Comparison in Antioxidant Potential and Concentrations of Selected Bioactive Ingredients in Fruits of Lesser-Known Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Its Preparation
2.2. Bioactive Compound Measurements
2.2.1. Ascorbate Determination
2.2.2. Total Phenolic Content (TPC)
2.2.3. Trolox Equivalent Antioxidant Capacity (TEAC)
2.2.4. Statistical Analysis and Presentation of Data
3. Results and Discussion
3.1. Ascorbate and Phenolics
3.2. Total Antioxidant Capacity (TEAC)
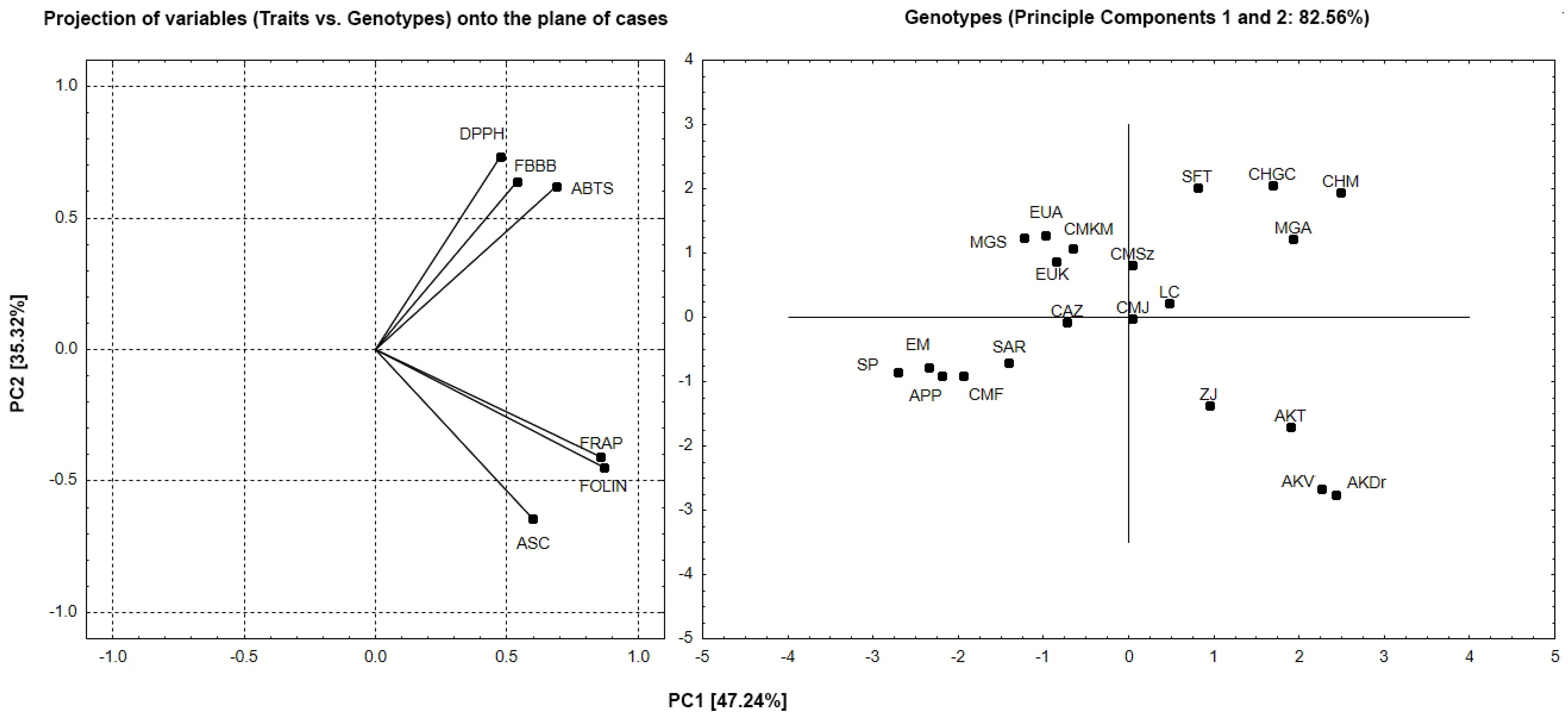
3.3. Multivariate PCA Biplot Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slavin, J.L.; Lioyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Česonienė, L.; Štreimikytė, P.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Viškelis, J.; Daubaras, R. Berries and leaves of Actinidia kolomikta (Rupr. & Maxim.) Maxim.: A source of phenolic compounds. Plants 2022, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Česonienė, L.; Januškevičė, V.; Saunoriūtė, S.; Liaudanskas, M.; Žvikas, V.; Krikštolaitis, R.; Viškelis, P.; Urbonavičienė, D.; Martusevičė, P.; Zych, M.; et al. Phenolic compounds in berries of winter-resistant Actinidia arguta Miq. and Actinidia kolomikta Maxim.: Evidence of antioxidative activity. Antioxidants 2024, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Khromykh, N.O.; Lykholat, Y.V.; Didur, O.O.; Sklyar, T.V.; Davydov, V.R.; Lavrentieva, K.V.; Lykholat, T.Y. Phytochemical profiles, antioxidant and antimicrobial activity of Actinidia polygama and A. arguta fruits and leaves. Biosyst. Divers. 2022, 30, 39–45. [Google Scholar] [CrossRef]
