Transcriptome Reveals the Key Genes Related to the Metabolism of Volatile Sulfur-Containing Compounds in Lentinula edodes Mycelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture
2.2. Microstructure Analysis by Scanning Electron Microscope (SEM)
2.3. Flavor Extraction and Analysis
2.4. Enzyme Activities
2.5. RNA-seq and Transcriptomes Analysis
2.6. Transcriptome Validation
2.7. Molecular Docking and Molecular Dynamic Simulation
2.8. Statistical Analysis
3. Results
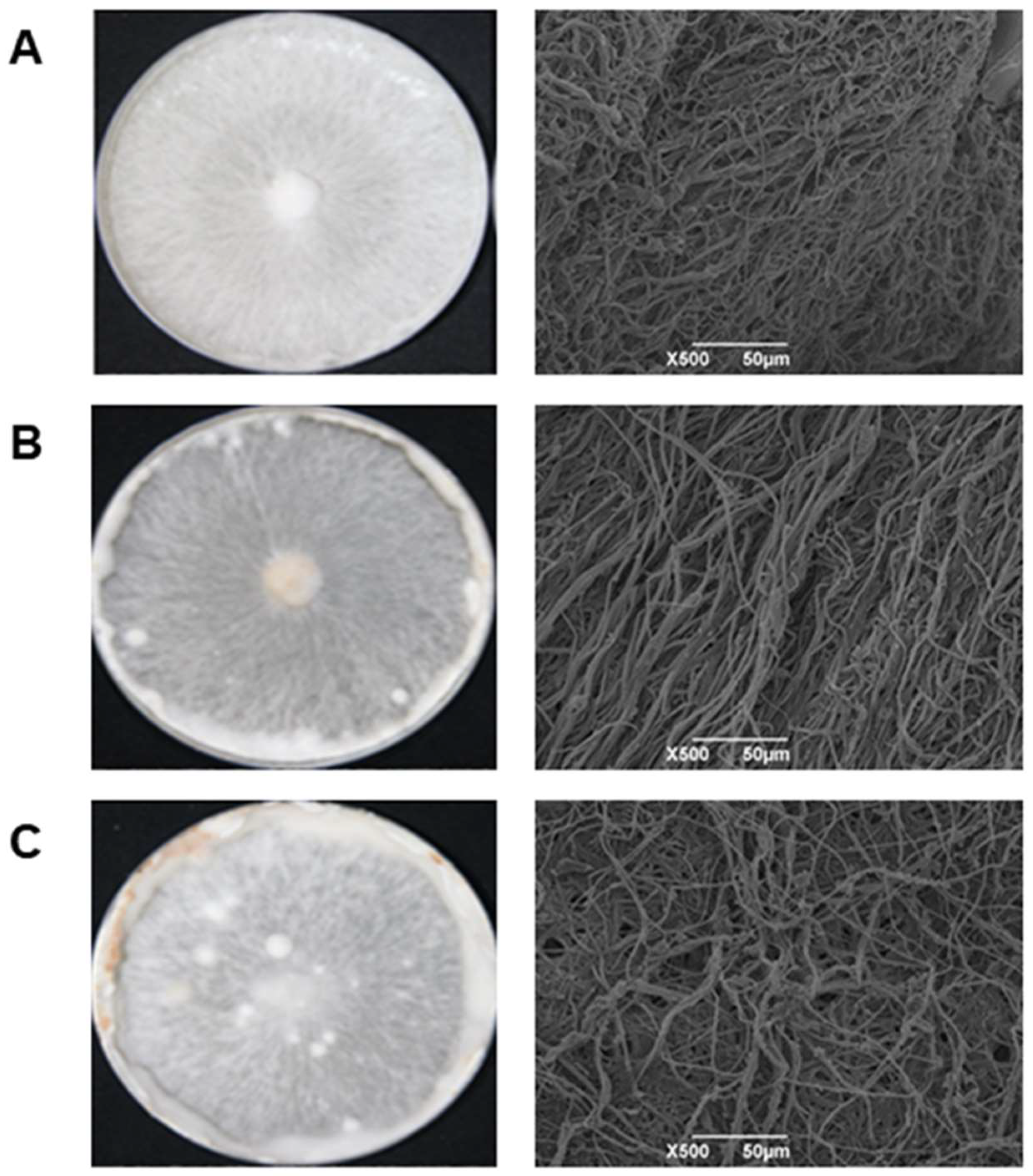
3.1. Morphology and Microstructure Analysis of Mycelium
3.2. Analysis of Volatile Components in Mycelium
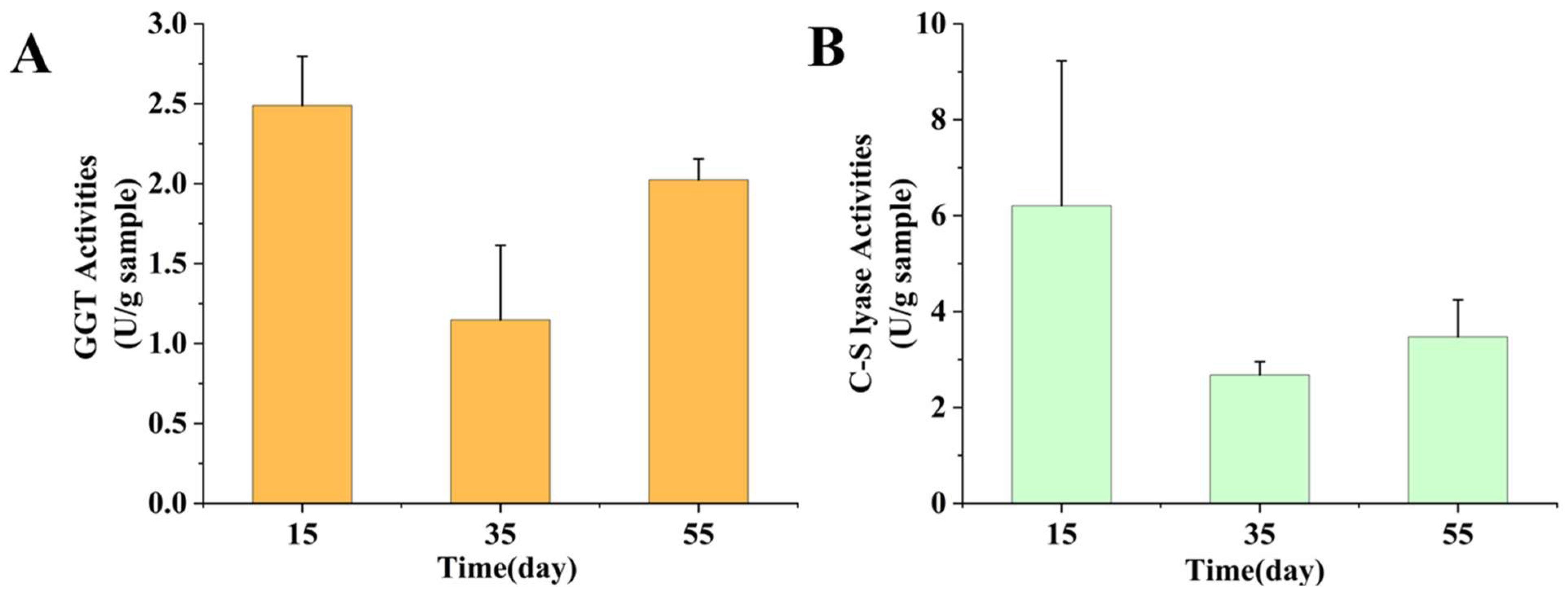
3.3. Enzyme Activities Analysis
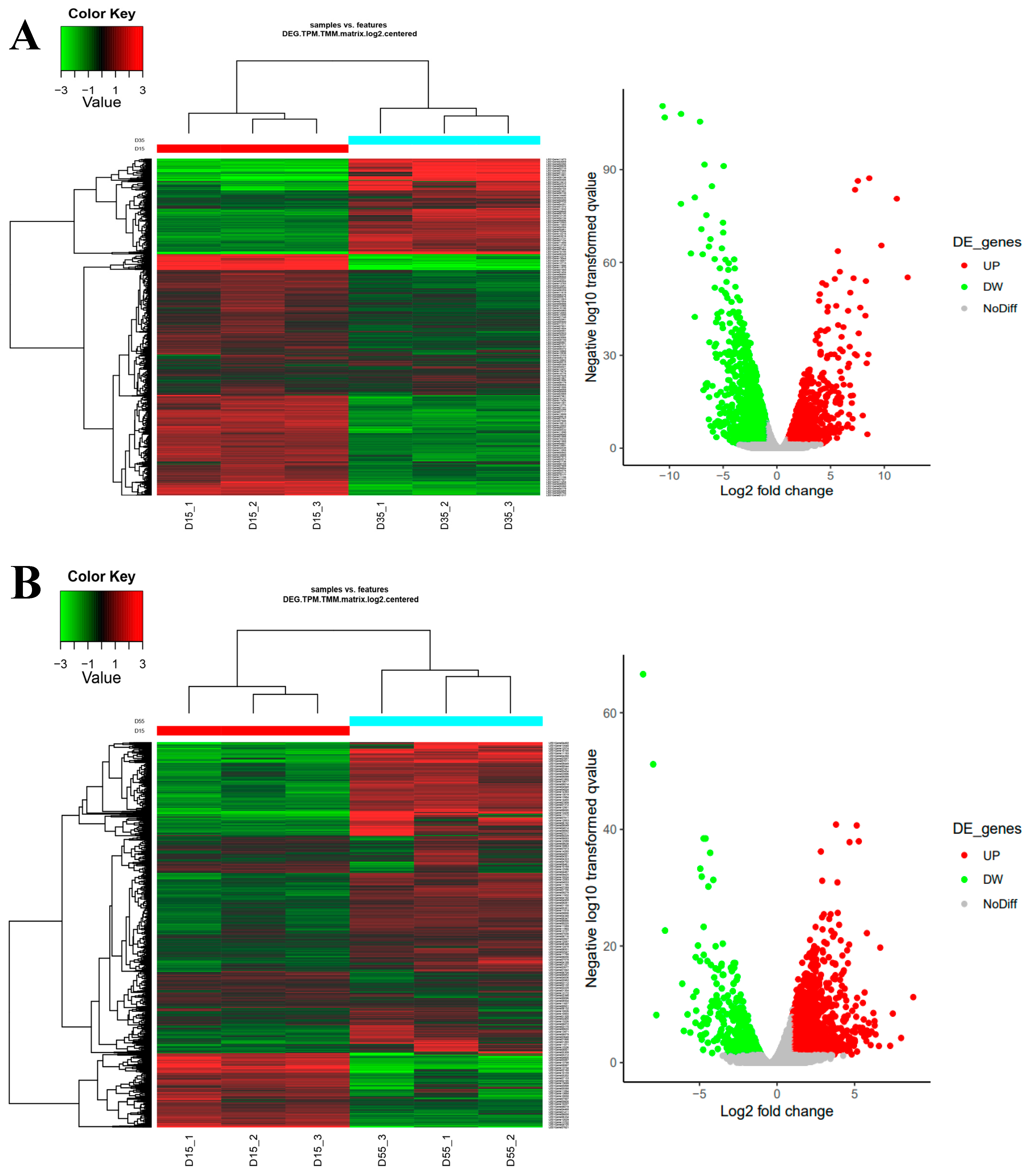
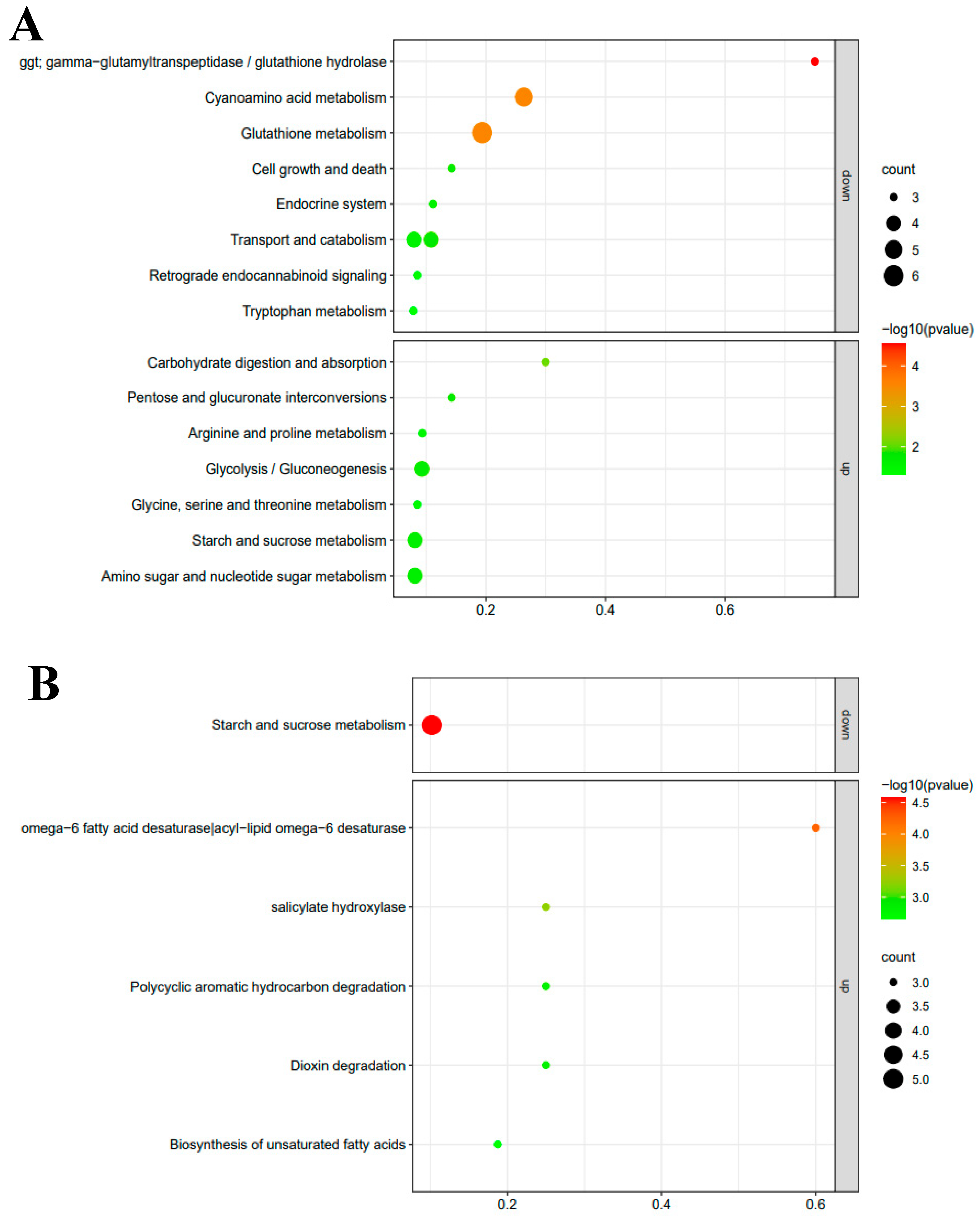
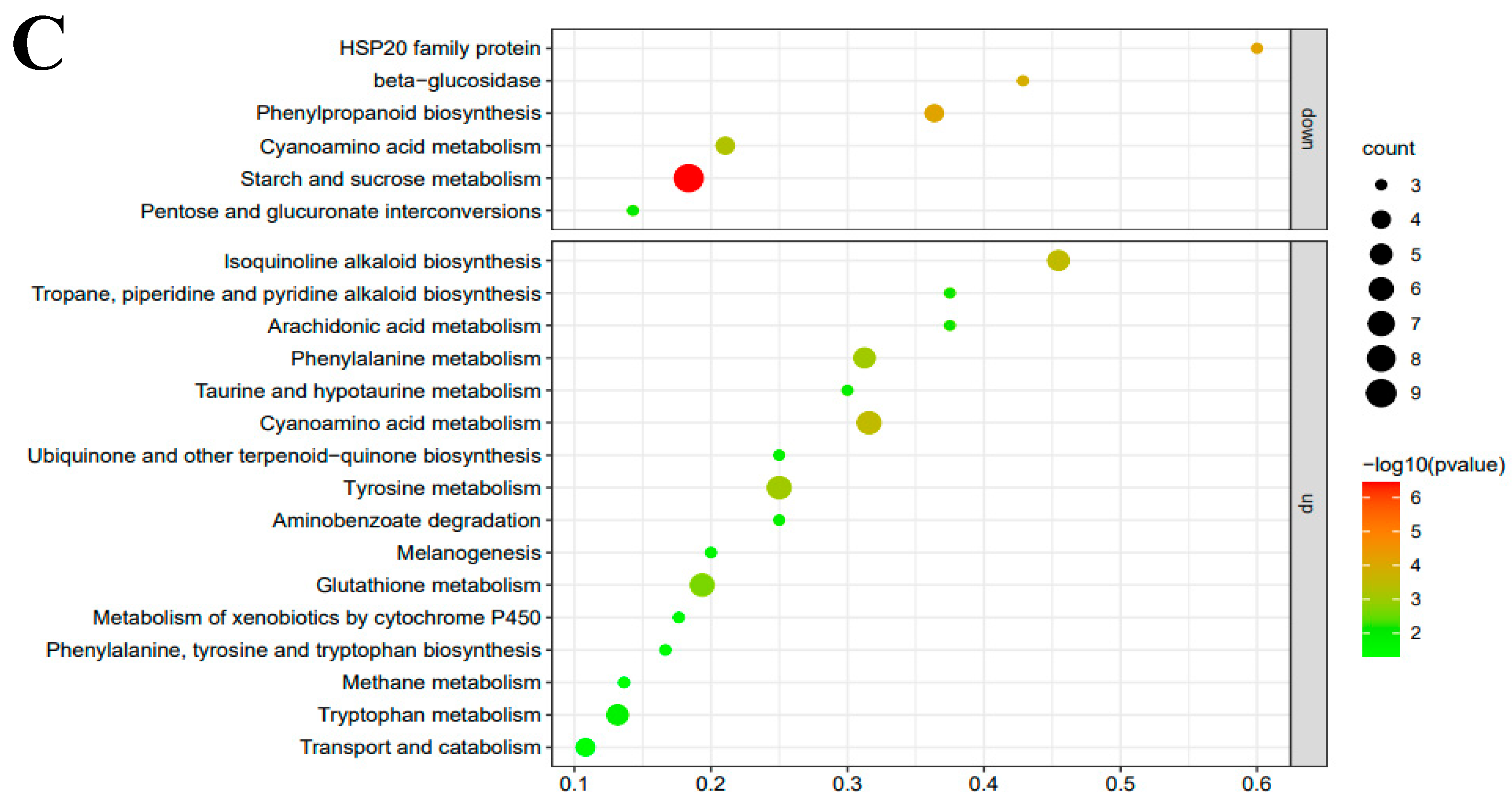
3.4. RNA-seq and Transcriptomes Analysis
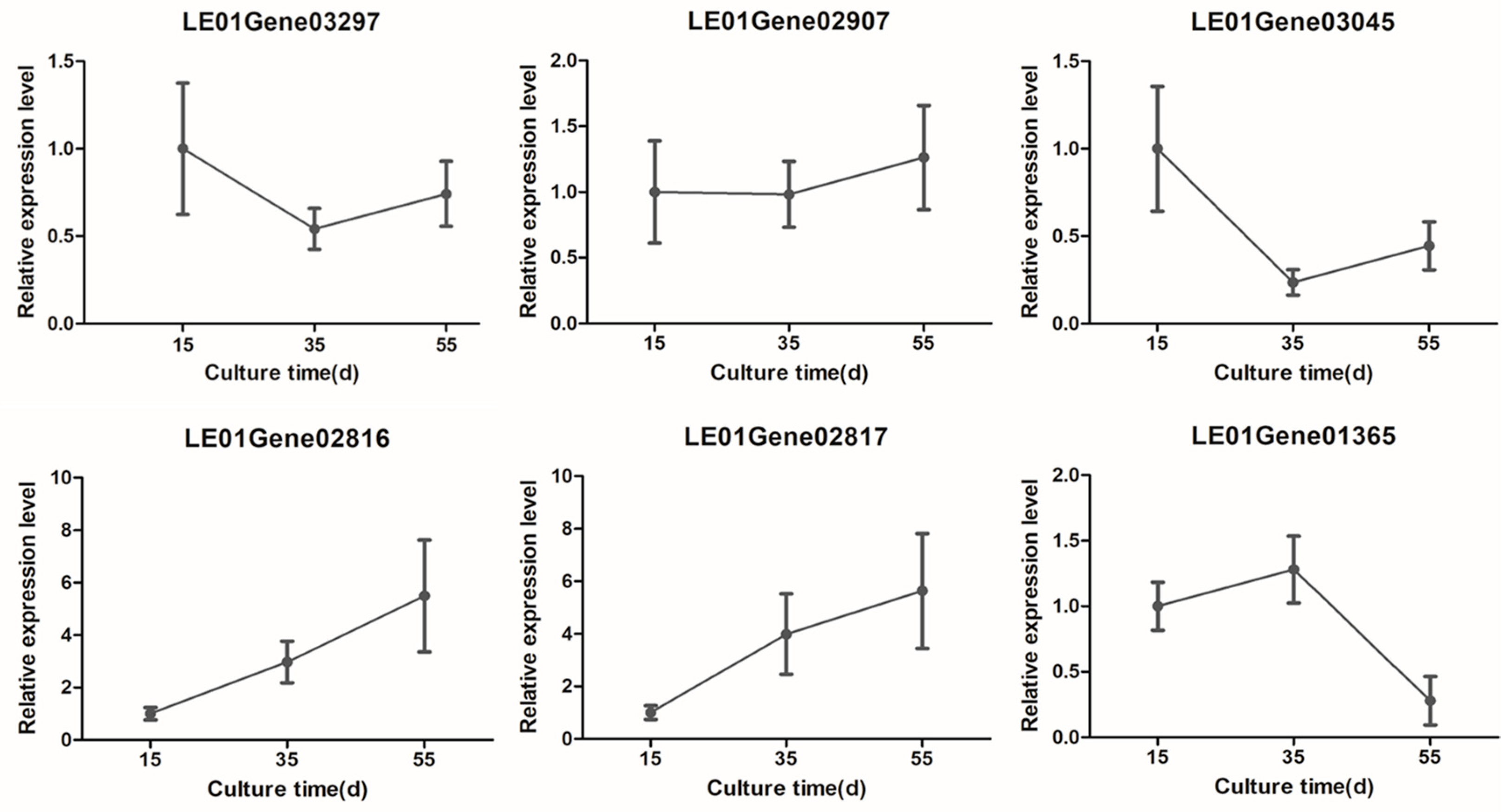
3.5. Transcriptome Validation
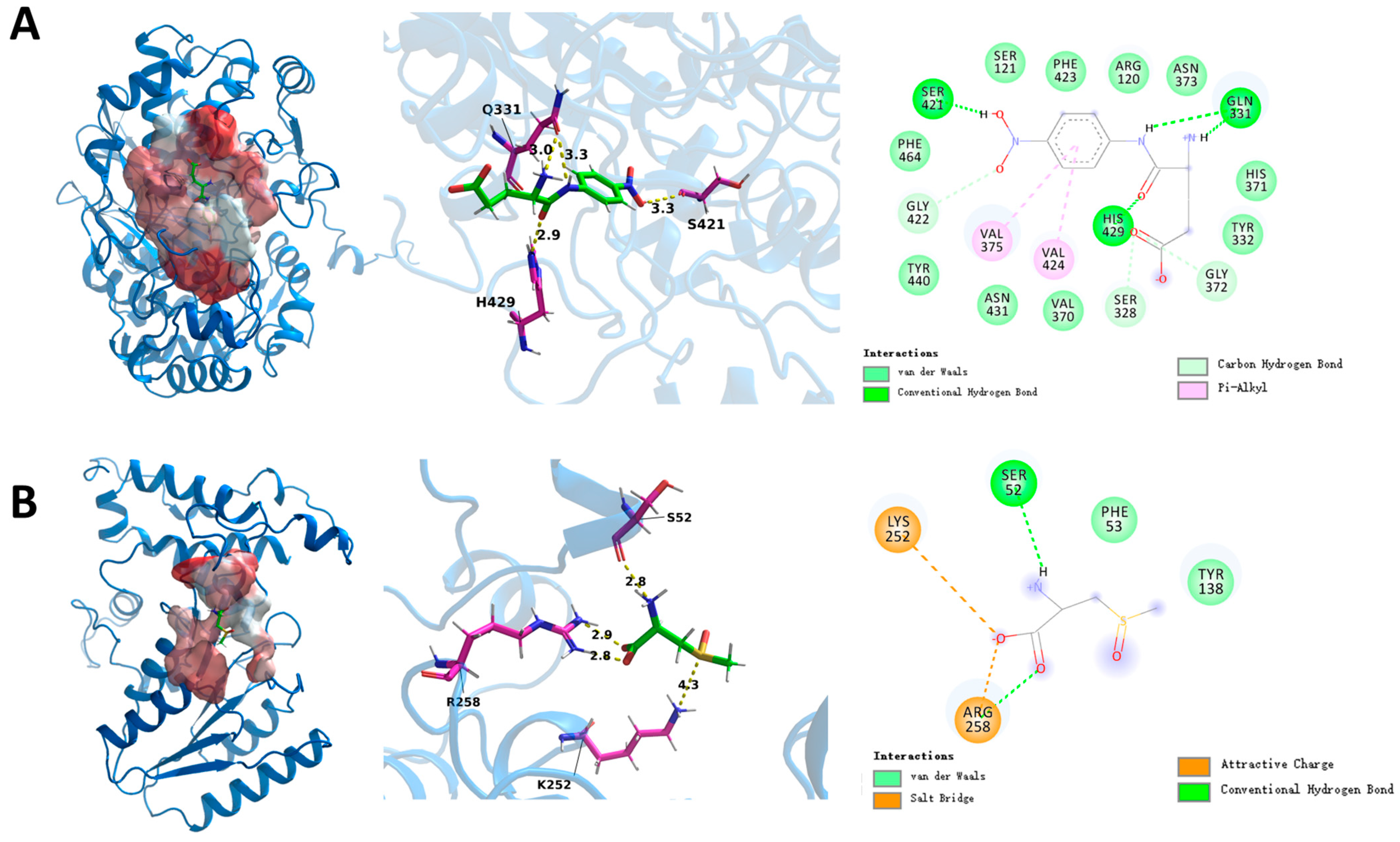
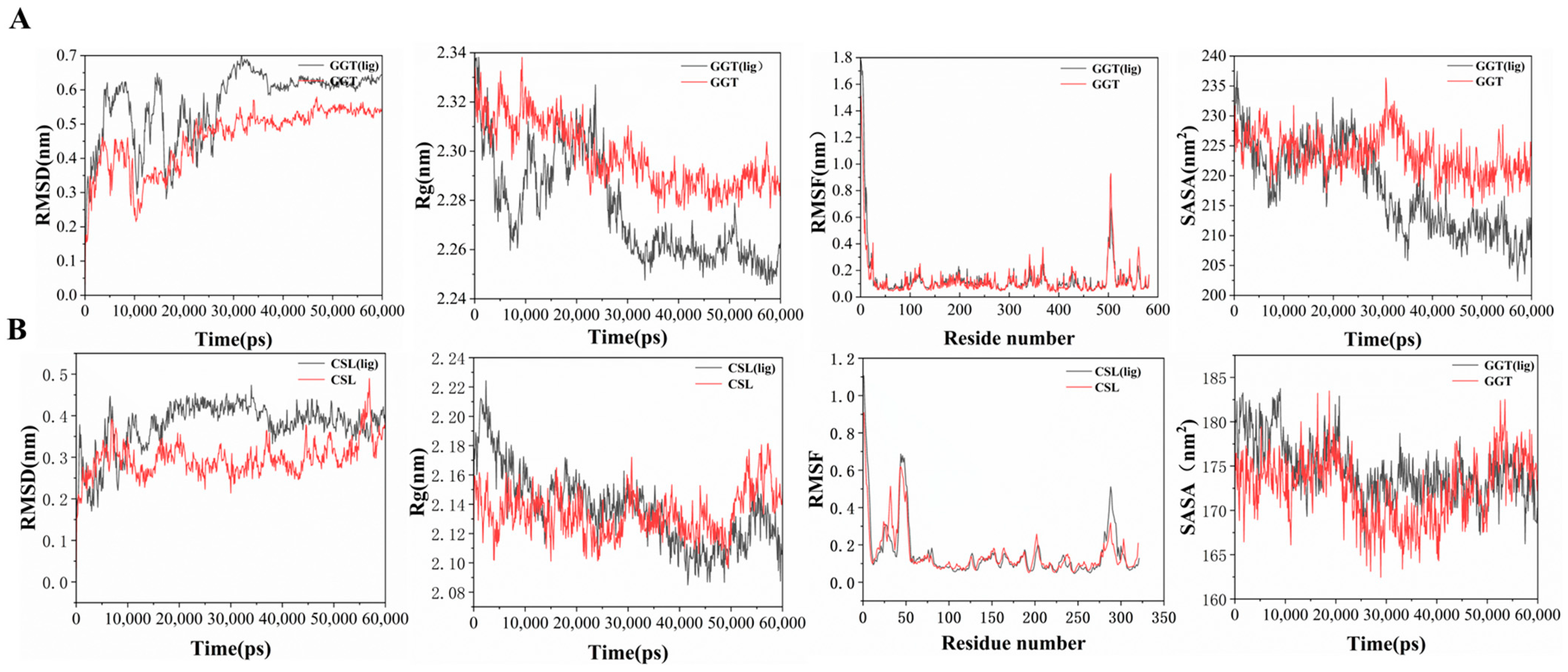
3.6. Mechanisms of Leggt3 and Lecsl3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, L.; Xin, G.; Hou, Z.; Zhao, X.; Xu, H.; Bao, X.; Xia, R.; Li, Y.; Li, L. Biosynthetic Mechanism of Key Volatile Biomarkers of Harvested Lentinula edodes Triggered by Spore Release. J. Agric. Food Chem. 2021, 69, 9350–9361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Huang, D. Dietary Organosulfur-Containing Compounds and Their Health-Promotion Mechanisms. Annu. Rev. Food Sci. Technol. 2022, 13, 287–313. [Google Scholar] [CrossRef]
