The Role of Nondigestible Oligosaccharides in Alleviating Human Chronic Diseases by Regulating the Gut Microbiota: A Review
Abstract
1. Introduction
2. Classification and Sources of Oligosaccharides
3. The Impact of Different NDOs on the Diversity of the Gut Microbiota
3.1. Degree of Polymerization (DP)
3.2. Glycosidic Bond
3.3. Linear and Branching Structures
4. The Impact of Different NDOs on the Diversity of the Gut Microbiota
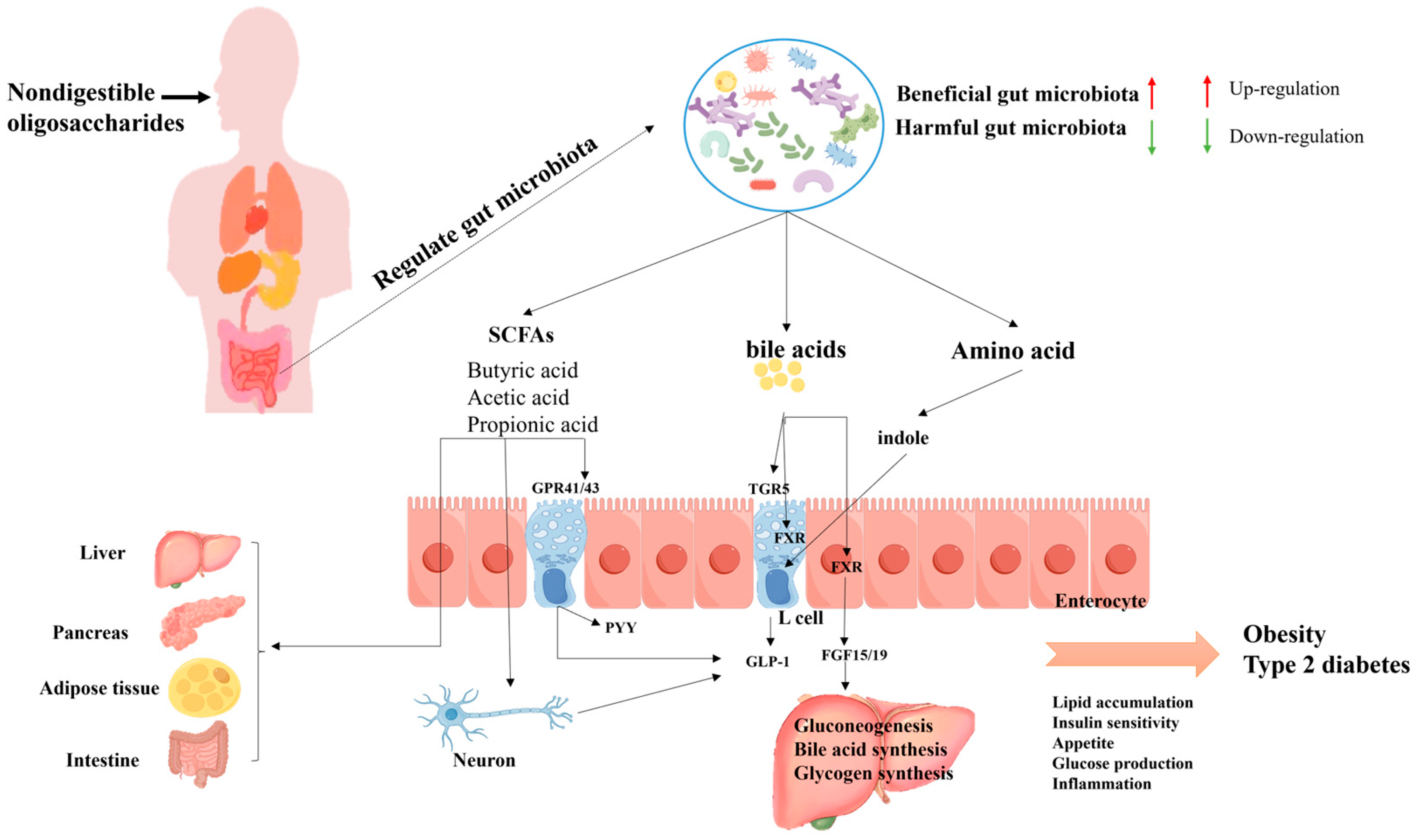
4.1. T2DM
4.2. Obesity
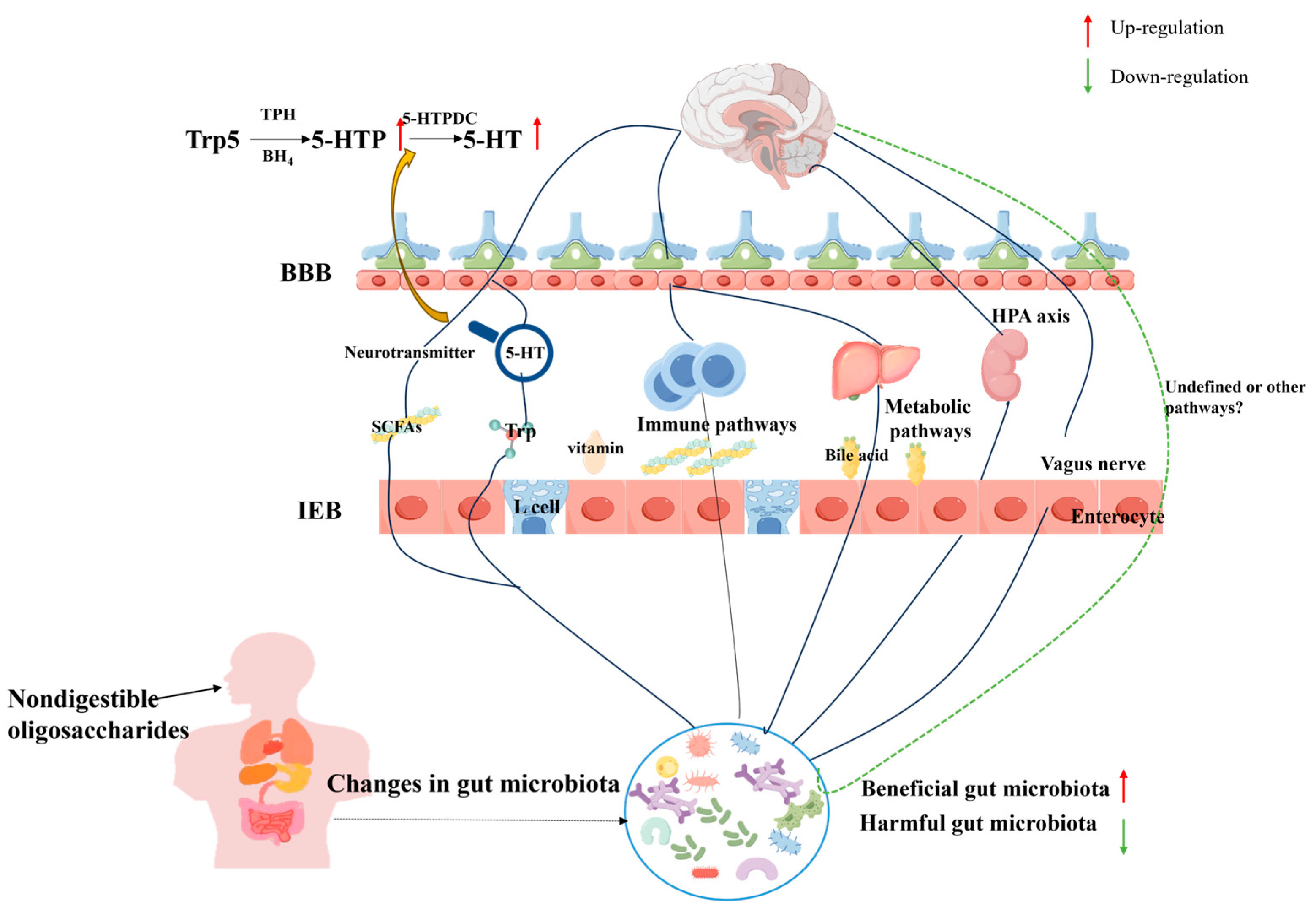
4.3. Depression
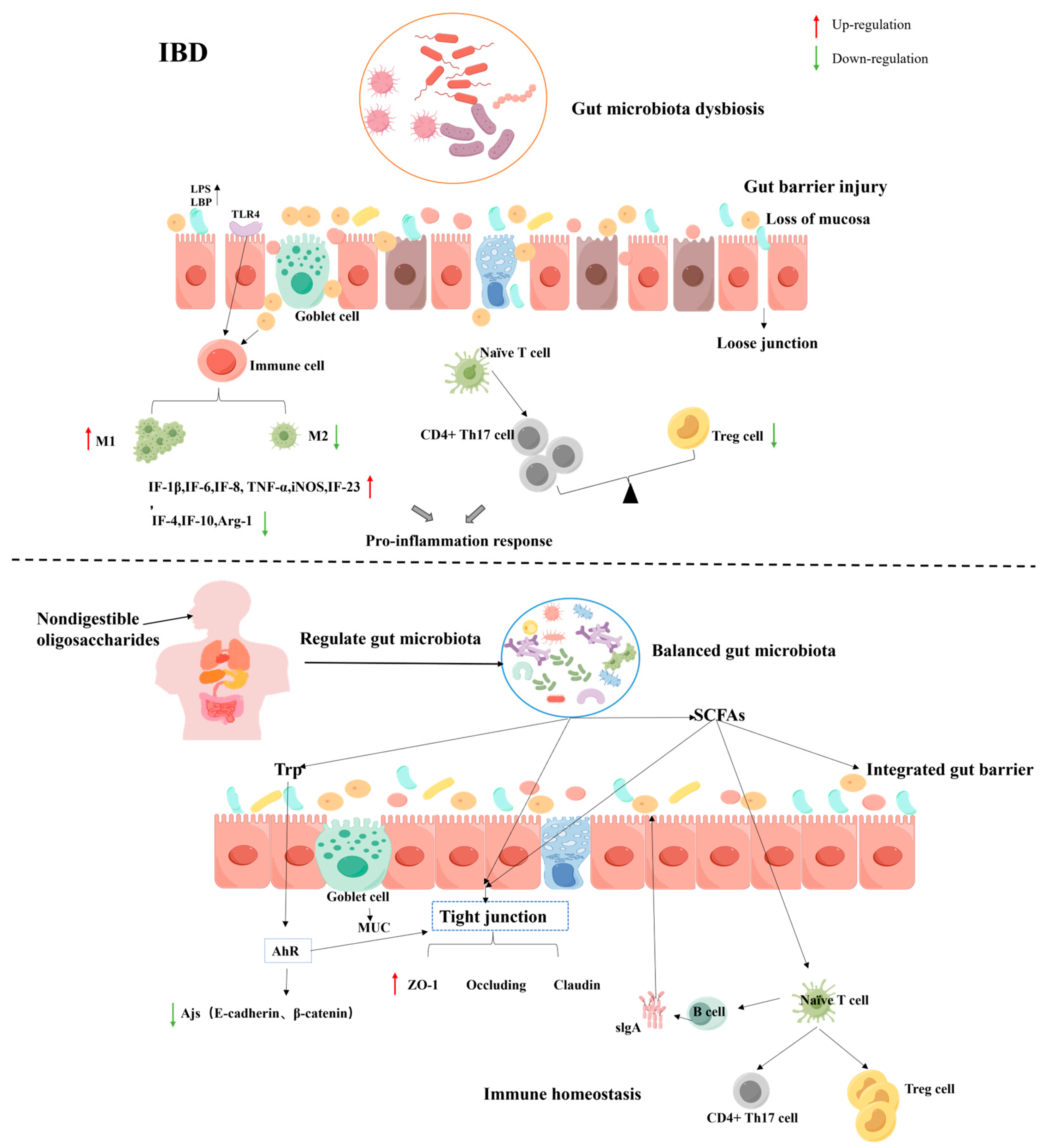
4.4. Inflammatory Bowel Disease (IBD)
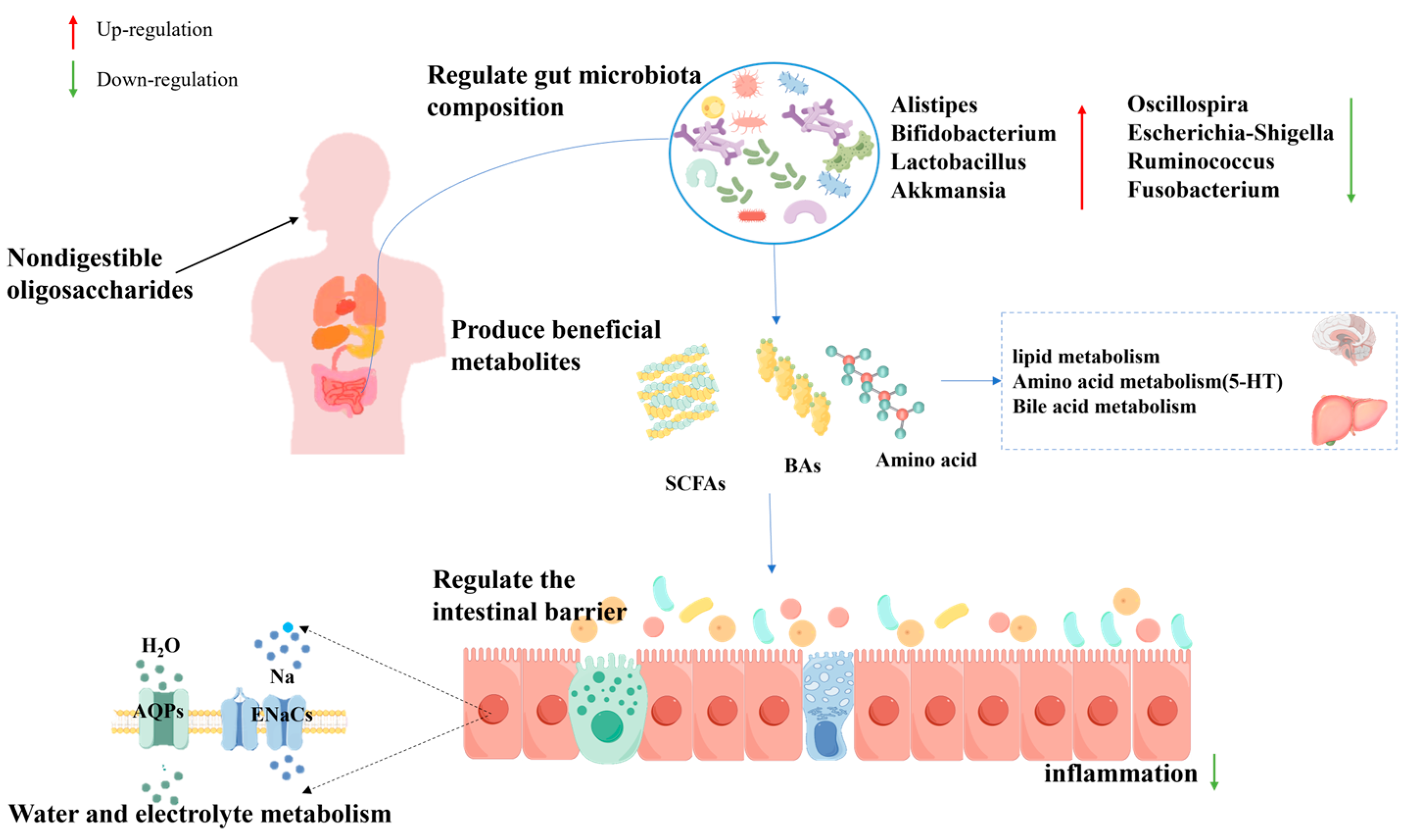
4.5. Constipation
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis a Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Casen, C.; Valeur, J.; Hausken, T.; Hatlebakk, J.G. Responses to Faecal Microbiota Transplantation in Female and Male Patients with Irritable Bowel Syndrome. World J. Gastroenterol. 2021, 27, 2219–2237. [Google Scholar] [CrossRef]
- He, L.A.; Chen, R.P.; Zhang, B.Z.; Zhang, S.; Khan, B.A.; Zhu, D.; Wu, Z.Z.; Xiao, C.X.; Chen, B.L.; Chen, F.W.; et al. Fecal Microbiota Transplantation Treatment of Autoimmune-Mediated Type 1 Diabetes Mellitus. Front. Immunol. 2022, 13, 930872. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a Healthy Microbiota Reduces Amyloid and Tau Pathology in an Alzheimer’s Disease Animal Model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.F.; Deng, Z.L.; Luo, W.H.; He, X.X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, L.; Lei, L.; Zhu, Y.; Xu, J.; Liu, L. Fecal Microbiota Transplantation Ameliorates Abdominal Obesity through Inhibiting Microbiota-Mediated Intestinal Barrier Damage and Inflammation in Mice. Microbiol. Res. 2024, 282, 127654. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut Microbial Metabolites as Multi-Kingdom Intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Chassaing, B.; Miles-Brown, J.; Pellizzon, M.; Ulman, E.; Ricci, M.; Zhang, L.M.; Patterson, A.D.; Vijay-Kumar, M.; Gewirtz, A.T. Lack of Soluble Fiber Drives Diet-Induced Adiposity in Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G528–G541. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary Intervention Impact on Gut Microbial Gene Richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Zhu, J.H.; Ren, H.H.; Zhong, H.Z.; Li, X.P.; Zou, Y.Q.; Han, M.; Li, M.L.; Madsen, L.; Kristiansen, K.; Xiao, L. An Expanded Gene Catalog of Mouse Gut Metagenomes. Msphere 2021, 6, e01119-20. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Nie, X.-R.; Gan, R.-Y.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In Vitro Digestion and Fecal Fermentation Behaviors of a Pectic Polysaccharide from Okra (Abelmoschus esculentus) and Its Impacts on Human Gut Microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Xu, X.; Jiang, H.; Cai, C.; Yu, G. Odd-Numbered Agaro-Oligosaccharides Alleviate Type 2 Diabetes Mellitus and Related Colonic Microbiota Dysbiosis in Mice. Carbohyd. Polym. 2020, 240, 116261. [Google Scholar] [CrossRef]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.Z.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1063. [Google Scholar] [CrossRef]
- Villablanca, E.J.; Selin, K.; Hedin, C.R.H. Mechanisms of Mucosal Healing: Treating Inflammatory Bowel Disease without Immunosuppression? Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.T.; Wang, Y.L.; He, K.X.; Zhu, S. Microbial Sensing in the Intestine. Protein Cell 2023, 14, 824–860. [Google Scholar] [CrossRef] [PubMed]
- Rajca, S.; Grondin, V.; Louis, E.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Dupas, J.L.; Pillant, H.; Picon, L.; et al. Alterations in the Intestinal Microbiome (Dysbiosis) as a Predictor of Relapse after Infliximab Withdrawal in Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 978–986. [Google Scholar] [PubMed]
