Towards Reducing Food Wastage: Analysis of Degradation Products Formed during Meat Spoilage under Different Conditions
Abstract
1. Introduction
2. Materials and Methods
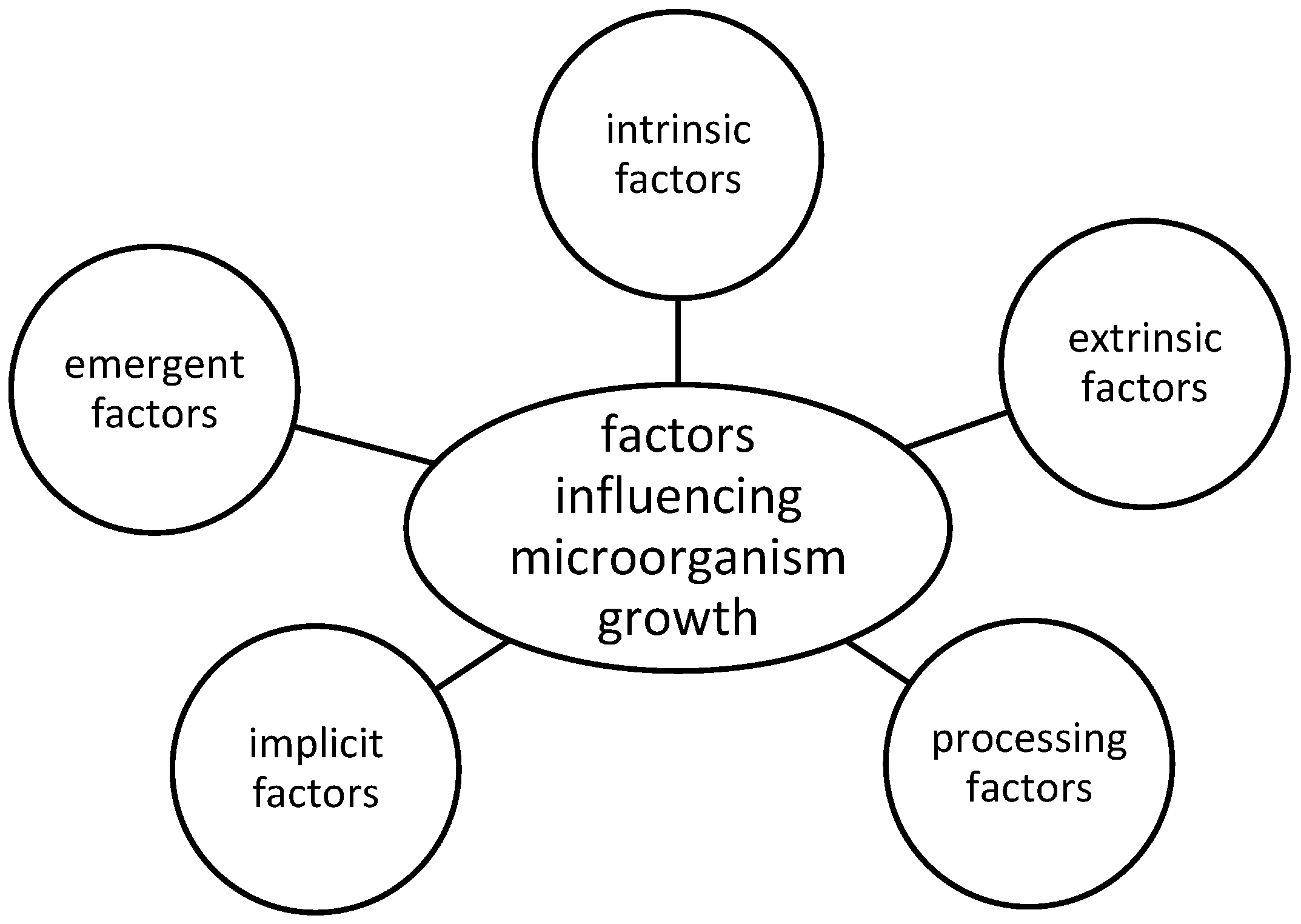
3. Basics of Microbial Spoilage in Meat—Influencing Factors
3.1. Extrinsic Factors
3.2. Processing Factors
3.3. Intrinsic Factors
3.4. Implicit Factors
4. Degradation Products That Can Be Indicated during Meat Spoilage
4.1. Aldehydes
4.1.1. Temperature
4.1.2. Atmosphere
4.1.3. Meat Type
4.2. Alcohols
4.2.1. Temperature
4.2.2. Atmosphere
4.2.3. Meat Type
4.3. Ketones
4.3.1. Temperature
4.3.2. Atmosphere
4.3.3. Meat Type
4.4. Sulfur Compounds
4.4.1. Temperature
4.4.2. Atmosphere
4.4.3. Meat Type
4.5. Esters
4.5.1. Temperature
4.5.2. Atmosphere
4.5.3. Meat Type
4.6. Carboxylic Acids
4.6.1. Temperature
4.6.2. Atmosphere
4.6.3. Meat Type
4.7. Biogenic Amines
4.7.1. Temperature
4.7.2. Atmosphere
4.7.3. Meat Type
4.7.4. Biogenic Amine Index
4.8. Total Volatile Basic Nitrogen
4.8.1. Temperature
4.8.2. Atmosphere
4.8.3. Meat Type
5. Discussion
5.1. Aldehydes
5.2. Alcohols
5.3. Ketones
5.4. Sulfur Compounds
5.5. Esters
5.6. Carboxylic Acids
5.7. Biogenic Amines
5.8. Total Volatile Basic Nitrogen
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis: Advanced Evaluation Methods, Techniques, and Technologies; Mandal, P., Biswas, A., Eds.; Academic Press: London, UK, 2020; pp. 307–334. [Google Scholar]
- Fraqueza, M.J.; Ferreira, M.C.; Barreto, A.S. Spoilage of light (PSE-like) and dark turkey meat under aerobic or modified atmosphere package: Microbial indicators and their relationship with total volatile basic nitrogen. Br. Poult. Sci. 2008, 49, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Williams, M.; Bissett, A.; Ross, T.; Bowman, J.P. Effect of abattoir, livestock species and storage temperature on bacterial community dynamics and sensory properties of vacuum packaged red meat. Food Microbiol. 2021, 94, 103648. [Google Scholar] [CrossRef]
- Rimbach, G.; Nagursky, J.; Erbersdobler, H.F. Erbersdobler, Lebensmittel-Warenkunde für Einsteiger, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Fresán, U.; Mejia, M.A.; Craig, W.J.; Jaceldo-Siegl, K.; Sabaté, J. Meat Analogs from Different Protein Sources: A Comparison of Their Sustainability and Nutritional Content. Sustainability 2019, 11, 3231. [Google Scholar] [CrossRef]
- Mandal, P.; Biswas, A. Meat Quality Analysis: Advanced Evaluation Methods, Techniques, and Technologies; Academic Press: London, UK, 2020; Available online: https://books.google.de/books?hl=de&lr=&id=22CqDwAAQBAJ&oi=fnd&pg=PP1&dq=Meat+quality+analysis+advanced&ots=HF1ynlQPly&sig=jxca6bOHJ9UFNNoIJyBKRkrdIAc#v=onepage&q=Meat%20quality%20analysis%20advanced&f=false (accessed on 6 March 2024).
