Optimizing Encapsulation of Active Compounds of Carrot By-Product in TPP-Chitosomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Quantification of Phytochemical Compounds
2.3. Optimization of the Encapsulation of Phytochemical Compounds from Carrot Pomace in TPP-Chitosomes
2.4. Characterization of TPP-Chitosome Nanoparticles Encapsulating Phytochemical Compounds from Carrot Pomace
2.4.1. Particle Morphology, Size, and Zeta Potential
2.4.2. Encapsulation Efficiency of Phenolics and β- and α-Carotene
2.5. Statistical Analysis to Optimize Encapsulation of Phytochemical Compounds from Carrot Pomace
3. Results and Discussion
3.1. Active Contents of Extracts, the Visual Aspect of TPP-Chitosomes Containing Encapsulated Phenolics and Carotenoids
3.2. Encapsulation Efficiency of Phenolics
3.3. Encapsulation Efficiency of α- and β-Carotene
3.4. Zeta Potential of TPP-Chitosomes Containing Encapsulated Phenolics and Carotenoids
3.5. Particle Morphology and Size Distribution
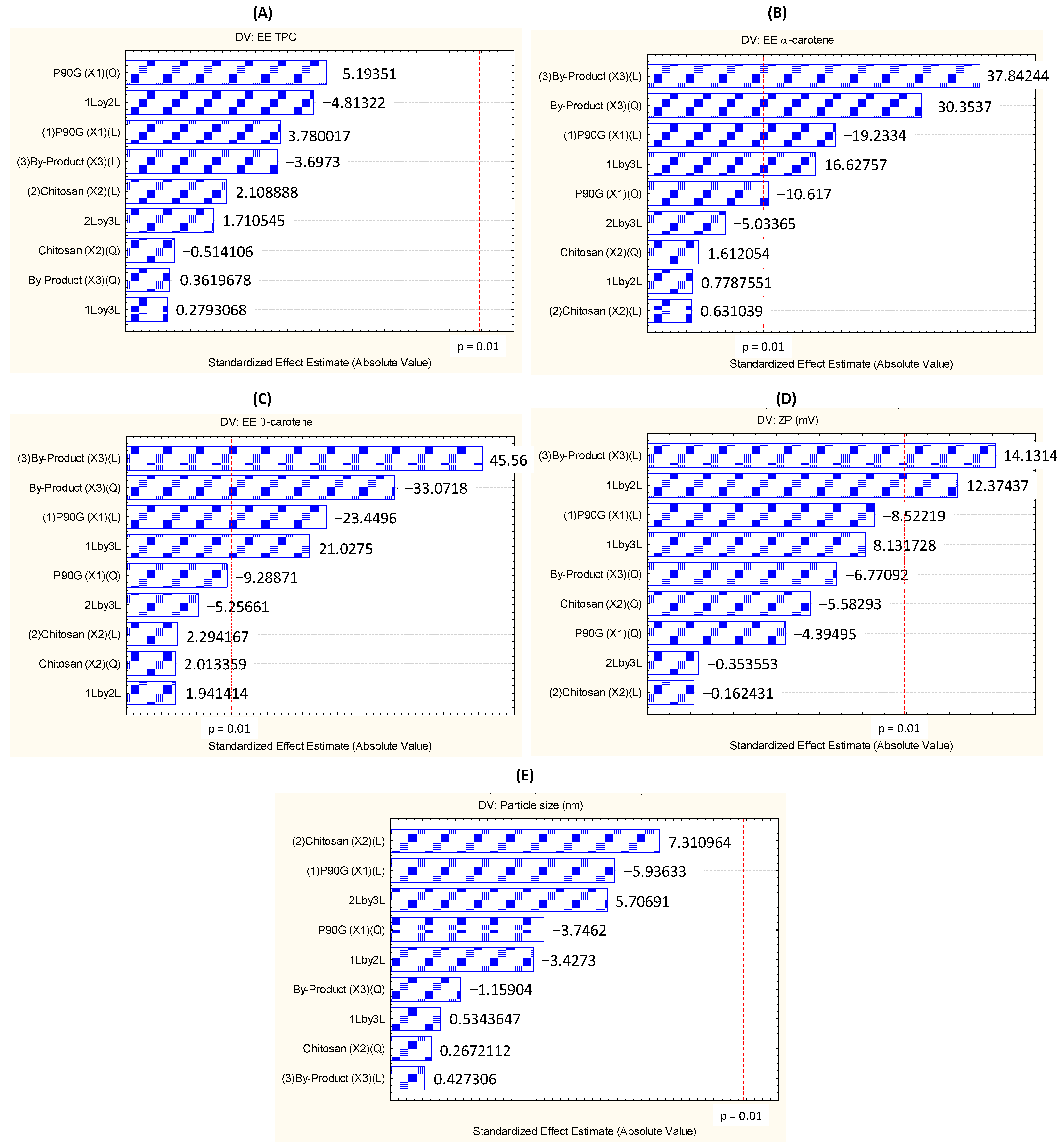
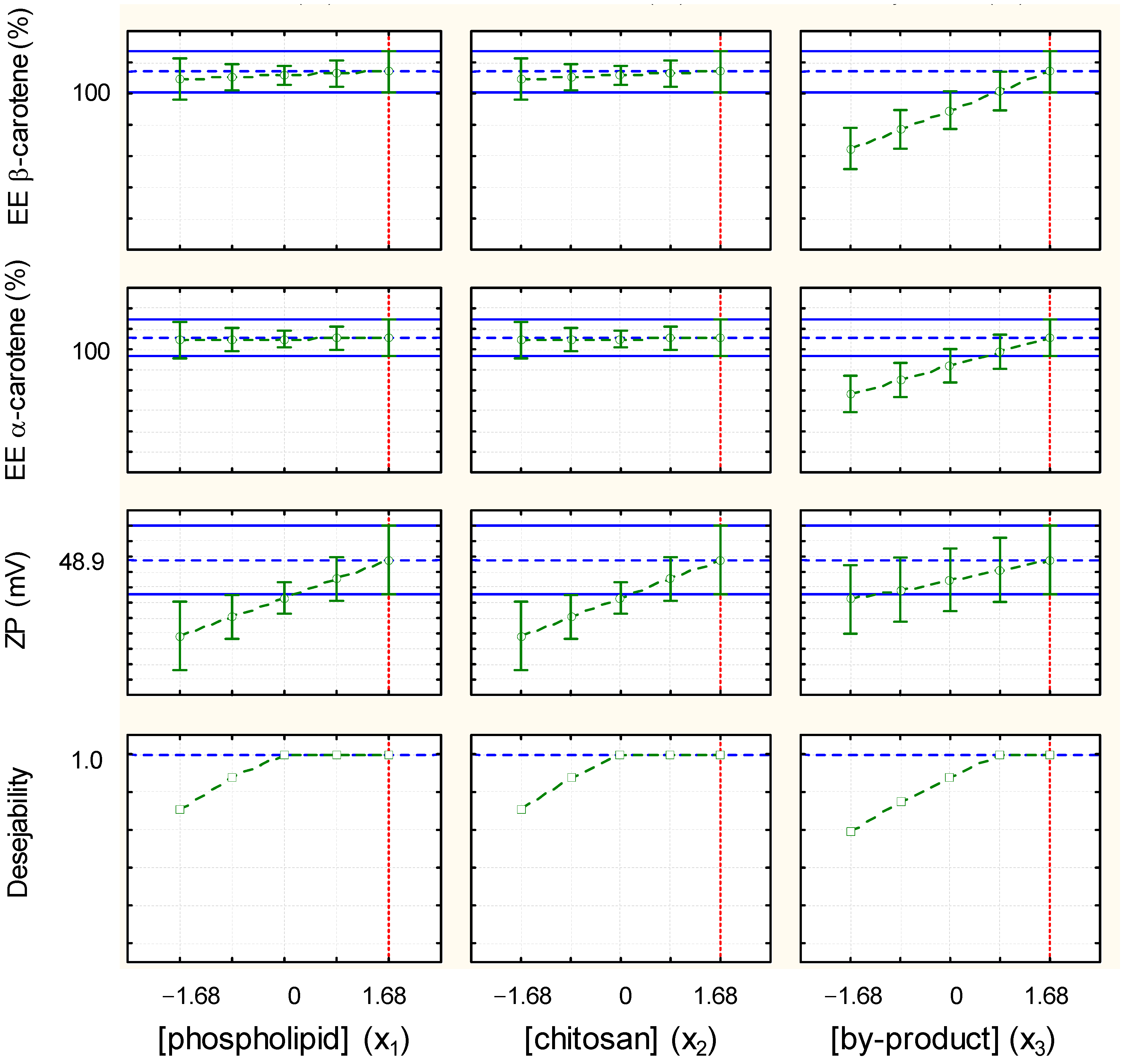
3.6. Optimization of the Encapsulation Efficiency of α- and β-Carotene and Zeta Potential of TPP-Chitosomes Containing Encapsulated Phenolics and Carotenoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). Food Waste Index: Report 2021. 2021. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/35280/FoodWaste.pdf (accessed on 3 February 2024).