- Zhang, W.; Lyu, M.Z.; Bessman, N.J.; Xie, Z.L.; Arifuzzaman, M.; Yano, H.; Parkhurst, C.N.; Chu, C.C.; Zhou, L.; Putzel, G.G.; et al. Gut-Innervating Nociceptors Regulate the Intestinal Microbiota to Promote Tissue Protection. Cell 2022, 185, 4170–4189. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastro Hepat. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.Y.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; van Dullemen, H.M.; et al. nterplay of Host Genetics and Gut Microbiota Underlying the Onset and Clinical Presentation of Inflammatory Bowel Disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Li, M.Z.; Yu, M.L.; Shen, M.Y.; Wang, Q.; Yu, Y.; Xie, J.H. Natural Polysaccharides Exhibit Anti-Tumor Activity by Targeting Gut Microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Mikolaitis, S.; Sedghi, S.; Chakradeo, P.S.; Engen, P.; Chlipala, G.; Green, S.; Keshavarzian, A. Mo1795 Dietary Treatment of Crohn’s Disease: A Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Gastroenterology 2016, 150 (Suppl. S1), S778. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.Y.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A Pyrosequencing Study in Twins Shows That Gastrointestinal Microbial Profiles Vary with Inflammatory Bowel Disease Phenotypes. Gastroenterology 2010, 139, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Padwad, Y. Probiotic Bacteria as Modulators of Cellular Senescence: Emerging Concepts and Opportunities. Gut Microbes 2020, 11, 335–349. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O. Effects of Probiotics and Prebiotics on Frailty and Ageing: A Narrative Review. Curr. Clin. Pharmacol. 2020, 15, 183–192. [Google Scholar]
- Setbo, E.; Campbell, K.; O’Cuiv, P.; Hubbard, R. Utility of Probiotics for Maintenance or Improvement of Health Status in Older People a Scoping Review. J. Nutr. Health Aging 2019, 23, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lye, H.S.; Lee, Y.T.; Ooi, S.Y.; Teh, L.K.; Lim, L.N.; Wei, L.K. Modifying Progression of Aging and Reducing the Risk of Neurodegenerative Diseases by Probiotics and Synbiotics. Front. Biosci. 2018, 10, 344–351. [Google Scholar]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M. Increased IgA and IgM Responses against Gut Commensals in Chronic Depression: Further Evidence for Increased Bacterial Translocation or Leaky Gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhang, X.; Yu, Z.H.; Zhang, Z.; Deng, M.; Zhao, J.H.; Ruan, B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef]
- Madan, A.; Thompson, D.; Fowler, J.C.; Ajami, N.J.; Salas, R.; Frueh, B.C.; Bradshaw, M.R.; Weinstein, B.L.; Oldham, J.M.; Petrosino, J.F. The Gut Microbiota Is Associated with Psychiatric Symptom Severity and Treatment Outcome among Individuals with Serious Mental Illness. J. Affect. Disord. 2020, 264, 98–106. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linlokken, A.; Wilson, R.; Rudi, K. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y.; Lu, Y.; Hao, H.; Liu, J.; Huang, R. Structural Features, Interaction with the Gut Microbiota and Anti-Tumor Activity of Oligosaccharides. RSC Adv. 2020, 10, 16339–16348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jin, M.; Wang, K.; Zhang, Z.; Shah, N.P.; Wei, H. Functional Oligosaccharide Fermentation in the Gut: Improving Intestinal Health and Its Determinant Factors-a Review. Carbohyd. Polym. 2022, 284, 119043. [Google Scholar] [CrossRef] [PubMed]
- Divyashri, G.; Karthik, P.; Murthy, T.P.K.; Priyadarshini, D.; Reddy, K.R.; Raghu, A.V.; Vaidyanathan, V.K. Non-Digestible Oligosaccharides-Based Prebiotics to Ameliorate Obesity: Overview of Experimental Evidence and Future Perspectives. Food Sci. Biotechnol. 2023, 32, 1993–2011. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, M.M.; Li, M.L.; Li, C.C.; Miao, M. Effects of Human Milk Oligosaccharides in Infant Health Based on Gut Microbiota Alteration. J. Agric. Food Chem. 2023, 71, 994–1001. [Google Scholar]
- Chen, X.; Hu, J.; Yang, J.; Yu, Q.; Chen, Y.; Shen, M.; Rong, L.; Xie, J. Human Milk Oligosaccharide 2′-Fucosyllactose Alleviates Dss-Induced Ulcerative Colitis via Improving Intestinal Barrier Function and Regulating Gut Microbiota. Food Biosci. 2024, 59, 104162. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.J.; Tan, Q.H.; Deng, X.Y.; Tsa, P.J.; Chen, P.H.; Ye, M.X.; Guo, J.; Su, Z.Q. Nondigestible Oligosaccharides with Anti-Obesity Effects. J. Agric. Food Chem. 2020, 68, 4–16. [Google Scholar] [CrossRef]
- Duffuler, P.; Bhullar, K.S.; Wu, J. Targeting Gut Microbiota in Osteoporosis: Impact of the Microbial-Based Functional Food Ingredients. Food Sci. Hum. Well 2024, 13, 1–15. [Google Scholar] [CrossRef]
