifolius and Chimonanthus zhejiangensis). Food Chem. 2022, 385, 132698. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, P.; Zhu, Z.; Chen, C.; Xu, X. Production of phenolic compounds and antioxidant activity via bioconversion of wheat straw by Inonotus obliquus under submerged fermentation with the aid of a surfactant. J. Sci. Food Agric. 2021, 101, 1021–1029. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, L.; Hong, X.; Huang, Y.; Lou, G.; Hou, Z.; Abozeid, A.; Wei, Y.; Yang, D. Metabolomic and antioxidant analyses of Salvia miltiorrhiza Bunge and Salvia prattii Hemsl. seeds. Nat. Prod. Res. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Cen, Y.; Yang, D.; Qi, Z.; Hou, Z.; Han, S.; Cai, Z.; Liu, K. Bivariate Correlation Analysis of the Chemometric Profiles of Chinese Wild Salvia miltiorrhiza Based on UPLC-Qqq-MS and Antioxidant Activities. Molecules 2018, 23, 538. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Maouni, A.; da Silva, L.P.; Saidi, R.; Legssyer, M.; Lamrani, Z.; Esteves da Silva, J.C.G. Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules 2023, 28, 1123. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Paul, S.; Acharya, K. Mushroom as the potential source of new generation of antioxidant: A review. Res. J. Pharm. Technol. 2013, 6, 496–505. [Google Scholar]
- Kozarski, M.S.; Klaus, A.S.; Niksic, M.P.; Van Griensven, L.J.L.D.; Vrvic, M.M.; Jakovljevic, D.M. Polysaccharides of higher fungi: Biological role, structure and antioxidative activity. Hem. Ind. 2014, 68, 305–320. [Google Scholar] [CrossRef]
- Valenzuela-Cobos, J.D.; Rodriguez-Grimon, R.O.; Zied, D.C.; Grijalva-Endara, A.; Garces-Moncayo, M.F.; Eugenia Garin-Aguilar, M.; Sanchez-Hernandez, A.; Valencia del Toro, G. Chemical composition and biological properties of Pleurotus spp. cultivated on peat moss and wheat straw. Emir. J. Food Agric. 2019, 31, 830–836. [Google Scholar] [CrossRef]
- Mihai, R.A.; Melo Heras, E.J.; Florescu, L.I.; Catana, R.D. The Edible Gray Oyster Fungi Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm a Potent Waste Consumer, a Biofriendly Species with Antioxidant Activity Depending on the Growth Substrate. J. Fungi 2022, 8, 274. [Google Scholar] [CrossRef]
- Zang, Q.; Chen, X.; Zhang, C.; Lin, M.; Xu, X. Improving crude protein and methionine production, selective lignin degradation and digestibility of wheat straw by Inonotus obliquus using response surface methodology. J. Sci. Food Agric. 2022, 102, 1146–1154. [Google Scholar] [CrossRef]
| Treatment | Content (g/100 g Dry Weight) | |||||
|---|---|---|---|---|---|---|
| Camellia Shell | Cottonseed Shell | Sawdust | Bran | Sucrose | Gypsum | |
| A1 | 0 | 39 | 39 | 20 | 1 | 1 |
| A2 | 10 | 29 | 39 | 20 | 1 | 1 |
| A3 | 20 | 19 | 39 | 20 | 1 | 1 |
| A4 | 30 | 9 | 39 | 20 | 1 | 1 |
| A5 | 35 | 4 | 39 | 20 | 1 | 1 |
| A6 | 39 | 0 | 39 | 20 | 1 | 1 |
| Sample | Mycelium Running (cm/d) | Total Yield (g/bag) | Biological Efficiency (%) |
|---|---|---|---|
| A1 | 0.54 ± 0.07 d | 415.10 ± 40.63 b | 74.13 ± 7.26 c |
| A2 | 0.36 ± 0.04 c | 412.20 ± 39.35 b | 73.57 ± 7.02 c |
| A3 | 0.35 ± 0.06 c | 407.83 ± 24.93 b | 72.83 ± 4.45 c |
| A4 | 0.33 ± 0.24 bc | 307.10 ± 53.87 b | 54.84 ± 9.62 b |
| A5 | 0.29 ± 0.20 ab | 246.07 ± 19.59 b | 43.94 ± 3.50 b |
| A6 | 0.27 ± 0.24 a | 163.90 ± 21.51 a | 29.27 ± 3.84 a |
| Sample | Moisture Content (%) | Aqueous Extract Content (%) |
|---|---|---|
| A1 | 87.05 ± 0.95 a | 50.55 ± 1.24 b |