- Jaouhari, Y.; Travaglia, F.; Giovannelli, L.; Picco, A.; Oz, E.; Oz, F.; Bordiga, M. From Industrial Food Waste to Bioactive Ingredients: A Review on the Sustainable Management and Transformation of Plant-Derived Food Waste. Foods 2023, 12, 2183. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.L.; Zhang, M.; Wang, Y.; Adhikari, B. Novel technologies applied for recovery and value addition of high-value compounds from plant byproducts: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Nath, A.; Vatai, G.; Bánvölgyi, S. Functional Foods and Bioactive Compounds through Environmentally Benign Emerging Processes. Processes 2023, 11, 1182. [Google Scholar] [CrossRef]
- Embrapa. Cenoura (Daucus carota). 2008. 16p. Available online: https://www.embrapa.br/en/agencia-de-informacao-tecnologica/cultivos/cenoura (accessed on 10 February 2024).
- Kołodziejczyk, M.; Dobrzyński, J.; Ostrowska-Ligęza, E. Carrot (Daucus carota L.) and carrot products as a source of valuable bioactive compounds. Pol. J. Food Nutr. Sci. 2019, 69, 319–327. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 February 2024).
- Teixeira, L.J.Q. Campos Eletricos Pulsados de Alta Intensidade no Processamento de Suco de Cenoura. Ph.D. Thesis, Universidade Federal de Vicosa, Vicosa, Brazil, 2008. [Google Scholar]
- Papoutsis, K.; Edelenbos, M. Postharvest environmentally and human-friendly pre-treatments to minimize carrot waste in the supply chain caused by physiological disorders and fungi. Trends Food Sci. Technol. 2021, 112, 88–98. [Google Scholar] [CrossRef]
- Amin, S.; Jung, S.; Kang, I.; Duval, A. Valorization of Baby Carrot Processing Waste. J. Culin. Sci. Technol. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Šeregelj, V.; Vulić, J.; Ćetković, G.; Čanadanovć-Brunet, J.; Šaponjac, V.T.; Stajčić, S. Natural bioactive compounds in carrot waste for food applications and health benefits. Stud. Nat. Prod. Chem. 2020, 67, 307–344. [Google Scholar] [CrossRef]
- Gordon, H.T.; Bauernfeind, J.C. Carotenoids as food colorants. Crit. Rev. Food Sci. Nutr. 1982, 18, 59–97. [Google Scholar] [CrossRef]
- Ataei, M.; Maghsoudi, A.S.; Hassani, S. Dyes and colorants. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 997–1012. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Lehtonen, M.; Merinen, M.; Kilpeläinen, P.O.; Xu, C.; Willför, S.M.; Mikkonen, K.S. Phenolic residues in spruce galactoglucomannans improve stabilization of oil-in-water emulsions. J. Colloid Interface Sci. 2018, 512, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.A. Parâmetros Físico-Químicos e Sensoriais de Diferentes Variedades de Cenouras (Daucus carota L.) em Cultivo Orgânico. Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2002; p. 90. [Google Scholar]
- García-Casal, M.N.; Layrisse, M.; Solano, L.; Barón, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Vitamin A and betacarotene can improve nonheme iron absorption from rice, wheat and corn by humans. J. Nutr. 1998, 128, 646–650. [Google Scholar] [CrossRef]
- Suhr, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, Y.; Huang, Q. Edible nanoencapsulation vehicles for oral delivery of phytochemicals: A perspective paper. J. Agric. Food Chem. 2017, 65, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and light conditions affect stability of phenolic compounds of stored grape cane extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef]
- Tobal, T.M.; Rodrigues, L.V. Effect of storage on the bioactive compounds, nutritional composition and sensory acceptability of pitanga jams. Food Sci. Technol. 2019, 39, 581–587. [Google Scholar] [CrossRef]