- Bieniek, A.A.; Grygorieva, O.; Bielska, N. Biological properties of honeysuckle (Lonicera caerulea L.): A Review: The nutrition, health properties of honeysuckle. Agrobiodiversity Improv. Nutr. Health Life Qual. 2021, 5. Available online: https://agrobiodiversity.uniag.sk/scientificpapers/article/view/390 (accessed on 23 July 2024). [CrossRef]
- Sharma, A.; Lee, H.J. Lonicera caerulea: An updated account of its phytoconstituents and health-promoting activities. Trends Food Sci. Technol. 2021, 107, 130–149. [Google Scholar] [CrossRef]
- Cheng, Z.; Bao, Y.; Li, Z.; Wang, J.; Wang, M.; Wang, S.; Wang, Y.; Li, B. Lonicera caerulea (Haskap berries): A review of development traceability, functional value, product development status, future opportunities, and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 8992–9016. [Google Scholar] [CrossRef]
- Guo, L.; Qiao, J.; Zhang, L.; Yan, W.; Zhang, M.; Lu, Y.; Wang, Y.; Ma, H.; Liu, Y.; Zhang, Y.; et al. Critical review on anthocyanins in blue honeysuckle (Lonicera caerulea L.) and their function. Plant Physiol. Biochem. 2023, 204, 108090. [Google Scholar] [CrossRef]
- Watychowicz, K.; Janda, K.; Jakubczyk, K.; Wolska, J. Chaenomeles–health promoting benefits. Rocz. Panstw. Zakl. Hig. 2017, 68, 217–227. Available online: https://roczniki.pzh.gov.pl/pdf-182474-102970?filename=Chaenomeles%20_%20health.pdf (accessed on 20 July 2024).