- Sutherland, J. Modelling food spoilage. In Woodhead Publishing in Food Science and Technology, Food Preservation Techniques; Bøgh-Sørensen, L., Zeuthen, P., Eds.; CRC Press: Boca Raton, FL, USA; Cambridge, UK, 2003; pp. 451–474. [Google Scholar]
- GEMET; Degradation Product. Available online: https://www.eionet.europa.eu/gemet/en/concept/2042 (accessed on 6 February 2023).
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Dave, D.; Ghaley, A.E. Meat Spoilage Mechanisms and Preservation Techniques: A Critical Review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45 Pt A, 83–102. [Google Scholar] [CrossRef]
- Regez, P.; Gallo, L.; Schmitt, R.E.; Schmidt-Lorenz, W. Microbial Spoilage of Refrigerated Fresh Broilers. III. Effect of Storage Temperature on the Microbial Association of Poultry Carcasses. Lebensmittel − Wissenschaft + Technologie = Food Science + Technology. 1988. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19891350480 (accessed on 6 February 2023).
- EC 2073/2005; 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. European Union: Luxembourg, 2005.
- Franke, C. Untersuchung der Dynamik Flüchtiger Organischer Verbindungen von Schutzgas-Verpacktem Fleisch als Grundlage für Intelligente Verpackungen; Universitätsbibliothek der TU München: München, Germany, 2018. [Google Scholar]
- Borch, E.; Kant-Muermans, M.L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef] [PubMed]
- ISO 16779:2015; Sensory Analysis—Assessment (Determination and Verification) of the Shelf Life of Foodstuffs. International Organisation for Standardisation: Berlin, Germany, 2015.
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage. Foods 2018, 7, 132. [Google Scholar] [CrossRef]
- Statista, Wie Häufig Treffen die Folgenden Gründe auf Sie zu, Wenn Sie Lebensmittel Wegwerfen?: (Mindestens Gelegentlich). Available online: https://de.statista.com/statistik/daten/studie/486235/umfrage/umfrage-zu-gruenden-fuer-das-wegwerfen-von-lebensmitteln-in-deutschland/ (accessed on 6 March 2024).
- Schumann, B.; Schmid, M. Packaging concepts for fresh and processed meat—Recent progresses. Innov. Food Sci. Emerg. Technol. 2018, 47, 88–100. [Google Scholar] [CrossRef]
- Boukid, F. Smart Food Packaging: An Umbrella Review of Scientific Publications. Coatings 2022, 12, 1949. [Google Scholar] [CrossRef]
- EFSA. Guidelines on submission of a dossier for safety evaluation by the EFSA of active or intelligent substances present in active and intelligent materials and articles intended to come into contact with food. EFS2 2009, 7, 1208. [Google Scholar] [CrossRef]
- Chen, S.; Brahma, S.; Mackay, J.; Cao, C.; Aliakbarian, B. The role of smart packaging system in food supply chain. J. Food Sci. 2020, 85, 517–525. [Google Scholar] [CrossRef] [PubMed]
- COMMISSION REGULATION (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food: 450/2009. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R0450 (accessed on 6 March 2024).
- Flores, Y.; Pelegrín, C.J.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Use of herbs and their bioactive compounds in active food packaging. In Aromatic Herbs in Food; Academic Press: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Intelligent Packaging: Concepts and Applications. J. Food Sci. 2005, 70, R1–R10. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Fletcher, B.; Mullane, K.; Platts, P.; Todd, E.; Power, A.; Roberts, J.; Chapman, J.; Cozzolino, D.; Chandra, S. Advances in meat spoilage detection: A short focus on rapid methods and technologies. CyTA—J. Food 2018, 16, 1037–1044. [Google Scholar] [CrossRef]
- Cloak, O.M.; Duffy, G.; Sheridan, J.J.; Blair, I.S.; McDowell, D.A. A survey on the incidence of Campylobacter spp. and the development of a surface adhesion polymerase chain reaction (SA-PCR) assay for the detection of Campylobacter jejuni in retail meat products. Food Microbiol. 2001, 18, 287–298. [Google Scholar] [CrossRef]
- Ajaykumar, V.J.; Mandal, P.K. Modern concept and detection of spoilage in meat and meat products. Meat Qual. Anal. 2020, 335–349. [Google Scholar] [CrossRef]
- Weng, X.; Luan, X.; Kong, C.; Chang, Z.; Li, Y.; Zhang, S.; Al-Majeed, S.; Xiao, Y. A Comprehensive Method for Assessing Meat Freshness Using Fusing Electronic Nose, Computer Vision, and Artificial Tactile Technologies. J. Sens. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Miller, K.; Reichert, C.L.; Schmid, M. Biogenic Amine Detection Systems for Intelligent Packaging Concepts: Meat and Meat Products. Food Rev. Int. 2021, 39, 2543–2567. [Google Scholar] [CrossRef]
- Horak, I.; Engelbrecht, G.; van Rensburg, P.J.J.; Claassens, S. Microbial metabolomics: Essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. J. Appl. Microbiol. 2019, 127, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-T. Active and Intelligent Packaging Materials. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Wang, G.-Y.; Wang, H.-H.; Han, Y.-W.; Xing, T.; Ye, K.-P.; Xu, X.-L.; Zhou, G.-H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N. Fresh Meat Spoilage and Modified Atmosphere Packaging (MAP); CRC Press: Boca Raton, FL, USA; Cambridge, UK, 2005. [Google Scholar]
- Guerrero, I.; Chabela, L.P. MEAT AND POULTRY | Spoilage of Cooked Meats and Meat Products. In Encyclopedia of Food Microbiology; Robinson, R.K., Batt, C.A., Eds.; Acadamic Press: San Diego, CA, USA, 2000; pp. 1266–1272. [Google Scholar]