- Navarro, D.; Abelilla, J.J.; Stein, H.H. Structures and Characteristics of Carbohydrates in Diets Fed to Pigs: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-Digestible Oligosaccharides: A Review. Carbohyd. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Caselato, V.; Dos Santos, E.; Sgarbieri, V. The Importance of Prebiotics in Functional Foods and Clinical Practice. Food Nutr. Sci. 2011, 2, 22019. [Google Scholar]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological Aspects of the Production of Fructo and Galacto-Oligosaccharides. Enzymatic Synthesis and Hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wu, Y.J.; Liu, X.Y.; Liu, B.; Cao, H.; Yu, H.; Sarker, S.D.; Nahar, L.; Xiao, J.B. Functional Properties, Structural Studies and Chemo-Enzymatic Synthesis of Oligosaccharides. Trends Food Sci. Technol. 2017, 66, 135–145. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as Functional Foods: A Review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Precup, G.; Venus, J.; Heiermann, M.; Schneider, R.; Pop, I.D.; Vodnar, D.C. Chemical and Enzymatic Synthesis of Biobased Xylo-Oligosaccharides and Fermentable Sugars from Wheat Straw for Food Applications. Polymers 2022, 14, 1336. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an Emerging Prebiotic: Microbial Synthesis, Utilization, Structural Characterization, Bioactive Properties, and Applications. Compr. Rev. Food Sci. F 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Nopvichai, C.; Charoenwongpaiboon, T.; Luengluepunya, N.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Production and Purification of Mannan Oligosaccharide with Epithelial Tight Junction Enhancing Activity. Peerj 2019, 7, e7206. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y. Characterization of Galacto-Oligosaccharides Using High-Performance Anion Exchange Chromatography-Tandem Mass Spectrometry. J. Sep. Sci. 2021, 44, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.P.; Le, N.A.T.; Tran, P.T.; Bui, D.D.; Nguyen, Q.H. Preparation of Water-Soluble Chitosan Oligosaccharides by Oxidative Hydrolysis of Chitosan Powder with Hydrogen Peroxide. Heliyon 2023, 9, e19565. [Google Scholar] [CrossRef]
- Milani, C.; Lugli, G.A.; Duranti, S.; Turroni, F.; Mancabelli, L.; Ferrario, C.; Mangifesta, M.; Hevia, A.; Viappiani, A.; Scholz, M.; et al. Bifidobacteria Exhibit Social Behavior through Carbohydrate Resource Sharing in the Gut. Sci. Rep. 2015, 5, 15782. [Google Scholar] [CrossRef]
- Zúñiga, M.; Yebra, M.J.; Monedero, V. Complex Oligosaccharide Utilization Pathways in Lactobacillus. Curr. Issues Mol. Biol. 2021, 40, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Li, R.Q.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Rastall, R.A. Prebiotics: Metabolism, Structure, and Function. Funct. Food Rev. 2011, 3, 93–106. [Google Scholar]
- Singh, R.D.; Muir, J.; Arora, A. Concentration of Xylooligosaccharides with a Low Degree of Polymerization Using Membranes and Their Effect on Bacterial Fermentation. Biofuel Bioprod. Bior 2021, 15, 61–73. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Li, Z.; Yan, B.; Pei, W.; Wu, H. The Preparation Technology and Application of Xylo-Oligosaccharide as Prebiotics in Different Fields: A Review. Front. Nutr. 2022, 9, 996811. [Google Scholar] [CrossRef]
- Sanz, M.L.; Gibson, G.R.; Rastall, R.A. Influence of Disaccharide Structure on Prebiotic Selectivity In Vitro. J. Agric. Food Chem. 2005, 53, 5192–5199. [Google Scholar] [CrossRef]
- Suzuki, N.; Aiba, Y.; Takeda, H.; Fukumori, Y.; Koga, Y. Superiority of 1-Kestose, the Smallest Fructo-Oligosaccharide, to a Synthetic Mixture of Fructo-Oligosaccharides in the Selective Stimulating Activity on Bifidobacteria. Biosci. Microflora 2006, 25, 109–116. [Google Scholar] [CrossRef]
- Böger, M.; van Leeuwen, S.S.; van Bueren, A.L.; Dijkhuizen, L. Structural Identity of Galactooligosaccharide Molecules Selectively Utilized by Single Cultures of Probiotic Bacterial Strains. J. Agric. Food Chem. 2019, 67, 13969–13977. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Wang, T.; Su, Y.; Huang, C.; Lai, C.; Yong, Q. Evaluation of Prebiotic Ability of Xylo-Oligosaccharide Fractions with Different Polymerization Degrees from Bamboo Shoot Shells. Food Bioprod. Process 2024, 143, 202–211. [Google Scholar] [CrossRef]
- Immerzeel, P.; Falck, P.; Galbe, M.; Adlercreutz, P.; Karlsson, E.N.; Stålbrand, H. Extraction of Water-Soluble Xylan from Wheat Bran and Utilization of Enzymatically Produced Xylooligosaccharides by Lactobacillus, Bifidobacterium and Weissella spp. LWT-Food Sci. Technol. 2014, 56, 321–327. [Google Scholar] [CrossRef]