- Schmidberger, P.C.; Schieberle, P. Changes in the Key Aroma Compounds of Raw Shiitake Mushrooms (Lentinula edodes) Induced by Pan-Frying as Well as by Rehydration of Dry Mushrooms. J. Agric. Food Chem. 2020, 68, 4493–4506. [Google Scholar] [CrossRef]
- Wen, X.; Li, W.; Li, W.; Chen, W.; Zhang, Z.; Wu, D.; Yang, Y. Quality characteristics and non-volatile taste formation mecha nism of Lentinula edodes during hot air drying. Food Chem. 2022, 393, 133378. [Google Scholar] [CrossRef]
- Xi, J.; Chen, X.; Du, J.; Zhong, L.; Hu, Q.; Zhao, L. Biosynthesis, behavior and fate of volatile organic sulfide in Lentinus edodes (Berk.) upon hot-air drying treatment. Food Chem. 2023, 412, 135528. [Google Scholar] [CrossRef] [PubMed]
- Sneeden, E.Y.; Harris, H.H.; Pickering, I.J.; Prince, R.C.; Johnson, S.; Li, X.; Block, E.; George, G.N. The sulfur chemistry of shiitake mushroom. J. Am. Chem. Soc. 2004, 126, 458–459. [Google Scholar] [CrossRef]
- Xu, L.; Fang, X.; Wu, W.; Chen, H.; Mu, H.; Gao, H. Effects of high-temperature pre-drying on the quality of air-dried shiitake mushrooms (Lentinula edodes). Food Chem. 2019, 285, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, X.-Y.; Chen, L.-F.; Bian, Y.-B.; Yang, H.; Ibrahim, S.A.; Huang, W. A novel cysteine desulfurase influencing or ganosulfur compounds in Lentinula edodes. Sci Rep. 2015, 5, 10047. [Google Scholar] [CrossRef]