- Stellato, G.; La Storia, A.; de Filippis, F.; Borriello, G.; Villani, F.; Ercolini, D. Overlap of Spoilage-Associated Microbiota between Meat and the Meat Processing Environment in Small-Scale and Large-Scale Retail Distributions. Appl. Environ. Microbiol. 2016, 82, 4045–4054. [Google Scholar] [CrossRef]
- in’t Veld, J.H.H. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Mann, E.; Wetzels, S.U.; Pinior, B.; Metzler-Zebeli, B.U.; Wagner, M.; Schmitz-Esser, S. Psychrophile spoilers dominate the bacterial microbiome in musculature samples of slaughter pigs. Meat Sci. 2016, 117, 36–40. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Geornaras, I.; Sofos, J. Microbiology of Land Muscle Foods. In Handbook of Food Science, Technology, and Engineering, Taylor & Francis; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Wickramasinghe, N.N.; Ravensdale, J.; Coorey, R.; Chandry, S.P.; Dykes, G.A. The Predominance of Psychrotrophic Pseudomonads on Aerobically Stored Chilled Red Meat. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, N.N.; Ravensdale, J.T.; Coorey, R.; Dykes, G.A.; Chandry, P.S. In situ characterisation of biofilms formed by psychrotrophic meat spoilage pseudomonads. Biofouling 2019, 35, 840–855. [Google Scholar] [CrossRef]
- Ercolini, D.; Ferrocino, I.; Nasi, A.; Ndagijimana, M.; Vernocchi, P.; La Storia, A.; Laghi, L.; Mauriello, G.; Guerzoni, M.E.; Villani, F. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions. Appl. Environ. Microbiol. 2011, 77, 7372–7381. [Google Scholar] [CrossRef] [PubMed]
- Balamatsia, C.C.; Paleologos, E.K.; Kontominas, M.G.; Savvaidis, I.N. Correlation between microbial flora, sensory changes and biogenic amines formation in fresh chicken meat stored aerobically or under modified atmosphere packaging at 4 degrees C: Possible role of biogenic amines as spoilage indicators. Antonie Van Leeuwenhoek 2006, 89, 9–17. [Google Scholar] [CrossRef]
- Hilgarth, M.; Lehner, E.M.; Behr, J.; Vogel, R.F. Diversity and anaerobic growth of Pseudomonas spp. isolated from modified atmosphere packaged minced beef. J. Appl. Microbiol. 2019, 127, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Ferranti, P.; Mauriello, G.; Villani, F. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int. J. Food Microbiol. 2010, 142, 120–131. [Google Scholar] [CrossRef]
- Yushina, Y.K.; Bataeva, D.S.; Zaiko, E.V.; Machova, A.A.; Velebit, B. Bacterial populations and volatile organic compounds associated with meat spoilage. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 12114. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Hultman, J.; Parshintsev, J.; Riekkola, M.-L.; Björkroth, J. Development of spoilage bacterial community and volatile compounds in chilled beef under vacuum or high oxygen atmospheres. Int. J. Food Microbiol. 2016, 223, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Blixt, Y.; Borch, E. Comparison of shelf life of vacuum-packed pork and beef. Meat Sci. 2002, 60, 371–378. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Kagkli, D.M.; Nychas, G.-J.E. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiol. 2010, 27, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Wambui, J.; Stephan, R. Relevant Aspects of Clostridium estertheticum as a Specific Spoilage Organism of Vacuum-Packed Meat. Microorganisms 2019, 7, 142. [Google Scholar] [CrossRef]
- Broda, D.M.; Delacy, K.M.; Bell, R.; Braggins, T.J.; Cook, R.L. Psychrotrophic Clostridium spp. associated with ‘blown pack’ spoilage of chilled vacuum-packed red meats and dog rolls in gas-impermeable plastic casings. Int. J. Food Microbiol. 1996, 29, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Boskovic, M.; Dokmanovic, M.; Lazic, I.B.; Ledina, T.; Suvajdzic, B.; Baltic, M.Z. Vacuum and Modified Atmosphere Packaging Effect on Enterobacteriaceae Behaviour in Minced Meat. J. Food Process. Preserv. 2017, 41, e12837. [Google Scholar] [CrossRef]
- Moschonas, G.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. The effect of storage temperature and inoculum level on the time of onset of ‘blown pack’ spoilage. J. Appl. Microbiol. 2010, 108, 532–539. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, D.; Zheng, X.; Wen, X.; Yan, T.; Zhang, Z.; Hou, C. Effects of Different Storage Temperatures on the Physicochemical Properties and Bacterial Community Structure of Fresh Lamb Meat. Food Sci. Anim. Resour. 2021, 41, 509–526. [Google Scholar] [CrossRef]
- Kennedy, J.; Jackson, V.; Blair, I.S.; McDowell, D.A.; Cowan, C.; Bolton, D.J. Food safety knowledge of consumers and the microbiological and temperature status of their refrigerators. J. Food Prot. 2005, 68, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.-J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, S.; Albrecht, A.; Petersen, B.; Kreyenschmidt, J. Influence of cold chain interruptions on the shelf life of fresh pork and poultry. Int. J. Food Sci. Technol. 2012, 47, 1639–1646. [Google Scholar] [CrossRef]
- Sørheim, O.; Nissen, H.; Nesbakken, T. The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide. Meat Sci. 1999, 52, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Holy, A. The influence of extrinsic factors on the microbiological spoilage pattern of ground beef. Int. J. Food Microbiol. 1988, 6, 269–280. [Google Scholar] [CrossRef]
- FSIS, U. FSIS Safety and Security Guidelines for the Transportation and Distribution of Meat, Poultry, and Egg Products. 2002. Available online: https://www.ams.usda.gov/sites/default/files/media/FSIS%20Safety%20and%20Security%20Guidelines%20for%20the%20Transportation%20and%20Distribution%20of%20Meat%2C%20Poultry%2C%20and%20Egg%20Products.pdf (accessed on 6 March 2024).