- Zhang, R.; Li, S.; Zhu, Z.; He, J. Recent advances in valorization of Chaenomeles fruit: A review of botanical profile, phytochemistry, advanced extraction technologies and bioactivities. Trends Food. Sci. Technol. 2019, 91, 467–482. [Google Scholar] [CrossRef]
- Xu, R.; Kuang, M.; Li, N. Phytochemistry and pharmacology of plants in the genus Chaenomeles. Arch. Pharm. Res. 2023, 46, 825–854. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.; Kostić, A.Ž.; Kamiloglu, S.; Tomas, M.; Mićanović, N.; Capanoglu, E. Chemical composition, nutritional and health related properties of the medlar (Mespilus germanica L.): From medieval glory to underutilized fruit. Phytochem. Rev. 2023, 22, 1663–1690. [Google Scholar] [CrossRef]
- Nistor, D.I.; Marc, R.A.; Mureșan, C.C. Phytochemistry, nutritional composition, health benefits and future prospects of Mespilus germanica L.(Medlar): A review. Food Chem. X 2024, 22, 101334. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical characterization, antioxidant activity, and phenolic compounds of hawthorn (Crataegus spp.) fruits species for potential use in food applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Nazir, N.; Zahoor, M.; Nisar, M. A review on traditional uses and pharmacological importance of genus Elaeagnus species. Botl. Rev. 2020, 86, 247–280. [Google Scholar] [CrossRef]
- Xie, P.J.; You, F.; Huang, L.X.; Zhang, C.H. Comprehensive assessment of phenolic compounds and antioxidant performance in the developmental process of jujube (Ziziphus jujuba Mill.). J. Funct. Foods 2017, 36, 233–242. [Google Scholar] [CrossRef]
- Kazimierski, M.; Reguła, J.; Molska, M. Cornelian cherry (Cornus mas L.)—characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar] [CrossRef]
- Miao, J.; Zhao, C.; Li, X.; Chen, X.; Mao, X.; Huang, H.; Wang, T.; Gao, W. Chemical composition and bioactivities of two common Chaenomeles fruits in China: Chaenomeles speciosa and Chaenomeles sinensis. J. Food. Sci. 2016, 81, H2049–H2058. [Google Scholar] [CrossRef]
- Żurek, N.; Karatsai, O.; Rędowicz, M.J.; Kapusta, I.T. Polyphenolic compounds of Crataegus berry, leaf, and flower extracts affect viability and invasive potential of human glioblastoma cells. Molecules 2021, 26, 2656. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active compounds, antioxidant activity and α-glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Xiao, Z.; Sun, A.; Zhao, M.; Wang, Y.; Zhang, Y. Polyphenols in twenty cultivars of blue honeysuckle (Lonicera caerulea L.): Profiling, antioxidant capacity, and α-amylase inhibitory activity. Food Chem. 2023, 421, 136148. [Google Scholar] [CrossRef]
- Orsavová, J.; Sytařová, I.; Mlček, J.; Mišurcová, L. Phenolic compounds, vitamins C and E and antioxidant activity of edible honeysuckle berries (Lonicera caerulea L. var. kamtschatica Pojark) in relation to their origin. Antioxidants 2022, 11, 433. [Google Scholar] [CrossRef]