- Lee, S.J.; Wong, M. Nano-and microencapsulation of phytochemicals. In Nano-and Microencapsulation for Foods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 117–165. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, A.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Food Funct. 2021, 12, 31–48. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Goula, A.M. Encapsulation of bioactive compounds through electrospinning/electrospraying and its effect on food preservation. Food Eng. Rev. 2013, 5, 238–250. [Google Scholar]
- Iwamoto, K.; Sunamoto, J. Liposomal membranes. Adsorption of polysaccharides on liposomal membranes as monitored by fluorescence depolarization. J. Biochem. 1982, 91, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Carbone, C.; Ennas, G.; Puglisi, G.; Fadda, A.M.; Manconi, M. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid Interface Sci. 2016, 461, 69–78. [Google Scholar] [CrossRef]
- Iida, A.S.L.; Luz, K.N.; Barros-Alexandrino, T.T.; F’avaro-Trindade, C.S.; de Pinho, S.C.; Assis, O.B.G.; Martelli-Tosi, M. Investigation of TPP-Chitosomes particles structure and stability as encapsulating agent of cholecalciferol. Polímeros 2019, 29, e2019049. [Google Scholar] [CrossRef]
- Esposto, B.S.; Pinho, S.G.B.; Thomazini, M.; Ramos, A.P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. TPP-chitosomes as potential encapsulation system to protect carotenoid-rich extract obtained from carrot by-product: A comparison with liposomes and chitosomes. Food Chem. 2022, 397, 133857. [Google Scholar] [CrossRef] [PubMed]
- Esposto, B.S.; Tessaro, L.; Aguilar, G.J.; Sobral, P.J.d.A.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Chitosan films activated with phytochemicals extracted from carrot by-product encapsulated in TPP chitosomes. Int. J. Food Sci. Technol. 2024, 59, 1384–1393. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sarkar, B.C.; Sharma, H.K.; Jha, S.K. Colour kinetics and storage characteristics of carrot, pulse, and rice by-product based extrudates. Br. Food J. 2012, 114, 1279–1296. [Google Scholar] [CrossRef]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT Food Sci. Technol. 2017, 85, 37–44. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Bashir Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.u.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Taher, B.I.; Fickers, P.; Chniti, S.; Hassouna, M. Optimization of enzymatic hydrolysis and fermentation conditions for improved bioethanol production from potato peel residues. Biotechnol. Prog. 2017, 33, 397–406. [Google Scholar] [CrossRef]
- Carabajal, M.; Teglia, C.M.; Cerutti, S.; Culzoni, M.J.; Goicoechea, H.C. Applications of liquid-phase microextraction procedures to complex samples assisted by response surface methodology for optimization. Microchem. J. 2019, 152, 104436. [Google Scholar] [CrossRef]
- Bernardi, A.V.; Gerolamo, L.E.; Dinamarco, T.M.; Tapia-Blácido, D.R. Optimization of biomass saccharification processes with experimental design tools for 2G ethanol production: A review. Biofuels Bioprod. Biorefining 2023, 17, 1789–1806. [Google Scholar] [CrossRef]
- Machado, A.R.; Pinheiro, A.C.; Vicente, A.A.; Souza-Soares, L.A.; Cerqueira, M.A. Liposomes loaded with phenolic extracts of Spirulina LEB-18: Physicochemical characterization and behavior under simulated gastrointestinal conditions. Food Res. Int. 2019, 120, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.L.; Qian, J.Q.; Zhao, C.Y.; Yang, H.Y.; Zhao, X.H.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Planejamento de experimentos e otimização de processos. Casa do Espírito Amigo Fraternidade Fé e Amor. Campinas. SP. 2014. Available online: https://www.lojacarita.com.br/loja/product/planejamento-de-experimentos-e-otimizacao-de-processos/ (accessed on 15 February 2024).