- 853/200; Regulation (EC) No2004 OF The European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for on the Hygiene of Foodstuffs. Official Journal of the European Union: Luxembourg, 2004.
- 2017/1981; REGULATION C.O.M.S.I.N. 2017/1981 of 31 October 2017 Amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as Regards Temperature Conditions during Transport of Meat. Official Journal of the European Union: Luxembourg, 2017.
- Farber, J.M. Microbiological Aspects of Modified-Atmosphere Packaging Technology—A Review 1. J. Food Prot. 1991, 54, 58–70. [Google Scholar] [CrossRef] [PubMed]
- McMillin, K.W. Advancements in meat packaging. Meat Sci. 2017, 132, 153–162. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of Modified Atmosphere Packaging and Active/Smart Technologies to Red Meat and Poultry: A Review. Food Bioprocess Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- McMillin, K.W. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43–65. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, L.; Sun, Y.; Ren, F.; Chen, S.; Zhang, H.; Guo, H. Changes in the microbiota of lamb packaged in a vacuum and in modified atmospheres during chilled storage analysed by high-throughput sequencing. Meat Sci. 2016, 121, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Luong, N.-D.M.; Coroller, L.; Zagorec, M.; Membré, J.-M.; Guillou, S. Spoilage of Chilled Fresh Meat Products during Storage: A Quantitative Analysis of Literature Data. Microorganisms 2020, 8, 1198. [Google Scholar] [CrossRef]
- Dhananjayan, R.; Han, I.Y.; Acton, J.C.; Dawson, P.L. Growth depth effects of bacteria in ground turkey meat patties subjected to high carbon dioxide or high oxygen atmospheres. Poult. Sci. 2006, 85, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Dousset, X.; Jaffrès, E.; Zagorec, M. Spoilage: Bacterial Spoilage. In Encyclopedia of Food and Health; Caballero, B., Toldrá, F., Finglas, P.M., Eds.; Elsevier Science: Burlington, UK, 2015; pp. 106–112. [Google Scholar]
- Church, N. Developments in modified-atmosphere packaging and related technologies. Trends Food Sci. Technol. 1994, 5, 345–352. [Google Scholar] [CrossRef]
- Pennacchia, C.; Ercolini, D.; Villani, F. Spoilage-related microbiota associated with chilled beef stored in air or vacuum pack. Food Microbiol. 2011, 28, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Mendoza, M.; Maté, J.I. Whey protein isolate edible films with essential oils incorporated to improve the microbial quality of poultry. J. Sci. Food Agric. 2013, 93, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Delgado-Pando, G.; Linton, M.; Patterson, M.F.; Koidis, A. Synergism between high-pressure processing and active packaging against Listeria monocytogenes in ready-to-eat chicken breast. Innov. Food Sci. Emerg. Technol. 2015, 27, 41–47. [Google Scholar] [CrossRef]
- Tornuk, F.; Hancer, M.; Sagdic, O.; Yetim, H. LLDPE based food packaging incorporated with nanoclays grafted with bioactive compounds to extend shelf life of some meat products. LWT—Food Sci. Technol. 2015, 64, 540–546. [Google Scholar] [CrossRef]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essent. Oil Readyto-Eat Meat. Foods 2016, 5, 57. [Google Scholar]
- Tørngren, M.A.; Darré, M.; Gunvig, A.; Bardenshtein, A. Case studies of packaging and processing solutions to improve meat quality and safety. Meat Sci. 2018, 144, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Jessen, B.; Lammert, L. Biofilm and disinfection in meat processing plants. Int. Biodeterior. Biodegrad. 2003, 51, 265–269. [Google Scholar] [CrossRef]
- Arnold, J.W.; Silvers, S. Comparison of poultry processing equipment surfaces for susceptibility to bacterial attachment and biofilm formation. Poult. Sci. 2000, 79, 1215–1221. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.-J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: Causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Fanning, S.; Whyte, P.; Kerry, J.; Bolton, D. Comparison of hot versus cold boning of beef carcasses on bacterial growth and the risk of blown pack spoilage. Meat Sci. 2017, 125, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.; Donnison, A.; Brightwell, G. Factors affecting microbial spoilage and shelf-life of chilled vacuum-packed lamb transported to distant markets: A review. Meat Sci. 2014, 98, 71–80. [Google Scholar] [CrossRef]
- Stefanello, A.; Gasperini, A.M.; Copetti, M.V. Ecophysiology of OTA-producing fungi and its relevance in cured meat products. Curr. Opin. Food Sci. 2022, 45, 100838. [Google Scholar] [CrossRef]
- Preetha, S.S.; Narayanan, R. Factors Influencing the Development of Microbes in Food. Ash 2020, 7, 57–77. [Google Scholar] [CrossRef]
- Chmiel, M.; Roszko, M.; Hać-Szymańczuk, E.; Adamczak, L.; Florowski, T.; Pietrzak, D.; Cegiełka, A.; Bryła, M. Time evolution of microbiological quality and content of volatile compounds in chicken fillets packed using various techniques and stored under different conditions. Poult. Sci. 2020, 99, 1107–1116. [Google Scholar] [CrossRef]
- Sun, X.; Young, J.; Liu, J.-H.; Newman, D. Prediction of pork loin quality using online computer vision system and artificial intelligence model. Meat Sci. 2018, 140, 72–77. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Mallouchos, A.; Panagou, E.Z.; Nychas, G.-J.E. The dynamics of the HS/SPME-GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015, 193, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, O.S.; Iliopoulos, V.; Mallouchos, A.; Panagou, E.Z.; Chorianopoulos, N.; Tassou, C.C.; Nychas, G.-J.E. Spoilage Potential of Pseudomonas (P. fragi, P. putida) and LAB (Leuconostoc mesenteroides, Lactobacillus sakei) Strains and Their Volatilome Profile During Storage of Sterile Pork Meat Using GC/MS and Data Analytics. Foods 2020, 9, 633. [Google Scholar] [CrossRef]