- Lei, X.; Gao, S.; Feng, X.; Huang, Z.; Bian, Y.; Huang, W.; Liu, Y. Effects of GGT and C-S Lyase on the Generation of Endogenous Formaldehyde in Lentinula edodes at Different Growth Stages. Molecules 2019, 24, 4203. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Lei, X.Y.; Yang, H.; Ibrahim, S.A.; Huang, W. Purification and characterisation of two enzymes related to endogenous formaldehyde in Lentinula edodes. Food Chem. 2013, 138, 2174–2179. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodriguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Guillamon, E.; Garcia-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Rostagno, M.A.; Villares, A.; Martinez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Nielsen, J.B.; Wei, Y. Harnessing synthetic biology for mushroom farming. Trends Biotechnol. 2023, 41, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Rathore, H.; Prasad, S.; Kapri, M.; Tiwari, A.; Sharma, S. Medicinal importance of mushroom mycelium: Mechanisms and applications. J. Funct. Foods 2019, 56, 182–193. [Google Scholar] [CrossRef]
- Park, Y.-J.; Oh, T.-S.; Jang, M.-J. Effect of adding amino acids on the production of Gamma-Aminobutyric Acid (GABA) by mycelium of Lentinula edodes. Int. J. Food Eng. 2019, 15, 20180287. [Google Scholar] [CrossRef]