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Joy, J.M.; Amruth, P.; Rosemol, J.M.; Pavan Kumar, D.; Renuka, R.V. Anandan. Liposome mediated encapsulation and role of chitosan on modulating liposomal stability to deliver potential bioactives—A review. Food Hydrocoll. Health 2023, 4, 100142. [Google Scholar] [CrossRef]
- Hassani, S.; Laouini, A.; Fessi, H.; Charcosset, C. Preparation of chitosan–TPP nanoparticles using microengineered membranes—Effect of parameters and encapsulation of tacrine. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 34–43. [Google Scholar] [CrossRef]



| Sample | P90G, mg/mL (X1) * | Chitosan, mg/mL (X2) * | By-Product, g/100 mL of Ethanol (X3) * |
|---|---|---|---|
| 1 | 1.5 (−1) | 4.0 (−1) | 6.0 (−1) |
| 2 | 3.5 (+1) | 4.0 (−1) | 6.0 (−1) |
| 3 | 1.5 (−1) | 8.0 (+1) | 6.0 (−1) |
| 4 | 3.5 (+1) | 8.0 (+1) | 6.0 (−1) |
| 5 | 1.5 (−1) | 4.0 (−1) | 12 (+1) |
| 6 | 3.5 (+1) | 4.0 (−1) | 12 (+1) |
| 7 | 1.5 (−1) | 8.0 (+1) | 12 (+1) |
| 8 | 3.5 (+1) | 8.0 (+1) | 12 (+1) |
| 9 | 0.8 (−1.68) | 6.0 (0) | 9.0 (0) |
| 10 | 4.2 (+1.68) | 6.0 (0) | 9.0 (0) |
| 11 | 2.5 (0) | 2.6 (−1.68) | 9.0 (0) |
| 12 | 2.5 (0) | 9.4 (+1.68) | 9.0 (0) |
| 13 | 2.5 (0) | 6.0 (0) | 4.0 (−1.68) |
| 14 | 2.5 (0) | 6.0 (0) | 14.0 (+1.68) |
| 15 | 2.5 (0) | 6.0 (0) | 9.0 (0) |
| 16 | 2.5 (0) | 6.0 (0) | 9.0 (0) |
| 17 | 2.5 (0) | 6.0 (0) | 9.0 (0) |
| By-Product, g/100 mL of Ethanol (x3) | TPC μg GAE/g | TPC μg GAE/mL | β-Carotene μg/g | β-Carotene μg/mL | α-Carotene μg/g | α-Carotene μg/mL |
|---|---|---|---|---|---|---|
| 4 | 168 ± 1 | 6.7 ± 0.1 | 129 ± 5 | 5.2 ± 0.1 | 51 ± 1 | 2.1 ± 0.1 |
| 6 | 200 ± 3 | 12.1 ± 0.2 | 116 ± 11 | 7.1 ± 0.1 | 47 ± 3 | 2.9 ± 0.1 |
| 9 | 164 ± 20 | 14.9 ± 1.8 | 96 ± 8 | 8.9 ± 0.7 | 38 ± 2 | 3.5 ± 0.1 |
| 12 | 175 ± 20 | 21.1 ± 2.3 | 57 ± 4 | 7.0 ± 0.3 | 23 ± 1 | 2.8 ± 0.1 |
| 14 | 143 ± 5 | 20.0 ± 0.1 | 49 ± 1 | 8.2 ± 1.3 | 22 ± 1 | 3.5 ± 0.4 |
| Sample | Phenolics EE (%) | β-Carotene EE (%) | α-Carotene EE (%) | D1 (nm) | % Area—D1 | D2 (nm) | % Area—D2 | D3 (nm) | % Area—D3 | ζ (mV) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 88 | 98 | 97 | 53 | 13 | 339 | 87 | - | - | +35 ± 4 |
| 2 | 97 | 87 | 85 | 111 | 41 | 442 | 58 | - | - | +17 ± 4 |
| 3 | 100 | 98 | 97 | 64 | 14 | 547 | 86 | - | - | +24 ± 1 |
| 4 | 85 | 90 | 89 | 164 | 53 | 719 | 31 | 3740 | 16 | +24 ± 3 |
| 5 | 88 | 99 | 98 | 332 | 30 | 565 | 22 | - | - | +36 ± 3 |
| 6 | 90 | 99 | 99 | 250 | 90 | 4940 | 10 | +30 ± 3 | ||
| 7 | 92 | 100 | 98 | 156 | 31 | 748 | 69 | 5560 | 0,5 | +25 ± 1 |
| 8 | 94 | 98 | 97 | 385 | 30 | 631 | 70 | - | - | +36 ± 4 |
| 9 | 80 | 99 | 98 | 76 | 12 | 534 | 88 | - | - | +37 ± 3 |