- La Storia, A.; Ferrocino, I.; Torrieri, E.; Di Monaco, R.; Mauriello, G.; Villani, F.; Ercolini, D. A combination of modified atmosphere and antimicrobial packaging to extend the shelf-life of beefsteaks stored at chill temperature. Int. J. Food Microbiol. 2012, 158, 186–194. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 1739. [Google Scholar] [CrossRef]
- Saraiva, C.; Oliveira, I.; Silva, J.A.; Martins, C.; Ventanas, J.; García, C. Implementation of multivariate techniques for the selection of volatile compounds as indicators of sensory quality of raw beef. J. Food Sci. Technol. 2015, 52, 3887–3898. [Google Scholar] [CrossRef]
- Nieminen, T.T.; Dalgaard, P.; Björkroth, J. Volatile organic compounds and Photobacterium phosphoreum associated with spoilage of modified-atmosphere-packaged raw pork. Int. J. Food Microbiol. 2016, 218, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhan, G.; Pan, D.; Zhou, G.; Wang, Y.; He, J.; Cao, J. Charactering the spoilage mechanism of “three sticks” of Jinhua ham. Food Sci. Hum. Wellness 2022, 11, 1322–1330. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Zhou, D.-Y.; Li, A.; Zhao, M.-T.; Hu, Y.-Y.; Li, D.-Y.; Xie, H.-K.; Zhao, Q.; Hu, X.-P.; Zhang, J.-H. Fereidoon Shahidi Effects of temperature and heating time on the formation of aldehydes during the frying process of clam assessed by an HPLC-MS/MS method. Food Chem. 2020, 308, 125650. [Google Scholar] [CrossRef] [PubMed]
- Mayr, D.; Margesin, R.; Klingsbichel, E.; Hartungen, E.; Jenewein, D.; Schinner, F.; Märk, T.D. Rapid detection of meat spoilage by measuring volatile organic compounds by using proton transfer reaction mass spectrometry. Appl. Environ. Microbiol. 2003, 69, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.B.; Brooks, J.C.; Martin, J.N.; Tittor, A.; Miller, M.F.; Brashears, M.M. The impact of packaging system and temperature abuse on the shelf life characteristics of ground beef. Meat Sci. 2014, 97, 1–10. [Google Scholar] [CrossRef]
- Cayuela, J.; Gil, M.; Ban, S.; Garrido, M. Effect of vacuum and modified atmosphere packaging on the quality of pork loin. Eur. Food Res. Technol. 2004, 219, 316–320. [Google Scholar] [CrossRef]
- John, L.; Cornforth, D.; Carpenter, C.E.; Sorheim, O.; Pettee, B.C.; Whittier, D.R. Color and thiobarbituric acid values of cooked top sirloin steaks packaged in modified atmospheres of 80% oxygen, or 0.4% carbon monoxide, or vacuum. Meat Sci. 2005, 69, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, A.-G.; Walgraeve, C.; Vanderroost, M.; van Langenhove, H.; Devlieghere, F.; de Meulenaer, B. Non-Destructive Measurement of Volatile Organic Compounds in Modified Atmosphere Packaged Poultry Using SPME-SIFT-MS in Tandem with Headspace TD-GC-MS. Food Anal. Methods 2018, 11, 848–861. [Google Scholar] [CrossRef]
- Sirocchi, V.; Caprioli, G.; Cecchini, C.; Coman, M.M.; Cresci, A.; Maggi, F.; Papa, F.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Biogenic amines as freshness index of meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis. Int. J. Food Sci. Nutr. 2013, 64, 921–928. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, M.; Li, Z.; Peng, X. Evaluation of Freshness in Determination of Volatile Organic Compounds Released from Pork by HS-SPME-GC-MS. Food Anal. Methods 2018, 11, 1321–1329. [Google Scholar] [CrossRef]
- Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Mauriello, G.; Villani, F.; Ercolini, D. Spoilage-related activity of Carnobacterium maltaromaticum strains in air-stored and vacuum-packed meat. Appl. Environ. Microbiol. 2011, 77, 7382–7393. [Google Scholar] [CrossRef]
- Borch, E.; Agerhem, H. Chemical, microbial and sensory changes during the anaerobic cold storage of beef inoculated with a homofermentative Lactobacillus sp. or a Leuconostoc sp. Int. J. Food Microbiol. 1992, 15, 99–108. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Ukuku, D.O.; Yuk, H.-G. Identification and Quantification of Volatile Chemical Spoilage Indexes Associated with Bacterial Growth Dynamics in Aerobically Stored Chicken. J. Food Sci. 2016, 81, M2006–M2014. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Pearson’s correlation coefficient. BMJ 2012, 345, e4483. [Google Scholar] [CrossRef]
- Stutz, H.; Silverman, G.J.; Angelini, P.; Levin, R.E. Bacteria and Volatile Compounds Associated with Ground Beef Spoilage. J. Food Sci. 1991, 56, 1147–1153. [Google Scholar] [CrossRef]
- Nychas, G.J.; Arkoudelos, J.S. Microbiological and physicochemical changes in minced meats under carbon dioxide, nitrogen or air at 3 °C. Int. J. Food Sci. Technol. 1990, 25, 389–398. [Google Scholar] [CrossRef]
- Mansur, A.R.; Seo, D.-H.; Song, E.-J.; Song, N.-E.; Hwang, S.H.; Yoo, M.; Nam, T.G. Identifying potential spoilage markers in beef stored in chilled air or vacuum packaging by HS-SPME-GC-TOF/MS coupled with multivariate analysis. LWT—Food Sci. Technol. 2019, 112, 108256. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Nurjuliana, M.; Man, Y.B.C.; Hashim, D.M.; Mohamed, A.K.S. Rapid identification of pork for halal authentication using the electronic nose and gas chromatography mass spectrometer with headspace analyzer. Meat Sci. 2011, 88, 638–644. [Google Scholar] [CrossRef]
- Chen, L.; Mardiansyah, S.T.; Kuuliala, L.; Somrani Achouri, M.; Walgraeve, C.; Demeestere, K.; Devlieghere, F. Identification of Volatile Spoilage Indicators for Pork Packaged under Modified Atmospheres. Dublin. 2022. Available online: https://biblio.ugent.be/publication/01GJ59ACV4XBS7A3MACD9QS05M (accessed on 6 March 2024).