- Falck, P.; Precha-Atsawanan, S.; Grey, C.; Immerzeel, P.; Stålbrand, H.; Adlercreutz, P.; Karlsson, E.N. Correction to Xylooligosaccharides from Hardwood and Cereal Xylans Produced by a Thermostable Xylanase as Carbon Sources for Lactobacillus Brevis and Bifidobacterium Adolescentis. J. Agric. Food Chem. 2013, 61, 7333–7340. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Pembroke, J.T. Brevundimonas Spp: Emerging Global Opportunistic Pathogens. Virulence 2018, 9, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.G.; Cao, Y.P.; Wang, C.T. In Vitro Fermentation of Xylooligosaccharides from Wheat Bran Insoluble Dietary Fiber by Bifidobacteria. Carbohyd. Polym. 2010, 82, 419–423. [Google Scholar] [CrossRef]
- Grimoud, J.; Durand, H.; Courtin, C.; Monsan, P.; Ouarné, F.; Theodorou, V.; Rogues, C. In Vitro Screening of Probiotic Lactic Acid Bacteria and Prebiotic Glucooligosaccharides to Select Effective Synbiotics. Anaerobe 2010, 16, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Kohmoto, T.; Kikuchi, H.; Shiota, M.; Iino, H.; Mitsuoka, T. Effects of Isomaltooligosaccharides with Different Degrees of Polymerization on Human Fecal Bifidobactcria. Biosci. Biotechnol. Biochem. 1994, 58, 2288–2290. [Google Scholar] [CrossRef]
- Sanz, M.L.; Côté, G.L.; Gibson, G.R.; Rastall, R.A. Influence of Glycosidic Linkages and Molecular Weight on the Fermentation of Maltose-Based Oligosaccharides by Human Gut Bacteria. J. Agric. Food Chem. 2006, 54, 9779–9784. [Google Scholar] [CrossRef]
- Ladirat, S.E.; Schols, H.A.; Nauta, A.; Schoterman, M.H.C.; Schuren, F.H.J.; Gruppen, H. In vitro fermentation of galacto-oligosaccharides and its specific size-fractions using non-treated and amoxicillin-treated human inoculum. Bioact. Carbohydr. Diet. Fibre 2014, 3, 59–70. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu. Rev. Food Sci. Technol. 2015, 6, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.M.; Barrangou, R.; Abou Hachem, M.; Lahtinen, S.; Goh, Y.J. Transcriptional and functional analysis of galactooligosaccharide uptake by lacS in Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2011, 108, 17785–17790. [Google Scholar] [CrossRef]
- Wiater, A.; Wasko, A.; Adamczyk, P.; Gustaw, K.; Pleszczynska, M.; Wlizlo, K.; Skowronek, M.; Tomczyk, M.; Szczodrak, J. Prebiotic Potential of Oligosaccharides Obtained by Acid Hydrolysis of α-(1→3)-Glucan from Laetiporus sulphureus: A Pilot Study. Molecules 2020, 25, 5542. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Li, N.; Astmann, T.; Oh, J.-H.; van Pijkeren, J.-P.; Pan, X. Facile and Efficient Chemical Synthesis of Gluco-Oligosaccharides (Glcos) with Diverse Glycosidic Linkages as Potential Prebiotics to Promote the Growth of Probiotic Bacteria. Food Res. Int. 2023, 165, 112436. [Google Scholar] [CrossRef]
- Zeng, M.J.; Oh, J.H.; van Pijkeren, J.P.; Pan, X.J. Selective Utilization of Gluco-Oligosaccharides by Lactobacilli: A Mechanism Study Revealing the Impact of Glycosidic Linkages and Degree of Polymerization on Their Utilization. Int. J. Food Sci. 2024, 89, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Djouzi, Z.; Andlueux, C. Compared Effects of Three Oligosaccharides on Metabolism of Intestinal Microflora in Rats Inoculated with a Human Faecal Flora. Br. J. Nutr. 2007, 78, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective Stimulation of Bifidobacteria in the Human Colon by Oligofructose and Inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Deguchi, Y.; Matsumoto, K.; Kimura, M.; Onodera, N.; Yajima, T. Influence of Galactooligosaccharides on the Human Fecal Microflora. J. Nutr. Sci. Vitaminol. 1993, 39, 635–640. [Google Scholar] [CrossRef]
- Dumortier, V.; Brassart, C.; Bouquelet, S. Purification and properties of α-d-galactosidase from Bifidobacterium bifidum exhibiting a transgalactosylation reaction. Biotechnol. Appl. Biochem. 1994, 19, 341–354. [Google Scholar]
- Kittibunchakul, S.; Maischberger, T.; Domig, K.J.; Kneifel, W.; Nguyen, H.M.; Haltrich, D.; Nguyen, H. Fermentability of a Novel Galacto-Oligosaccharide Mixture by Lactobacillus spp. And Bifidobacterium spp. Molecules 2018, 23, 3352. [Google Scholar] [CrossRef] [PubMed]
- Cardelle-Cobas, A.; Corzo, N.; Olano, A.; Peláez, C.; Requena, T.; Avila, M. Galactooligosaccharides Derived from Lactose and Lactulose: Influence of Structure on Lactobacillus, Streptococcus and Bifidobacterium Growth. Int. J. Food Microbiol. 2011, 149, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kabel, M.A.; Kortenoeven, L.; Schols, H.A.; Voragen, A.G. In Vitro Fermentability of Differently Substituted Xylo-Oligosaccharides. J. Agric. Food Chem. 2002, 50, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.J.; Gibson, G.R.; Rastall, R.A. Carbohydrate Preferences of Bifidobacterium Species Isolated from the Human Gut. Curr. Issues Intest. Microbiol. 2003, 4, 71–75. [Google Scholar]