- Cao, X.; Liu, C.; Zhang, M.; Bi, R.; Fu, M.; Korik, E.; Chen, J.; Gao, J.; Semak, I.; Liu, J. Bovine lactoferrin and Lentinus edodes mycelia polysaccharide complex: The formation and the activity to protect islet β cells. Int. J. Biol. Macromol. 2021, 191, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.-C.; Wang, J.-B.; Feng, J.; Wu, D.; Zhang, Z.; Zhang, J.-S.; Yang, Y. Effects of enzymatic reaction on the generation of key aroma volatiles in shiitake mushroom at different cultivation substrates. Food Sci. Nutr. 2021, 9, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, Y.; Li, F.; Liu, P. Active polysaccharides from Lentinula edodes and Pleurotus ostreatus by addition of corn straw and xylosma sawdust through solid-state fermentation. Int. J. Biol. Macromol. 2023, 228, 647–658. [Google Scholar] [CrossRef]
- Yu, C.-X.; Zhang, Y.-R.; Ren, Y.-F.; Zhao, Y.; Song, X.-X.; Yang, H.-L.; Chen, M.-J. Composition and contents of fatty acids and amino acids in the mycelia of Lentinula edodes. Food Sci. Nutr. 2023, 11, 4038–4046. [Google Scholar] [CrossRef]
- Wang, W.-K.; Zhu, Y.; Tang, Y.; Lu, N.; Song, J.-L.; Yuan, W.-D.; Jia, Y. Non-Volatile Taste Components of Different Cultivated Mushrooms at Mycelia, Primordium, and Fruit Body Cultivation Stages. Int. J. Food Prop. 2016, 19, 1938–1948. [Google Scholar] [CrossRef]
- Chen, W.C.; Li, W.; Yang, Y.; Yu, H.L.; Zhou, S.; Feng, J.; Li, X.B.; Liu, Y.F. Analysis and Evaluation of Tasty Components in the Pileus and Stipe of Lentinula edodes at Different Growth Stages. J. Agric. Food Chem. 2015, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Chen, X.; Han, D.; Han, J.; Wang, L.; Ren, A.; Yu, H.; Zhao, M. Lenthionine, a Key Flavor Substance in Lentinula edodes, Is Regulated by Cysteine under Drought Stress. J. Agric. Food Chem. 2021, 69, 12645–12653. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Gong, Y.H.; Cai, Y.L.; Liu, W.; Zhou, Y.; Xiao, Y.; Xu, Z.Y.; Liu, Y.; Lei, X.Y.; Wang, G.Z.; et al. Genome Sequence of the Edible Cultivated Mushroom Lentinula edodes (Shiitake) Reveals Insights into Lignocellulose Degradation. PLoS ONE 2016, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, X.; Ren, A.; Shi, D.K.; Shi, L.; Zhu, J.; Yu, H.S.; Zhao, M.W. Heat stress-induced reactive oxygen species participate in the regulation of HSP expression, hyphal branching and ganoderic acid biosynthesis in Ganoderma lucidum. Microbiol Res. 2018, 209, 43–54. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.-X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Greenberg, E.P. Bacterial communication: Tiny teamwork. Nature 2003, 424, 134. [Google Scholar] [CrossRef]
- Boulianne, R.P.; Liu, Y.; Aebi, M.; Lu, B.C.; Kues, U. Fruiting body development in Coprinus cinereus: Regulated expression of two galectins secreted by a non-classical pathway. Microbiology 2000, 146, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Kues, U.; Liu, Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biotechnol. 2000, 54, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-B.; Zhang, Z.-Y.; Xin, G.; Sun, B.-X.; Bao, X.-J.; Wei, Y.-Y.; Zhao, X.-M.; Xu, H.-R. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Dermiki, M.; Phanphensophon, N.; Mottram, D.S.; Methven, L. Contributions of non-volatile and volatile compounds to the umami taste and overall flavour of shiitake mushroom extracts and their application as flavor enhancers in cooked minced meat. Food Chem. 2013, 141, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: Relationship between sensory evaluations and amounts of odorous components. J. Wood Sci. 2004, 50, 358–364. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.-C.; Wang, J.-B.; Feng, J.; Wu, D.; Zhang, Z.; Zhang, J.-S.; Yang, Y. Screening candidate genes related to volatile synthesis in shiitake mushrooms and construction of regulatory networks to effectively improve mushroom aroma. J. Sci. Food Agric. 2021, 101, 5618–5626. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, G.; Wang, C.; Gong, Y.; Bian, Y.; Zhou, Y. Selection and validation of reference genes for qRT-PCR in Lentinula edodes under different experimental conditions. Genes 2019, 10, 647. [Google Scholar] [CrossRef]
- Gholami, S.; Bordbar, A.-K. Exploring binding properties of naringenin with bovine β-lactoglobulin: A fluorescence, molecular docking and molecular dynamics simulation study. Biophys. Chem. 2014, 187–188, 33–42. [Google Scholar] [CrossRef]