- Skender, A.; Hadžiabulić, S.; Ercisli, S.; Hasanbegović, J.; Dedić, S.; Almeer, R.; Sayed, A.A.; Ullah, R.; Assouguem, A. Morphological and biochemical properties in fruits of naturally grown cornelian cherry (Cornus mas L.) genotypes in Northwest Bosnia and Herzegovina. Sustainability 2022, 14, 4579. [Google Scholar] [CrossRef]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlček, J.; Balla, Š.; Szekeres, L.; Žitný, R.; Zitka, O.; Adam, V.; Kizek, R. Evaluation of polyphenolic profile and nutritional value of non-traditional fruit species in the Czech Republic—A comparative study. Molecules 2012, 17, 8968–8981. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.N.; Dey, P.; Singhal, R.K.; Sahu, C.; Jena, D.; Nanda, S.P.; Chauhan, J. Plant Phenolics: As Antioxidants and Potent Compounds Under Multiple Stresses. In Plant Phenolics in Abiotic Stress Management; Lone, R., Khan, S., Mohammed Al-Sadi, A., Eds.; Springer: Singapore, 2023; pp. 215–234. [Google Scholar] [CrossRef]
- Spinola, V.; Llorent-Martinez, E.J.; Castilho, P.C. In the case of vitamin C, precise detection methods are not always used. J. Chrom. A 2014, 1369, 2–17. [Google Scholar] [CrossRef]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Sawicka, M.; Latocha, P.; Łata, B. Peel to flesh bioactive compounds ratio affect apple antioxidant potential and cultivar functional properties. Agriculture 2023, 13, 478. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Ree, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Česonienė, L.; Labokas, J.; Jasutienė, I.; Šarkinas, A.; Kaškonienė, V.; Kaškonas, P.; Kazernavičiūtė, R.; Pažereckaitė, A.; Daubaras, R. Bioactive compounds, antioxidant, and antibacterial properties of Lonicera caerulea berries: Evaluation of 11 cultivars. Plants 2021, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Liu, X.; Chen, M.; Bai, B.; Yang, Y.; Bo, T.; Fan, S. The separation, purification, structure identification, and antioxidant activity of Elaeagnus umbellata polysaccharides. Molecules 2023, 28, 6468. [Google Scholar] [CrossRef] [PubMed]
- Khassaf, M.; McArdle, A.; Esanu, C.; Vasilaki, A.; McArdle, F.; Griffiths, R.D.; Brodie, D.A.; Jackson, M.J. Effect of vitamin C supplements on antioxidant defense and stress proteins in human lymphocytes and skeletal muscle. J. Physiol. 2003, 549, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Pranckietis, V.; Paulauskiene, A.; Jureviciene, V.; Taraseviciene, Z.; Pranckietiene, I. Breeding and processing of Lithuanian cultivars of Actinidia kolomikta (Maxim. & Rupr.)Maxim. fruits grown in organic conditions. Zesz. Probl. Postęp. Nauk Rol. 2009, 536, 177–183. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.researchgate.net/profile/Aurelija_Paulauskiene/publication/236875946_Breeding_and_processing_of_lithuanian_cultivars_of_Actinidia_kolomikta_(Maxim._and_Rupr.)_Maxim._fruits_grown_in_organic_conditions/links/00b4953623c96b81ff000000%3Forigin%3Dpublication_detail&ved=2ahUKEwjKreP9i8OHAxXSCRAIHaATCPwQFnoECBoQAQ&usg=AOvVaw0pBQK1uIPXSBs-6cIokmT1 (accessed on 25 July 2024).