- Englyst, H.N.; Hay, S.; MacFarlane, G.T. Polysaccharide breakdown by mixed populations of human faecal bacteria. FEMS Microbiol. Lett. 1987, 95, 163–171. [Google Scholar] [CrossRef]
- Ho, A.L.; Kosik, O.; Lovegrove, A.; Charalampopoulos, D.; Rastall, R.A. In Vitro Fermentability of Xylo-Oligosaccharide and Xylo-Polysaccharide Fractions with Different Molecular Weights by Human Faecal Bacteria. Carbohyd. Polym. 2018, 179, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mendis, M.; Martens, E.C.; Simsek, S. How Fine Structural Differences of Xylooligosaccharides and Arabinoxylooligosaccharides Regulate Differential Growth of Bacteroides Species. J. Agric. Food Chem. 2018, 66, 8398–8405. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Goumy, V.; Leclerc, M.; Del’homme, C.; Bernalier-Donadille, A. Characterization of the Xylan-Degrading Microbial Community from Human Faeces. FEMS Microbiol. Ecol. 2007, 61, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Reuhs, B.L.; Cantu-Jungles, T.M.; Tuncil, Y.E.; Kaur, A.; Terekhov, A.; Martens, E.C.; Hamaker, B.R. Corn Arabinoxylan Has a Repeating Structure of Subunits of High Branch Complexity with Slow Gut Microbiota Fermentation. Carbohyd. Polym. 2022, 289, 119435. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, D.; Li, C.; Yu, W.; Xie, G.; Zhang, Y.; Lu, W. Therapeutic Potential and Mechanism of Functional Oligosaccharides in Inflammatory Bowel Disease: A Review. Food Sci. Hum. Wellness 2023, 12, 2135–2150. [Google Scholar] [CrossRef]
- Kobayashi, M.; Funane, K.; Oguma, T. Inhibition of Dextran and Mutan Synthesis by Cycloisomaltooligosaccharides. Biosci. Biotechnol. Biochem. 1995, 59, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Sorndech, W.; Nakorn, K.N.; Tongta, S.; Blennow, A. Isomalto-Oligosaccharides: Recent Insights in Production Technology and Their Use for Food and Medical Applications. LWT-Food Sci. Technol. 2018, 95, 135–142. [Google Scholar] [CrossRef]
- Kaplan, H.; Hutkins, R.W. Fermentation of Fructooligosaccharides by Lactic Acid Bacteria and Bifidobacteria. Appl. Environ. Microb. 2000, 66, 2682–2684. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; YongLie, C.; QianBing, W. Health Benefit Application of Functional Oligosaccharides. Carbohyd. Polym. 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Song, Y.; Wu, M.S.; Tao, G.; Lu, M.W.; Lin, J.; Huang, J.Q. Feruloylated Oligosaccharides and Ferulic Acid Alter Gut Microbiome to Alleviate Diabetic Syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef] [PubMed]
- Jana, U.K.; Kango, N.; Pletschke, B. Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation. Front. Nutr. 2021, 8, 670817. [Google Scholar] [CrossRef] [PubMed]
- Weninger, S.N.; Ding, A.E.L.; Browne, E.N.; Frost, M.; Schiro, G.; Laubitz, D.; Duca, F.A. Longitudinal Characterization of the Gut Microbiota in the Diabetic Zdsd Rat Model and Therapeutic Potential of Oligofructose. Metabolites 2023, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Li, P.; Chen, M.X.; Luo, Y.M.; Prabhakar, M.; Zheng, H.M.; He, Y.; Qi, Q.; Long, H.Y.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in Healthy Young Population. Sci. Rep. 2017, 7, 111789. [Google Scholar] [CrossRef]
- Du, L.; Li, Q.; Yi, H.; Kuang, T.; Tang, Y.; Fan, G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 149, 112839. [Google Scholar] [CrossRef]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef]
- Gao, Z.G.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J.P. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.; Jonkers, D.M.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Lin, S.L.; Zheng, B.D.; Cheung, P.C.K. Short-Chain Fatty Acids in Control of Energy Metabolism. Crit. Rev. Food Sci. 2018, 58, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behavior. Arch. Physiol. Biochem. 2018, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Li, J.P.; Liu, W.; Warda, M.; Cui, B.; Abd El-Aty, A.M. Oligosaccharides Derived from Lycium Barbarum Ameliorate Glycolipid Metabolism and Modulate the Gut Microbiota Community and the Faecal Metabolites in a Type 2 Diabetes Mouse Model: Metabolomic Bioinformatic Analysis. Food Funct. 