| A2 | 89.02 ± 0.48 b | 43.33 ± 0.57 a |
| A3 | 89.26 ± 0.55 b | 45.04 ± 1.43 a |
| A4 | 88.95 ± 0.54 b | 43.69 ± 1.05 a |
| A5 | 87.73 ± 0.85 a | 43.10 ± 3.11 a |
| A6 | 86.69 ± 1.36 a | 45.61 ± 1.58 a |
| Sample | DPPH Free Radical Scavenging Rate (%) | ABTS Free Radical Scavenging Rate (%) |
|---|---|---|
| A1 | 61.77 ± 6.02 a | 40.41 ± 5.88 b |
| A2 | 73.90 ± 4.40 bc | 47.27 ± 1.79 cd |
| A3 | 82.70 ± 2.02 d | 30.96 ± 5.34 a |
| A4 | 76.30 ± 1.71 c | 45.97 ± 2.61 c |
| A5 | 77.63 ± 2.83 cd | 51.71 ± 4.59 de |
| A6 | 67.81 ± 7.70 ab | 54.89 ± 6.95 e |
| Component | Content (g/100 g Dry Weight) | |||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | |
| Asp | 2.02 ± 0.09 ab | 1.75 ± 0.05 a | 2.09 ± 0.18 b | 2.22 ± 0.05 bc | 2.23 ± 0.22 bc | 2.43 ± 0.10 c |
| Thr | 1.04 ± 0.02 ab | 0.94 ± 0.02 a | 1.10 ± 0.07 bc | 1.20 ± 0.01 cd | 1.22 ± 0.11 cd | 1.31 ± 0.03 d |
| Ser | 1.05 ± 0.03 ab | 0.92 ± 0.03 a | 1.09 ± 0.06 b | 1.17 ± 0.04 bc | 1.17 ± 0.11 bc | 1.27 ± 0.07 c |
| Glu | 4.77 ± 0.45 a | 4.75 ± 0.13 a | 5.65 ± 0.32 ab | 5.96 ± 0.39 b | 6.41 ± 0.85 b | 6.04 ± 0.59 b |
| Gly | 0.97 ± 0.02 a | 0.99 ± 0.02 ab | 1.11 ± 0.06 bc | 1.22 ± 0.03 cd | 1.25 ± 0.12 d | 1.31 ± 0.01 d |
| Ala | 1.14 ± 0.04 a | 1.13 ± 0.03 a | 1.25 ± 0.05 b | 1.36 ± 0.01 c | 1.40 ± 0.08 c | 1.51 ± 0.05 d |
| Val | 1.08 ± 0.00 ab | 1.03 ± 0.02 a | 1.18 ± 0.05 b | 1.32 ± 0.00 c | 1.34 ± 0.11 cd | 1.43 ± 0.03 d |
| Met | 0.21 ± 0.02 a | 0.20 ± 0.00 a | 0.24 ± 0.03 ab | 0.25 ± 0.02 ab | 0.26 ± 0.06 ab | 0.29 ± 0.03 b |
| Ile | 0.83 ± 0.01 ab | 0.81 ± 0.02 a | 0.92 ± 0.04 b | 1.04 ± 0.01 c | 1.06 ± 0.09 c | 1.15 ± 0.02 d |
| Leu | 1.32 ± 0.04 a | 1.34 ± 0.04 a | 1.45 ± 0.07 a | 1.67 ± 0.06 b | 1.68 ± 0.11 bc | 1.82 ± 0.02 c |
| Tyr | 0.47 ± 0.01 a | 0.51 ± 0.02 ab | 0.55 ± 0.04 ab | 0.60 ± 0.05 bc | 0.68 ± 0.08 c | 0.70 ± 0.06 c |
| Phe | 0.87 ± 0.03 a | 0.85 ± 0.02 a | 0.94 ± 0.05 a | 1.07 ± 0.05 b | 1.11 ± 0.10 b | 1.17 ± 0.06 b |
| Lys | 1.07 ± 0.06 a | 1.00 ± 0.05 a | 1.13 ± 0.07 a | 1.36 ± 0.07 b | 1.40 ± 0.15 b | 1.52 ± 0.12 b |
| His | 0.68 ± 0.09 ab | 0.53 ± 0.08 a | 0.58 ± 0.05 ab | 0.70 ± 0.08 ab | 0.64 ± 0.07 ab | 0.74 ± 0.09 b |
| Arg | 0.86 ± 0.06 a | 0.68 ± 0.03 ab | 0.90 ± 0.12 bc | 0.95 ± 0.04 bc | 0.93 ± 0.15 bc | 1.11 ± 0.07 c |
| Pro | 0.74 ± 0.05 a | 0.68 ± 0.02 a | 0.79 ± 0.06 ab | 0.91 ± 0.04 c | 0.90 ± 0.07 bc | 1.00 ± 0.05 c |
| Total | 19.13 ± 0.34 ab | 18.10 ± 0.36 a | 20.97 ± 1.22 bc | 23.00 ± 0.16 cd | 23.60 ± 2.26 d | 24.83 ± 0.33 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, W.; Lu, N.; Yu, J.; Chen, S.; Liang, Z. The Role of Camellia Shell Substrates in Modulating the Nutritional Characteristics of Pleurotus pulmonarius. Foods 2024, 13, 2946. https://doi.org/10.3390/foods13182946
Huang Y, Wang W, Lu N, Yu J, Chen S, Liang Z. The Role of Camellia Shell Substrates in Modulating the Nutritional Characteristics of Pleurotus pulmonarius. Foods. 2024; 13(18):2946. https://doi.org/10.3390/foods13182946
Chicago/Turabian StyleHuang, Yikai, Weike Wang, Na Lu, Jing Yu, Shaoning Chen, and Zongsuo Liang. 2024. "The Role of Camellia Shell Substrates in Modulating the Nutritional Characteristics of Pleurotus pulmonarius" Foods 13, no. 18: 2946. https://doi.org/10.3390/foods13182946
APA StyleHuang, Y., Wang, W., Lu, N., Yu, J., Chen, S., & Liang, Z. (2024). The Role of Camellia Shell Substrates in Modulating the Nutritional Characteristics of Pleurotus pulmonarius. Foods, 13(18), 2946. https://doi.org/10.3390/foods13182946