| 10 | 97 | 96 | 93 | 118 | 46 | 415 | 33 | 1064 | 17 | +26 ± 2 |
| 11 | 97 | 100 | 100 | 54 | 9 | 292 | 91 | - | - | +28 ± 1 |
| 12 | 96 | 100 | 100 | 176 | 46 | 723 | 52 | - | - | +33 ± 4 |
| 13 | 100 | 83 | 80 | 105 | 37 | 580 | 62 | 5560 | 0,3 | +22 ± 1 |
| 14 | 94 | 99 | 98 | 49 | 5 | 306 | 95 | - | - | +37 ± 3 |
| 15 | 94 | 100 | 100 | 120 | 30 | 545 | 70 | - | - | +33 ± 0 |
| 16 | 96 | 100 | 99 | 127 | 28 | 442 | 64 | 4205 | 8 | +34 ± 2 |
| 17 | 93 | 99 | 99 | 79 | 14 | 520 | 87 | - | - | +35 ± 2 |
| Coefficient | TPC EE (y1) | α-Carotene EE (y3) | β-Carotene EE (y2) | Particle Size (y4) | ζ (y5) |
|---|---|---|---|---|---|
| β0 | 95.15 | 99.44 | 99.63 | 501.6 | 34.13 * |
| Linear | |||||
| β1 | 1.45 | −2.08 * | −1.88 * | −86.3 | −2.31 |
| β2 | 0.81 | 0.07 | 0.18 | 106.3 | −0.04 |
| β3 | −1.42 | 4.09 * | 3.65 * | 0.6 | 3.83 * |
| Quadratic | |||||
| β11 | −2.20 | −1.26 * | −0.82 | −60.0 | −1.31 |
| β22 | −0.22 | 0.19 | 0.18 | −4.3 | −1.67 |
| β33 | 0.15 | −3.61 * | −2.92 * | −18.6 | −2.02 |
| Interaction | |||||
| β12 | −2.41 | 0.11 | 0.20 | −65.1 | 4.38 * |
| β13 | 0.14 | 2.35 * | 2.20 * | 10.2 | 2.88 |
| β23 | 0.86 | −0.71 | −0.55 | 108.4 | −0.13 |
| R2 | 0.49 | 0.94 | 0.94 | 0.79 | 0.92 |
| Fcalculated | 0.75 | 31.70 | 28.16 | 2.93 | 9.84 |
| Ftabulated1 | 6.72 | 5.32 | 5.41 | 6.72 | 6.51 |
| Flack of fit | - | 24.00 | 44.63 | 20.75 | |
| Ftabulated2 | - | 99.39 | 99.40 | 99.42 |
| Dependent Variable | EE of TPC (%) | EE of α-Carotene (%) | EE of β-Carotene (%) |
|---|---|---|---|
| Experimental value | 95 | 98 | 99 |
| Predicted value | - | 96 | 97 |
| RD (%) | - | +2 | +2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malagutti, E.; Pinho, S.G.B.; Thomazini, M.; Tapia-Blácido, D.R.; Martelli Tosi, M. Optimizing Encapsulation of Active Compounds of Carrot By-Product in TPP-Chitosomes. Foods 2024, 13, 2604. https://doi.org/10.3390/foods13162604
Malagutti E, Pinho SGB, Thomazini M, Tapia-Blácido DR, Martelli Tosi M. Optimizing Encapsulation of Active Compounds of Carrot By-Product in TPP-Chitosomes. Foods. 2024; 13(16):2604. https://doi.org/10.3390/foods13162604
Chicago/Turabian StyleMalagutti, Elisa, Sabrina Guarda Botelho Pinho, Marcelo Thomazini, Delia Rita Tapia-Blácido, and Milena Martelli Tosi. 2024. "Optimizing Encapsulation of Active Compounds of Carrot By-Product in TPP-Chitosomes" Foods 13, no. 16: 2604. https://doi.org/10.3390/foods13162604
APA StyleMalagutti, E., Pinho, S. G. B., Thomazini, M., Tapia-Blácido, D. R., & Martelli Tosi, M. (2024). Optimizing Encapsulation of Active Compounds of Carrot By-Product in TPP-Chitosomes. Foods, 13(16), 2604. https://doi.org/10.3390/foods13162604