2022, 13, 5416–5429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, W.; Ni, X.; Farag, M.A.; Capanoglu, E.; Zhao, C. Regulatory Mechanisms of the Green Alga Ulva Lactuca Oligosaccharide via the Metabolomics and Gut Microbiome in Diabetic Mice. Curr. Res. Food Sci. 2022, 5, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Osadchiy, V.; Mayer, E.A. Brain-Gut-Microbiome Interactions in Obesity and Food Addiction. Nat. Rev. Gastro Hepat. 2020, 17, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Long, J.; Yang, J.; Henning, S.M.; Woo, S.L.; Hsu, M.; Chan, B.; Heber, D.; Li, Z. Xylooligosaccharide Supplementation Decreases Visceral Fat Accumulation and Modulates Cecum Microbiome in Mice. J. Funct. Foods 2019, 52, 138–146. [Google Scholar] [CrossRef]
- Thiennimitr, P.; Yasom, S.; Tunapong, W.; Chunchai, T.; Wanchai, K.; Pongchaidecha, A.; Lungkaphin, A.; Sirilun, S.; Chaiyasut, C.; Chattipakorn, N.; et al. Lactobacillus Paracasei Hii01, Xylooligosaccharides, and Synbiotics Reduce Gut Disturbance in Obese Rats. Nutrition 2018, 54, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the Prebiotic Concept: Lessons from an Exploratory, Double Blind Intervention Study with Inulin-Type Fructans in Obese Women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Zhao, J.W.; Xie, F.; He, H.X.; Johnston, L.J.; Dai, X.F.; Wu, C.D.; Ma, X. Dietary Fiber-Derived Short-Chain Fatty Acids: A Potential Therapeutic Target to Alleviate Obesity-Related Nonalcoholic Fatty Liver Disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, M.; Tang, M.; Gao, C.; Wang, H.; Man, S.; Lu, F. Oligosaccharide and Short-Chain Fatty Acid: A Double-Edged Sword in Obese Mice by Regulating Food Intake and Fat Synthesis. Food Res. Int. 2022, 159, 111619. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Huang, X.; Cao, H.; Bai, Y.; Che, Q.; Nie, H.; Su, Z. Anti-Obesity Effects of Galacto-Oligosaccharides in Obese Rats. Eur. J. Pharmacol. 2022, 917, 174728. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan Oligosaccharide Attenuates Cognitive and Behavioral Disorders in the 5xfad Alzheimer’s Disease Mouse Model via Regulating the Gut Microbiota-Brain Axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Lun, W.; Zhou, J.; Bai, Y.; Che, Q.; Cao, H.; Guo, J.; Su, Z. Chitosan Oligosaccharide Activates Brown Adipose Tissue by Modulating the Gut Microbiota and Bile Acid Pathways Based on Faecal Microbiota Transplantation. J. Funct. Foods 2023, 108, 105731. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.M.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal Fxr Agonism Promotes Adipose Tissue Browning and Reduces Obesity and Insulin Resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile Acids in Glucose Metabolism in Health and Disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef]
- Calderon, G.; McRae, A.; Rievaj, J.; Davis, J.; Zandvakili, I.; Linker-Nord, S.; Burton, D.; Roberts, G.; Reimann, F.; Gedulin, B.; et al. Ileo-Colonic Delivery of Conjugated Bile Acids Improves Glucose Homeostasis via Colonic Glp-1-Producing Enteroendocrine Cells in Human Obesity and Diabetes. Ebiomedicine 2020, 55, 102759. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Feng, S.; Liu, A.B.; Wang, H.; Zeng, X.; Yang, C.S. Protective Effects of α-Galacto-Oligosaccharides against a High-Fat/Western-Style Diet-Induced Metabolic Abnormalities in Mice. Food Funct. 2019, 10, 3660–3670. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Liu, F.; Zhang, P.; Muhammad, Z.; Pan, S. Role of the Gut Microbiota and Their Metabolites in Modulating the Cholesterol-Lowering Effects of Citrus Pectin Oligosaccharides in C57bl/6 Mice. J. Agric. Food Chem. 2019, 67, 11922–11930. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Khan, I.; Lin, Z.; Zhang, J.; Lee, M.Y.S.; Leong, W.; Hsiao, W.L.W.; Zheng, Y. Fructo-Oligosaccharides from Morinda Officinalis Remodeled Gut Microbiota and Alleviated Depression Features in a Stress Rat Model. Phytomedicine 2020, 67, 153157. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the microbiota-gut-brain axis: Prebioticshave anxiolytic and antidepressant-like effects and reverse the impact of chronicstress in mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Campagnoli, L.I.M.; Chirumbolo, S.; Candiano, B.; Carrara, A.; Ricevuti, G.; Esposito, C.; Pascale, A. The Brain-Gut-Microbiota Interplay in Depression: A Key to Design Innovative Therapeutic Approaches. Pharmacol. Res. 2023, 192, 106799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Ling, Z.X.; Zhang, Y.H.; Mao, H.J.; Ma, Z.P.; Yin, Y.; Wang, W.H.; Tang, W.X.; Tan, Z.L.; Shi, J.F.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.P.; Lu, C.Z.; Huang, S.J.; Wu, X.Y. Chemical Components Isolated from the Roots of Morinda Officinalis. Chem. Nat. Compd. 2015, 51, 548–549. [Google Scholar] [CrossRef]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic Relationships of Butyrate-Producing Bacteria from the Human Gut. Appl. Environ. Microb. 2000, 66, 1654–1661. [Google Scholar] [CrossRef]