- Mansur, A.R.; Song, E.-J.; Cho, Y.-S.; Nam, Y.-D.; Choi, Y.-S.; Kim, D.-O.; Seo, D.-H.; Nam, T.G. Comparative evaluation of spoilage-related bacterial diversity and metabolite profiles in chilled beef stored under air and vacuum packaging. Food Microbiol. 2019, 77, 166–172. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Apetrei, C.; Lozano, J.; Anyogu, A. Potential use of electronic noses, electronic tongues and biosensors as multisensor systems for spoilage examination in foods. Trends Food Sci. Technol. 2018, 80, 71–92. [Google Scholar] [CrossRef]
- Zareian, M.; Böhner, N.; Loos, H.M.; Silcock, P.; Bremer, P.; Beauchamp, J. Evaluation of volatile organic compound release in modified atmosphere-packaged minced raw pork in relation to shelf-life. Food Packag. Shelf Life 2018, 18, 51–61. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Yuk, H.-G. Detection of volatile organic compounds as markers of chicken breast spoilage using HS-SPME-GC/MS-FASST. Food Sci. Biotechnol. 2015, 24, 361–372. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.J.; Gorraiz, C.; Purroy, A. Volatile Compounds of Raw Beef from 5 Local Spanish Cattle Breeds Stored Under Modified Atmosphere. J Food Sci. 2002, 67, 1580–1589. [Google Scholar] [CrossRef]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Eilamo, M.; Kinnunen, A.; Latva-Kala, K.; Ahvenainen, R. Effects of packaging and storage conditions on volatile compounds in gas-packed poultry meat. Food Addit. Contam. 1998, 15, 217–228. [Google Scholar] [CrossRef]
- Comi, G. Spoilage of Meat and Fish; University of Udine: Udine, Italy, 2017. [Google Scholar]
- Dainty, R.H. Chemical/biochemical detection of spoilage. Int. J. Food Microbiol. 1996, 33, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Koskela, J.; Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Pulkkinen, P.; Tenhu, H.; Nieminen, T.; Kilpelä, A.; Peltonen, J. Monitoring the quality of raw poultry by detecting hydrogen sulfide with printed sensors. Sens. Actuators B Chem. 2015, 218, 89–96. [Google Scholar] [CrossRef]
- Toldra, F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998, 49, S101–S110. [Google Scholar] [CrossRef]
- Martín, A.; Benito, M.J.; Aranda, E.; Ruiz-Moyano, S.; Córdoba, J.J.; Córdoba, M.G. Characterization by volatile compounds of microbial deep spoilage in Iberian dry-cured ham. J. Food Sci. 2010, 75, M360–M365. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, A.; Nychas, G.-J.E. Storage of poultry meat under modified atmospheres or vacuum packs: Possible role of microbial metabolites as indicator of spoilage. J. Appl. Bacteriol. 1994, 76, 163–172. [Google Scholar] [CrossRef]
- Rosa, M.D. Packaging Sustainability in the Meat Industry. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: London, UK, 2019; pp. 161–179. [Google Scholar]
- Hernandez, M.S.; Woerner, D.R.; Brooks, J.C.; Wheeler, T.L.; Legako, J.F. Influence of Aging Temperature and Duration on Spoilage Organism Growth, Proteolytic Activity, and Related Chemical Changes in Vacuum-Packaged Beef Longissimus. Meat and Muscle Biology 2022, 6. [Google Scholar] [CrossRef]
- Ayseli, M.T.; Filik, G.; Selli, S. Evaluation of volatile compounds in chicken breast meat using simultaneous distillation and extraction with odour activity value. J. Food Nutr. Res. 2014, 53, 137–142. Available online: http://openaccess.ahievran.edu.tr/xmlui/bitstream/handle/20.500.12513/4103/filikg%c3%b6khan-%202.pdf?sequence=1&isAllowed=y (accessed on 6 February 2023).
- Cortez-Vega, W.R.; Pizato, S.; Prentice, C. Quality of raw chicken breast stored at 5 °C and packaged under different modified atmospheres. J Food Saf. 2012, 32, 360–368. [Google Scholar] [CrossRef]
- Nam, K.; Ahn, D. Combination of aerobic and vacuum packaging to control lipid oxidation and off-odor volatiles of irradiated raw turkey breast. Meat Sci. 2003, 63, 389–395. [Google Scholar] [CrossRef]
- Li, M.; Tian, L.; Zhao, G.; Zhang, Q.; Gao, X.; Huang, X.; Sun, L. Formation of biogenic amines and growth of spoilage-related microorganisms in pork stored under different packaging conditions applying PCA. Meat Sci. 2014, 96 Pt A, 843–848. [Google Scholar] [CrossRef]
- Sawaya, W.N.; Elnawawy, A.S.; Al-Zenki, S.; Al-Otaibi, J.; Al-Omirah, H.; Al-Amiri, H. Storage Stability of Chicken as Affected by Map and Lactic Acid Treatment. J Food Sci. 1995, 60, 611–614. [Google Scholar] [CrossRef]
- Nychas, G.J.; Dillon, V.M.; Board, R.G. Glucose, the Key Substrate in the Microbiological Changes Occurring in Meat and Certain Meat Products. Biotechnol. Appl. Biochem. 1988, 10, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Rokka, M.; Eerola, S.; Smolander, M.; Alakomi, H.-L.; Ahvenainen, R. Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions. Food Control 2004, 15, 601–607. [Google Scholar] [CrossRef]
- Hutarova, Z.; Vecerek, V.; Steinhauserova, I.; Marsalek, P.; Borilova, G.; Forejtek, P. The effect of treating method of pithed pheasant on the content of biogenic amines in the meat during the course of storage. Poult. Sci. 2013, 92, 2182–2187. [Google Scholar] [CrossRef]
- Jairath, G.; Singh, P.K.; Dabur, R.S.; Rani, M.; Chaudhari, M. Biogenic amines in meat and meat products and its public health significance: A review. J. Food Sci. Technol. 2015, 52, 6835–6846. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Junior, C.A.C.; Canto, A.C.; Monteiro, M.L.G.; Franco, R.M. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT—Food Sci. Technol. 2015, 60, 15–21. [Google Scholar] [CrossRef]
- Silva, C.M.; Glória, M.A. Bioactive amines in chicken breast and thigh after slaughter and during storage at 4±1 °C and in chicken-based meat products. Food Chem. 2002, 78, 241–248. [Google Scholar] [CrossRef]