- Mehranfar, F.; Bordbar, A.-K.; Parastar, H. A combined spectroscopic, molecular docking and molecular dynamic simulation study on the interaction of quercetin with β-casein nanoparticles. J. Photochem. Photobiol. B 2013, 127, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Rafiq, H.; Rahman, M.U.; Li, J.; Liu, H.; Luo, S.; Arshad, T.; Wadood, A.; Chen, H.-F. Gain-of-Function SHP2 E76Q Mutant Recusing Autoinhibition Mechanism Associated with Juvenile Myelomonocytic Leukemia. J. Chem. Inf. Model 2019, 59, 3229–3239. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ji, R.; Ji, J.; Chen, F. Changes of metabolites of acrylamide and glycidamide in acrylamide-exposed rats pretreated with blueberry anthocyanins extract. Food Chem. 2019, 274, 611–619. [Google Scholar] [CrossRef]
- Ling, Y.-Y.; Ling, Z.-L.; Zhao, R.-L. Construction of a heat-resistant strain of Lentinus edodes by fungal Hsp20 protein over expression and genetic transformation. Front. Microbiol. 2022, 13, 1009885. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.-E.; Lee, Y.-W.; Son, H. Fungal cytochrome P450s and the P450 complement (cypome) of Fusarium graminearum. Toxins 2018, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.D.; Chang, C.-Y.; Ma, M.; Shen, B. Cytochromes P450 for natural product biosynthesis in Streptomyces: Sequence, structure, and function. Nat. Prod. Rep. 2017, 34, 1141–1172. [Google Scholar] [CrossRef]
- Chen, S.; Glawischnig, E.; Jorgensen, K.; Naur, P.; Jorgensen, B.; Olsen, C.-E.; Hansen, C.H.; Rasmussen, H.; Pickett, J.A.; Halkier, B.A. CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J. 2003, 33, 923–937. [Google Scholar] [CrossRef]