- Kunze, W.A.; Mao, Y.K.; Wang, B.X.; Huizinga, J.D.; Ma, X.L.; Forsythe, P.; Bienenstock, J. Lactobacillus Reuteri Enhances Excitability of Colonic Ah Neurons by Inhibiting Calcium-Dependent Potassium Channel Opening. J. Cell Mol. Med. 2009, 13, 2261–2270. [Google Scholar] [CrossRef]
- Li, X.S.; Ellis, M.L.; Knight, J. Oxalobacter Formigenes Colonization and Oxalate Dynamics in a Mouse Model. Appl. Environ. Microb. 2015, 81, 5048–5054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Han, Y.; Du, J.; Liu, R.Z.; Jin, K.T.; Yi, W. Microbiota-Gut-Brain Axis and the Central Nervous System. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. Mbio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.L.; Farzi, A.; Liu, Z.; Zhu, W.Y. Increasing Carbohydrate Availability in the Hindgut Promotes Hypothalamic Neurotransmitter Synthesis: Aromatic Amino Acids Linking the Microbiota-Brain Axis. J. Neurochem. 2019, 149, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of Gut Luminal Serotonin by Commensal Microbiota in Mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef]
- Min, Q.X.; Wang, Y.; Jin, T.C.; Zhu, L.; Wu, X.Y.; Li, Y.K.; Wang, Y.J.; Xu, N. Analysis of Intestinal Short-Chain Fatty Acid Metabolism Profile after Probiotics and Glp-1 Treatment for Type 2 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 892127. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Chiasson, J.L. Acarbose Raises Serum Butyrate in Human Subjects Withimpaired Glucose Tolerance. Br. J. Nutr. 2000, 84, 57–61. [Google Scholar] [CrossRef]
- Al-Khafaji, A.H.; Jepsen, S.D.; Christensen, K.R.; Vigsnæs, L.K. The Potential of Human Milk Oligosaccharides to Impact the Microbiota-Gut-Brain Axis through Modulation of the Gut Microbiota. J. Funct. Foods 2020, 74, 104176. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Guérin, S.; Coquery, N.; Nogret, I.; Formal, M.; Romé, V.; Le Normand, L.; Meurice, P.; Randuineau, G.; Guilloteau, P.; et al. Oral Sodium Butyrate Impacts Brain Metabolism and Hippocampal Neurogenesis, with Limited Effects on Gut Anatomy and Function in Pigs. FASEB J. 2018, 32, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The Intervention of Unique Plant Polysaccharides—Dietary Fiber on Depression from the Gut-Brain Axis. Int. J. Biol. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhou, X.; Tao, G.; Hao, W.; Wang, L.; Lan, Z.; Song, Y.; Wu, M.; Huang, J.Q. Ferulic Acid and Feruloylated Oligosaccharides Alleviate Anxiety and Depression Symptom via Regulating Gut Microbiome and Microbial Metabolism. Food Res. Int. 2022, 162, 111887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Gao, C.S.; Zhang, H.; Yang, J.; Wang, Y.P.; Pan, L.B.; Yu, H.; He, C.Y.; Luo, H.B.; Zhao, Z.X.; et al. Morinda Officinalis Oligosaccharides Increase Serotonin in the Brain and Ameliorate Depression via Promoting 5-Hydroxytryptophan Production in the Gut Microbiota. Acta Pharm. Sin. B 2022, 12, 3298–3312. [Google Scholar] [CrossRef]
- He, N.; Yang, Y.; Wang, H.; Liu, N.; Yang, Z.; Li, S. Unsaturated Alginate Oligosaccharides (UAOS) Protects against Dextran Sulfate Sodium-Induced Colitis Associated with Regulation of Gut Microbiota. J. Funct. Foods 2021, 83, 104536. [Google Scholar] [CrossRef]
- Tester, R.F.; Al-Ghazzewi, F.H. Beneficial Health Characteristics of Native and Hydrolysed Konjac (Amorphophallus konjac) Glucomannan. J. Sci. Food Agric. 2016, 96, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zheng, C.; Zheng, J.H.; Xu, S.C. The Relationship between Intestinal Flora Changes and Osteoporosis in Rats with Inflammatory Bowel Disease and the Improvement Effect of Probiotics. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5697–5702. [Google Scholar]