- Silva, A.M.; Macedo, C.; Latocha, P.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Valorization of Actinidia spp. by-products and wastes for nutraceutical applications. In Nutraceutics from Agri-Food by Products; Spizzirri, U.G., Ed.; Scrivener Publishing LLC: Baverly, MA, USA, 2023. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Byczkiewicz, S.; Szwajgier, D.; Kobus-Cisowska, J.; Szczepaniak, O.; Szulc, P. Comparative examination of bioactive phytochemicals in quince (Chaenomeles) fruits and their in vitro antioxidant activity. Emir. J. Food Agric. 2021, 33, 293–302. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Szot, I.; Łysiak, G.P.; Sosnowska, B. The beneficial effects of anthocyanins from cornelian cherry (Cornus mas L.) fruits and their possible uses: A review. Agriculture 2023, 14, 52. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.X.; Tsim, K.W.K. A review of dietary Ziziphus jujuba fruit (Jujube): Developing health food supplements for brain protection. Evid. Based Complement. Alternat. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef]
- Tahergorabi, Z.; Abedini, M.R.; Mitra, M.; Fard, M.H.; Beydokhti, H. Ziziphus jujuba: A red fruit with promising anticancer activities. Phcog. Rev. 2015, 9, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kostecka, M.; Szot, I.; Czernecki, T.; Szot, P. Vitamin C content of new ecotypes of cornelian cherry (Cornus mas L.) determined by various analytical methods. Acta Sci. Pol. Hort. Cult. 2017, 16, 53–61. Available online: https://czasopisma.up.lublin.pl/index.php/asphc/article/view/2348/1635 (accessed on 25 July 2024). [CrossRef]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. Cornus mas (L.) fruit as a potential source of natural health-promoting compounds: Physico-chemical characterisation of bioactive components. Plant Foods Hum. Nutr. 2018, 73, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Yarılgaç, T.; Kadim, H.; Ozturk, B. Role of maturity stages and modified-atmosphere packaging on the quality attributes of cornelian cherry fruits (Cornus mas L.) throughout shelf life. J. Sci. Food Agric. 2019, 99, 421–428. [Google Scholar] [CrossRef]
- Muradoğlu, F.; Gürsoy, S.; Yıldız, K. Quantification analysis of biochemical and phenolic composition in hawthorn (Crataegus spp.) fruits. Erwerbs-Obstbau 2019, 61, 89–194. [Google Scholar] [CrossRef]
- Bozhuyuk, M.R. Morphological and biochemical diversity in fruits of rowanberry (Sorbus aucuparia L.) genotypes. Erwerbs-Obstbau 2021, 63, 431–435. [Google Scholar] [CrossRef]
- Bieniek, A.; Piłat, B.; Szałkiewicz, M.; Markuszewski, B.; Gojło, E. Evaluation of yield, morphology and quality of fruits of cherry silverberry (Elaeagnus multiflora Thunb.) biotypes under conditions of north-eastern Poland. Pol. J. Nat. Sci. 2017, 32, 61–70. [Google Scholar]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Tessa, G.; Donno, D.; Gamba, G.; Mellano, M.G.; Beccaro, G.L. Local and underutilised fruits as a source of nutraceutical molecules: Bioactive compounds in Mespilus germanica L. Eur. Food. Res. Technol. 2021, 247, 2861–2868. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Ciałek, S.; Styczyńska, M.; Oziembłowski, M. Functional properties of fruits of common medlar (Mespilus germanica L.) extract. Appl. Sci. 2021, 11, 7528. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Jakljevic, K.; Veberic, R.; Hudina, M.; Rusjan, D. Changes in the fruit quality parameters of medlar fruit (Mespilus germanica L.) after heat treatment, storage, freezing or hoarfrost. Foods 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Variation in Bioactive Compounds and Antioxidant Activity of Rubus Fruits at Different Developmental Stages. Foods 2022, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Szot, I.; Łysiak, G.P.; Sosnowska, B.; Chojdak-Łukasiewicz, J. Health-promoting properties of anthocyanins from cornelian cherry (Cornus mas L.) fruits. Molecules 2024, 29, 449. [Google Scholar] [CrossRef]
- Klymenko, S.; Kucharska, A.Z.; Sokół-Łętowska, A.; Piórecki, N.; Przybylska, D.; Grygorieva, O. Iridoids, flavonoids, and antioxidant capacity of Cornus mas, C. officinalis, and C. mas × C. officinalis fruits. Biomolecules 2021, 11, 776. [Google Scholar] [CrossRef]
- Aurori, M.; Niculae, M.; Hanganu, D.; Pall, E.; Cenariu, M.; Vodnar, D.C.; Bunea, A.; Fit, N.; Andrei, S. Phytochemical Profile, Antioxidant, Antimicrobial and Cytoprotective Effects of Cornelian Cherry (Cornus mas L.) Fruit Extracts. Pharmaceuticals 2023, 16, 420. [Google Scholar] [CrossRef]
| Genotype | Common Name | Family | Fruit Origin | Cultivar Origin |
|---|---|---|---|---|
| Actinidia kolomikta ‘Dr Szymanowski’ | Arctic kiwi | Actinidiaceae | Own collection | Poland |
| Actinidia kolomikta ‘Talin’ | Arctic kiwi | Actinidiaceae | Own collection | Lithuania |
| Actinidia kolomikta ‘Vitakola’ | Arctic kiwi | Actinidiaceae | Own collection | Czech Republic |
| Actinidia polygama ‘Pomarantseva’ | Silvervine | Actinidiaceae | Own collection | Ukraine |
| Chanomeles × californica ‘Gold Calif’ | Japanese quince | Rosaceae | Carya Nursery, Poland | Ukraine |
| Chanomeles × californica ‘Maksym’ | Japanese quince | Rosaceae | Carya Nursery, Poland | Ukraine |
| Cornus mas ‘Szafer’ | Edible dogwood | Cornaceae | Carya Nursery, Poland | Poland |
| Cornus mas ‘Flava’ | Edible dogwood | Cornaceae | Carya Nursery, Poland | Unknown origin |
| Cornus mas ‘Jolico’ | Edible dogwood | Cornaceae | Own collection | Austria |
| Cornus mas ‘Korałłowyj Marka’ | Edible dogwood | Cornaceae | Carya Nursery, Poland | Ukraine |
| Crategus × anomala ‘Zbigniew’ | Hawthorn | Rosaceae | Carya Nursery, Poland | Ukraine |
| Elaeagnus multiflora ‘Sweet Scarlet’ | Cherry silverberry | Elaeagnaceae | Carya Nursery, Poland | Ukraine |
| Elaeagnus umbellate ‘K2’ | Japanese silverberry | Elaeagnaceae | Carya Nursery, Poland | Poland |
| Elaeagnus umbellata ‘Amber’ | Japanese silverberry | Elaeagnaceae | Carya Nursery, Poland | USA |
| Lonicera caerulea var. kamtschatica ‘Atut’ | Honeysuckle | Caprifoliaceae | Carya Nursery, Poland | Poland |
| Mespilus germanica ‘Süssmispel’ | Common medlar | Rosaceae | Carya Nursery, Poland | Germany |