- Hull, A.K.; Vij, R.; Celenza, J.L. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2379–2384. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Pan, F.; Huang, W.; Gao, S.; Feng, X.; Chang, M.; Chen, L.; Bian, Y.; Tian, W.; Liu, Y. Transcriptome Reveals the Key Genes Related to the Metabolism of Volatile Sulfur-Containing Compounds in Lentinula edodes Mycelium. Foods 2024, 13, 2179. https://doi.org/10.3390/foods13142179
Li Z, Pan F, Huang W, Gao S, Feng X, Chang M, Chen L, Bian Y, Tian W, Liu Y. Transcriptome Reveals the Key Genes Related to the Metabolism of Volatile Sulfur-Containing Compounds in Lentinula edodes Mycelium. Foods. 2024; 13(14):2179. https://doi.org/10.3390/foods13142179
Chicago/Turabian StyleLi, Zheng, Fei Pan, Wen Huang, Shuangshuang Gao, Xi Feng, Meijie Chang, Lianfu Chen, Yinbing Bian, Wenli Tian, and Ying Liu. 2024. "Transcriptome Reveals the Key Genes Related to the Metabolism of Volatile Sulfur-Containing Compounds in Lentinula edodes Mycelium" Foods 13, no. 14: 2179. https://doi.org/10.3390/foods13142179
APA StyleLi, Z., Pan, F., Huang, W., Gao, S., Feng, X., Chang, M., Chen, L., Bian, Y., Tian, W., & Liu, Y. (2024). Transcriptome Reveals the Key Genes Related to the Metabolism of Volatile Sulfur-Containing Compounds in Lentinula edodes Mycelium. Foods, 13(14), 2179. https://doi.org/10.3390/foods13142179