- Schirone, M.; Esposito, L.; D’onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Moral, A. Free amino acids and biogenic amines in red and white muscle of tuna stored in controlled atmospheres. Amino Acids 2004, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food--existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef]
- Ahmad, W.; Mohammed, G.I.; Al-Eryani, D.A.; Saigl, Z.M.; Alyoubi, A.O.; Alwael, H.; Bashammakh, A.S.; O’sullivan, C.K.; El-Shahawi, M.S. Biogenic Amines Formation Mechanism and Determination Strategies: Future Challenges and Limitations. Crit. Rev. Anal. Chem. 2019, 50, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Amino acid availability as an influential factor on the biogenic amine formation in dry fermented sausages. Food Control 2014, 36, 76–81. [Google Scholar] [CrossRef]
- Krizek, A.R.; Smith, J.S.; Phebus, R.K. Biogenic Amine Formation in Fresh Vacuum-Packaged Beef Stored at −2 °C and 2 °C for 100 Days. J. Food Prot. 1995, 58, 284–288. [Google Scholar] [CrossRef]
- Geornaras, I.; Dykes, G.A.; von Holy, A. Biogenic amine formation by poultry-associated spoilage and pathogenic bacteria. Lett. Appl. Microbiol. 1995, 21, 164–166. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Bonadio, M.; Lorusso, V.; Romano, P. Role of biogenic amines as index of freshness in beef meat packed with different biopolymeric materials. Food Res. Int. 2009, 42, 1147–1152. [Google Scholar] [CrossRef]
- Moreira, A.P.S.; Giombelli, A.; Labanca, R.A.; Nelson, D.L.; Glória, M.B.A. Effect of aging on bioactive amines, microbial flora, physico-chemical characteristics, and tenderness of broiler breast meat. Poult. Sci. 2008, 87, 1868–1873. [Google Scholar] [CrossRef]
- Rosinská, D.; Lehotay, J. INFLUENCE OF TEMPERATURE ON PRODUCTION OF BIOGENIC AMINES IN PORK, BEEF, AND POULTRY AND THEIR HPLC DETERMINATION AFTER POSTCOLUMN DERIVATIZATION. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 609–619. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Durlu-Özkaya, F.; Ayhan, K.; Vural, N. Biogenic amines produced by Enterobacteriaceae isolated from meat products. Meat Sci. 2001, 58, 163–166. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Holzapfel, W.H. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Min, J.S.; Lee, S.O.; Jang, A.; Jo, C.; Lee, M. Control of microorganisms and reduction of biogenic amines in chicken breast and thigh by irradiation and organic acids. Poult. Sci. 2007, 86, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Roszko, M.; Hać-Szymańczuk, E.; Cegiełka, A.; Adamczak, L.; Florowski, T.; Pietrzak, D.; Bryła, M.; Świder, O. Changes in the microbiological quality and content of biogenic amines in chicken fillets packed using various techniques and stored under different conditions. Food Microbiol. 2022, 102, 103920. [Google Scholar] [CrossRef]
- Vinci, G.; Antonelli, M. Biogenic amines: Quality index of freshness in red and white meat. Food Control 2002, 13, 519–524. [Google Scholar] [CrossRef]
- Baston, O.; Barna, O.; Vasile, A. Microbiota and biogenic amines variation of chicken meat. Comparison between white and red meat. Ann. Food Sci. Technol. 2010, 2010, 69–73. [Google Scholar]
- Alessandroni, L.; Caprioli, G.; Faiella, F.; Fiorini, D.; Galli, R.; Huang, X.; Marinelli, G.; Nzekoue, F.; Ricciutelli, M.; Scortichini, S.; et al. A shelf-life study for the evaluation of a new biopackaging to preserve the quality of organic chicken meat. Food Chem. 2022, 371, 131134. [Google Scholar] [CrossRef] [PubMed]
- Dudnyk, I.; Janeček, E.-R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Cheng, W.; Sun, D.-W.; Cheng, J.-H. Pork biogenic amine index (BAI) determination based on chemometric analysis of hyperspectral imaging data. LWT—Food Sci. Technol. 2016, 73, 13–19. [Google Scholar] [CrossRef]
- Hernández-Jover, T.; Izquierdo-Pulido, M.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biogenic Amine Sources in Cooked Cured Shoulder Pork. J. Agric. Food Chem. 1996, 44, 3097–3101. [Google Scholar] [CrossRef]
- Mayr, D.; Margesin, R.; Schinner, F.; Märk, T. Detection of the spoiling of meat using PTR–MS. Int. J. Mass Spectrom. 2003, 223–224, 229–235. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Fu, L. Food Spoilage Microorganisms: Ecology and Control; CRC Press Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017. [Google Scholar]
- Randell, K.; Ahvenainen, R.; Latva-Kala, K.; Hurme, E.; Mattila-Sandholm, T.; Hyvönen, L. Modified Atmosphere-packed Marinated Chicken Breast and Rainbow Trout Quality as Affected by Package Leakage. J. Food Sci. 1995, 60, 667–672. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Blacha, I.; Krischek, C.; Klein, G. Influence of modified atmosphere packaging on meat quality parameters of turkey breast muscles. J. Food Prot. 2014, 77, 127–132. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M. Fabrication and characterization of alizarin colorimetric indicator based on cellulose-chitosan to monitor the freshness of minced beef. Sens. Actuators B Chem. 2019, 285, 519–528. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Alfaia, C.M.; Barreto, A.S. Biogenic amine formation in turkey meat under modified atmosphere packaging with extended shelf life: Index of freshness. Poult. Sci. 2012, 91, 1465–1472. [Google Scholar] [CrossRef]