| Mespilus germanica f. apyrena | Common medlar | Rosaceae | Carya Nursery, Poland | Natural origin |
| Sorbopyrus auricularis ‘Bulbiformis’ | The shipova | Rosaceae | Carya Nursery, Poland | Czech Republic |
| Sorbus aucuparia ‘Rosina’ | Rowan | Rosaceae | Carya Nursery, Poland | Germany |
| ×Sorbaronia fallax ‘Titan’ | Eesti | Rosaceae | Carya Nursery, Poland | Unknown origin |
| Zizipus jujuba | Jujube | Rhamnaceae | Kyiv Bot. Garden, Ukraine | Natural origin |
| Genotype | Acronym | ASC 1 ± SD | TPC (FOLIN) 2 ± SD | TPC (FBBB) 3 ± SD | FOLIN: FBBB Ratio |
|---|---|---|---|---|---|
| Actinidia kolomikta ‘Dr Szymanowski’ | AKDr | 782.2 ± 327 c | 260.0 ± 24.0 n | 25.5 ± 1.4 a | 10.2 |
| Actinidia kolomikta ‘Talin’ | AKT | 682.9 ± 1442 c | 188.9 ± 9.4 m | 42.0 ± 1.6 ab | 4.5 |
| Actinidia kolomikta ‘Vitakola’ | AKV | 298.3 ± 592 b | 309.6 ± 32.7 o | 51.6 ± 4.4 ab | 6.0 |
| Actinidia polygama ‘Pomarantseva’ | APP | 51.4 ± 20 a | 50.0 ± 10.1 a–f | 30.8 ± 2.9 a | 1.6 |
| Chanomeles × californica ‘Gold Calif’ | CHGC | 100.6 ± 16.8 a | 125.2 ± 23.5 i–k | 352.2 ± 28.9 h | 0.4 |
| Chanomeles × californica ‘Maksym’ | CHM | 133.7 ± 21.0 a | 169.8 ± 21.2 k–m | 460.4 ± 35.4 i | 0.4 |
| Cornus mas ‘Flava’ | CMF | 22.1 ± 2.5 a | 75.1 ± 16.2 c–h | 20.6 ± 1.9 a | 3.7 |
| Cornus mas ‘Jolico’ | CMJ | 51.7 ± 2.1 a | 101.5 ± 21.4 g–j | 180.1 ± 8.1 fg | 0.6 |
| Cornus mas ‘Korałłowyj Marka’ | CMKM | 26.4 ± 9.8 a | 43.7 ± 4.9 a–e | 72.7 ± 4.6 bc | 0.6 |
| Cornus mas ‘Szafer’ | CMSz | 29.7 ± 4.3 a | 90.5 ± 9.5 e–j | 156.4 ± 13.1 ef | 0.6 |
| Crategus × anomala ‘Zbigniew’ | CAZ | 33.0 ± 6.0 a | 90.9 ± 13.1 f–j | 207.0 ± 14.5 g | 0.4 |
| Elaeagnus multiflora ‘Sweet Scarlet’ | EM | 2.7 ± 0.13 a | 43.7 ± 6.8 a–d | 24.1 ± 5.6 a | 1.4 |
| Elaeagnus umbellata ‘K 2’ | EUK | 1.3 ± 0.3 a | 58.9 ± 26.3 b–g | 122.2 ± 16.0 de | 0.5 |
| Elaeagnus umbellata ‘Amber’ | EUA | 1.7 ± 0.4 a | 30.7 ± 9.2 a–c | 58.8 ± 4.0 a–c | 0.5 |
| Lonicera caerulea var. kamtschatica ‘Atut’ | LC | 50.9 ± 2.8 a | 135.9 ± 12.8 j–l | 312.3 ± 6.0 h | 0.4 |
| Mespilus germanica f. apyrena | MGA | 11.1 ± 1.3 a | 174.9 ± 25.1 l–m | 482.9 ± 29.5 i | 0.4 |
| Mespilus germanica ‘Süssmispel’ | MGS | 0.71 ± 0.1 a | 25.8 ± 3.3 ab | 26.9 ± 4.7 a | 1.0 |
| Sorbopyrus auricularis ‘Bulbiformis’ | SP | 5.0 ± 0.1 a | 6.6 ± 0.5 a | 29.6 ± 2.1 a | 0.2 |
| Sorbus aucuparia ‘Rosina’ | SAR | 64.5 ± 1.1 a | 79.3 ± 23.0 d–i | 80.1 ± 12.1 bc | 1.0 |
| ×Sorbaronia fallax ‘Titan’ | SFT | 17.6 ± 4.3 a | 108.8 ± 3.7 h–j | 325.7 ± 31.7 h | 0.3 |
| Zizipus jujuba | ZJ | 403.7 ± 4.1 b | 199.7 ± 25.2 m | 98.9 ± 13.2 cd | 2.0 |
| Average | 132.0 | 112.3 | 150.5 | ||
| Fold variation between genotypes | 1102 | 47 | 23 |
| TPC (FOLIN) | TPC (FBBB) | ASC | FRAP | ABTS | DPPH | |
|---|---|---|---|---|---|---|
| TPC (FOLIN) | X | 0.23 | 0.73 *** | 0.96 *** | 0.27 | 0.06 |
| TPC (FBBB) | X | −0.24 | 0.28 | 0.65 ** | 0.51 * | |
| ASC | X | 0.64 ** | 0.12 | −0.07 | ||
| FRAP | X | 0.27 | 0.05 | |||
| ABTS | X | 0.80 *** | ||||
| DPPH | X |
| Total Antioxidant Capacity, Assay Test | FRAP ± SD | ABTS ± SD | DPPH ± SD | |
|---|---|---|---|---|
| Genotype | Acronym | |||
| Actinidia kolomikta ‘Dr Szymanowski’ | AKDr | 68.2 ± 1.85 k | 30.9 ± 1.26 c–f | 53.0 ± 1.73 cde |
| Actinidia kolomikta ‘Talin’ | AKT | 53.6 ± 0.54 j | 45.9 ± 10.4 f–h | 53.5 ± 13.6 de |
| Actinidia kolomikta ‘Vitakola’ | AKV | 94.6 ± 1.35 l | 20.8 ± 0.57 b–d | 35.8 ± 13.1 a–d |
| Actinidia polygama ‘Pomarantseva’ | APP | 8.52 ± 0.29 b–d | 6.03 ± 0.47 ab | 8.66 ± 0.24 ab |
| Chanomeles × californica ‘Gold Calif’ | CHGC | 34.6 ± 3.40 g | 63.8 ± 13.6 i | 121.3 ± 13.5 hi |
| Chanomeles × californica ‘Maksym’ | CHM | 46.3 ± 1.24 i | 65.2 ± 2.07 i | 113.5 ± 13.7 g–i |
| Cornus mas ‘Flava’ | CMF | 11.3 ± 0.04 cd | 10.2 ± 0.38 ab | 9.84 ± 0.08 ab |
| Cornus mas ‘Jolico’ | CMJ | 41.9 ± 1.28 hi | 36.1 ± 0.48 e–g | 36.1 ± 2.10 b–d |
| Cornus mas ‘Korałłowyj Marka’ | CMKM | 11.7 ± 1.88 cd | 51.4 ± 5.23 hi | 71.6 ± 21.6 ef |
| Cornus mas ‘Szafer’ | CMSz | 31.1 ± 1.73 g | 36.5 ± 3.02 e–h | 93.3 ± 20.7 f–h |
| Crategus × anomala ‘Zbigniew’ | CAZ | 22.8 ± 0.97 f | 27.4 ± 0.60 c–e | 17.6 ± 1.01 a–c |
| Elaeagnus multiflora ‘Sweet Scarlet’ | EM | 9.89 ± 0.25 cd | 7.60 ± 0.59 ab | 6.00 ± 1.23 ab |
| Elaeagnus umbellata ‘K 2’ | EUK | 12.9 ± 1.92 de | 30.8 ± 6.43 c–f | 85.2 ± 15.2 c–g |
| Elaeagnus umbellata ‘Amber’ | EUA | 4.85 ± 0.95 ab | 44.7 ± 7.04 f–h | 90.6 ± 20.4 f–h |
| Lonicera caerulea var. kamtschatica ‘Atut’ | LC | 39.6 ± 0.43 h | 37.0 ± 0.47 e–h | 29.9 ± 2.46 a–d |
| Mespilus germanica f. apyrena | MGS | 56.8 ± 4.04 j | 47.6 ± 2.64 gh | 74.0 ± 18.1 ef |
| Mespilus germanica ‘Süssmispel’ | MGA | 1.83 ± 0.35 a | 36.4 ± 5.28 e–h | 106.8 ± 21.0 f–i |
| Sorbopyrus auricularis ‘Bulbiformis’ | SP | 8.26 ± 0.42 bc | 1.61 ± 0.21 a | 0.55 ± 0.18 a |
| Sorbus aucuparia ‘Rosina’ | SAR | 17.4 ± 1.62 e | 16.8 ± 2.29 bc | 12.2 ± 1.73 ab |
| ×Sorbaronia fallax ‘Titan’ | SFT | 24.8 ± 3.47 f | 42.0 ± 8.93 e–g | 139.2 ± 10.6 i |
| Zizipus jujuba | ZJ | 39.4 ± 0.37 h | 34.7 ± 1.04 fg | 33.2 ± 1.65 a–d |
| Average | 30.5 | 33.0 | 56.8 | |
| Fold variation between genotypes | 52 | 41 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łata, B.; Latocha, P.; Łaźny, R.; Gutfeld, A. Comparison in Antioxidant Potential and Concentrations of Selected Bioactive Ingredients in Fruits of Lesser-Known Species. Foods 2024, 13, 2926. https://doi.org/10.3390/foods13182926
Łata B, Latocha P, Łaźny R, Gutfeld A. Comparison in Antioxidant Potential and Concentrations of Selected Bioactive Ingredients in Fruits of Lesser-Known Species. Foods. 2024; 13(18):2926. https://doi.org/10.3390/foods13182926
Chicago/Turabian StyleŁata, Barbara, Piotr Latocha, Radosław Łaźny, and Anna Gutfeld. 2024. "Comparison in Antioxidant Potential and Concentrations of Selected Bioactive Ingredients in Fruits of Lesser-Known Species" Foods 13, no. 18: 2926. https://doi.org/10.3390/foods13182926
APA StyleŁata, B., Latocha, P., Łaźny, R., & Gutfeld, A. (2024). Comparison in Antioxidant Potential and Concentrations of Selected Bioactive Ingredients in Fruits of Lesser-Known Species. Foods, 13(18), 2926. https://doi.org/10.3390/foods13182926