- Qiao, L.; Tang, X.; Dong, J. A feasibility quantification study of total volatile basic nitrogen (TVB-N) content in duck meat for freshness evaluation. Food Chem. 2017, 237, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Holman, B.W.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Balamatsia, C.C.; Patsias, A.; Kontominas, M.G.; Savvaidis, I.N. Possible role of volatile amines as quality-indicating metabolites in modified atmosphere-packaged chicken fillets: Correlation with microbiological and sensory attributes. Food Chem. 2007, 104, 1622–1628. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Brink, B.T.; Damink, C.; Joosten, H.; Huis in ’t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; Dolatowski, Z.J. Biogenic amines in meat and fermented meat products. Acta Sci.Pol. Technol. Aliment. 2010, 9, 251–263. [Google Scholar]
- Magnaghi, L.R.; Alberti, G.; Capone, F.; Zanoni, C.; Mannucci, B.; Quadrelli, P.; Biesuz, R. Development of a Dye-Based Device to Assess the Poultry Meat Spoilage. Part II Array Act. J. Agric. Food Chem. 2020, 68, 12710–12718. [Google Scholar] [CrossRef]
- Hernández-Jover, T.; Izquierdo-Pulido, M.; Veciana-Nogués, M.T.; Mariné-Font, A.; Vidal-Carou, M.C. Effect of Starter Cultures on Biogenic Amine Formation during Fermented Sausage Production. J. Food Prot. 1997, 60, 825–830. [Google Scholar] [CrossRef]
| Preferred Growth Atmospheres | ||
|---|---|---|
| Aerobic Microorganisms | Anerobic Microorganisms | Facultative Anaerobic Microorganisms |
| Pseudomonas spp. [42,43,44,45,46,47,48] Acinetobacter spp. [42] Psychrobacter spp. [42] Moraxella spp. [42] Molds [36] | Lactic acid bacteria (e.g., Lactobacillus) [1,49,50,51] Clostridium spp. [53,54] b Brochothrix spp. [42,52] a/b | Enterobacteriaceae [42,55] a Yeasts [55] |
| Calculation Models for the BAI | BAI Value above Which Meat Is Considered Spoiled | Meat Type | Atmosphere | References |
|---|---|---|---|---|
| Putrescine + cadaverine + tyramine + histamine | >50 mg/kg | Pork | No specification | [138,154] |
| Chicken | Air, MAP (75% O2, 25% CO2), vacuum | [150] | ||
| Different meat types | No specification | [142] | ||
| (histamine + putrescin + cadaverine): (1 + spermine + spermidine) | >10 mg/kg (considered as low quality) | Meat in general | No specification | [155] |
| Putrescine + cadaverine + tyramine | No specification | Turkey | MAP (different compositions) | [143] |
| Chicken | Air, MAP (70% N2, 30% CO2) | [43] |
| Degradation Product | Related Microorganisms | Conditions in Which the Degradation Products Are (Frequently) Measured | ||
|---|---|---|---|---|
| Temperature | Atmosphere | Meat Type | ||
| Aldehydes | Pseudomonas spp. Carnobacterium spp. Enterobacteriaceae [11,90] | Increases with increasing temperature [15,94,101] | MAP (30–70% O2, 20–40% CO2, 10–30% N2) [98,106] Vacuum [168] Aerobic [103,168] | Beef [102] Pork [102] Poultry [102,107,169] |
| Alcohols | Pseudomonas spp. Carnobacterium spp. [1] | Various statements: increases with rising temperature [94] or indication at 4 °C, but not at higher temperatures (10 °C) [14] | Mainly aerobic [99,114] MAP with high amount of O2 (70% O2, 20% CO2, 10% N2) [98] Vacuum [168] | Beef [102] Chicken [111,170] |
| Ketones | Brochothrix thermosphacta Carnobacterium spp. Lactobacillus spp. [106] Photobacterium spp. [99] | Increases with increasing temperature [14] | MAP (70% O2, 20% CO2, 10% N2) [98] Vacuum [109] Aerobic [94] | Beef [49] Pork [91] Poultry [36,106], |
| Sulfur Compounds | Enterobacteriaceae Pseudomonas [127] | Increases with increasing temperature [126] Indication at 4 °C [119] Indication at room temperature [123] | MAP (30–60% O2, 40% CO2, 30% N2) [45,106] Aerobic [45,123] | Beef [106,113,123] Pork [119] Poultry [106,113,123] |
| Esters | Pseudomonas fragi [11,96] Pseudomonas spp. [96] | Mostly formed between—1.5 °C and 7 °C | MAP (60–80% O2, 20–40% CO2) [96,99] Aerobic [11,43,111] | Pork [95,99] Chicken [111] |
| Carboxylic acids | Brochothrix thermosphacta Carnobacterium spp. [131] | Indicated between—3.5 °C [98] and 21 °C [123] | MAP (80% O2, 20% CO2) [90] Aerobic [115,136] Vacuum [115] Increases with CO2 increase [136] | Beef [45,50,115,123,135] Chicken [90,115,123,135] |
| Biogenic amines | Enterobacteriaceae [138,145,154] Pseudomonas spp. [138,153] Lactic acid bacteria [127] | Increases with increasing temperature [142] Indicated below 6 °C [142,143] to—18 °C [155] | MAP (0–75% O2, 25–30% CO2, 0–70% N2) [46,145,146] Aerobic [46,146,161] Vacuum [161] | Beef [154,163] Pork [17] Poultry [17,145,146,155,163,164] Fish [138] Lamb [17] |
| Total volatile basic nitrogen | Pseudomonas spp. [120] | Indicated between—18 °C [91] and 28 °C [171] Increases faster with increasing temperatures [91] | MAP (0–50% O2, 0.03–100% CO2, 0–78% N2) [2,136,172] Aerobic [120] Vacuum [120,172] | Beef [136,173] Pork [91,136] Poultry [146,174,175] Seafood [176,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhlig, E.; Bucher, M.; Strenger, M.; Kloß, S.; Schmid, M. Towards Reducing Food Wastage: Analysis of Degradation Products Formed during Meat Spoilage under Different Conditions. Foods 2024, 13, 2751. https://doi.org/10.3390/foods13172751
Uhlig E, Bucher M, Strenger M, Kloß S, Schmid M. Towards Reducing Food Wastage: Analysis of Degradation Products Formed during Meat Spoilage under Different Conditions. Foods. 2024; 13(17):2751. https://doi.org/10.3390/foods13172751
Chicago/Turabian StyleUhlig, Elisa, Matthias Bucher, Mara Strenger, Svenja Kloß, and Markus Schmid. 2024. "Towards Reducing Food Wastage: Analysis of Degradation Products Formed during Meat Spoilage under Different Conditions" Foods 13, no. 17: 2751. https://doi.org/10.3390/foods13172751
APA StyleUhlig, E., Bucher, M., Strenger, M., Kloß, S., & Schmid, M. (2024). Towards Reducing Food Wastage: Analysis of Degradation Products Formed during Meat Spoilage under Different Conditions. Foods, 13(17), 2751. https://doi.org/10.3390/foods13172751