- Huang, G.; Ye, L.; Du, G.; Huang, Y.; Wu, Y.; Ge, S.; Yang, Z.; Zhu, G. Effects of Curcumin Plus Soy Oligosaccharides on Intestinal Flora of Rats with Ulcerative Colitis. Cell Mol. Biol. 2017, 63, 20–25. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Yong, Y.; Niu, X.; Gao, Y.; Zhou, Q.; Xie, H.; Liu, X.; Li, Y.; Yu, Z.; et al. Enhancing Gut Barrier Integrity: Upregulation of Tight Junction Proteins by Chitosan Oligosaccharide through the ERK1/2 Signaling Pathway. Nutrition 2024, 124, 112428. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 00277. [Google Scholar]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut Microbiota in Ulcerative Colitis: Insights on Pathogenesis and Treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Xia, B.; Liu, X.; Li, Z.; Ren, J.; Liu, X. The Effects of Microbiota-Targeted Approaches in Inflammatory Bowel Disease: Probiotics, Probiotic Foods, and Prebiotics. Curr. Opin. Food Sci. 2023, 49, 100956. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.J.; Chang, P.V. Microbial Tryptophan Metabolites Regulate Gut Barrier Function via the Aryl Hydrocarbon Receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef] [PubMed]
- Youssef, I.M.; Khalil, H.A.; Shakoori, A.M.; Bagadood, R.M.; Alyahyawi, A.Y.; Alhazzaa, R.A.; Fakiha, K.G.; Nasr, S.; Abo-Samra, M.A.; Hassan, M.S.; et al. Immune Response, Hematological Traits, Biochemical Blood Parameters, and Histological Status of Laying Hens Influenced by Dietary Chitosan- Oligosaccharides. Poult. Sci. 2023, 102, 102834. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.C.; Zhu, L.Q.; Lei, Z.W.; Song, Y.Q.; Tang, F.; Yin, Z.; Wang, J.; Huang, J.Q. Feruloylated Oligosaccharides Alleviate Dextran Sulfate Sodium-Induced Colitis In Vivo. J. Agric. Food Chem. 2019, 67, 9522–9531. [Google Scholar] [CrossRef] [PubMed]
- Suwannaporn, P.; Thepwong, K.; Tester, R.; Al-Ghazzewi, F.; Piggott, J.; Shen, N.; Chen, Z.; Chen, F.; Yang, J.; Zhang, D.; et al. Tolerance and Nutritional Therapy of Dietary Fibre from Konjac Glucomannan Hydrolysates for Patients with Inflammatory Bowel Disease (IBD). Bioact. Carbohydr. Diet. Fibre 2013, 2, 93–98. [Google Scholar] [CrossRef]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.F.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship between Microbiota of the Colonic Mucosa vs. Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients with Chronic Constipation. Gastroenterology 2016, 150, 367–379. [Google Scholar] [CrossRef]
- Kwon, J.I.; Park, Y.; Noh, D.O.; Suh, H.J.; Han, S.H. Complex-Oligosaccharide Composed of Galacto-Oligosaccharide and Lactulose Ameliorates Loperamide-Induced Constipation in Rats. Food Sci. Biotechnol. 2018, 27, 781–788. [Google Scholar] [CrossRef]
- Wang, L.; Pan, M.; Li, D.; Yin, Y.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Metagenomic Insights into the Effects of Oligosaccharides on the Microbial Composition of Cecal Contents in Constipated Mice. J. Funct. Foods 2017, 38, 486–496. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool Consistency Is Strongly Associated with Gut Microbiota Richness and Composition, Enterotypes and Bacterial Growth Rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lu, Y.H.; Lin, J.J.; Ko, L.Y. Effects of Isomalto-Oligosaccharides on Bowel Functions and Indicators of Nutritional Status in Constipated Elderly Men. J. Am. Coll. Nutr. 2001, 20, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Tseng, Y.H.; Kuo, Y.W.; Lee, M.C.; Chen, H.L. Long-Term Supplementation of Isomalto-Oligosaccharides Improved Colonic Microflora Profile, Bowel Function, and Blood Cholesterol Levels in Constipated Elderly People-a Placebo-Controlled, Diet-Controlled Trial. Nutrition 2011, 27, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Kong, M.; Cheng, X.; Lin, A.; Liu, H. Modulation of Gut Microbiota and Intestinal Metabolites by Lactulose Improves Loperamide-Induced Constipation in Mice. Eur. J. Pharm. Sci. 2021, 158, 105676. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Chen, J.; Miao, S.; Deng, K.; Liu, J.; Zeng, S.; Zheng, B.; Lu, X. Lotus Seed Oligosaccharides at Various Dosages with Prebiotic Activity Regulate Gut Microbiota and Relieve Constipation in Mice. Food Chem. Toxicol. 2019, 134, 110838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan Oligosaccharides Attenuate Loperamide-Induced Constipation through Regulation of Gut Microbiota in Mice. Carbohyd. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Yan, Q.; Li, Y.; Jiang, Z. Effect of Konjac Mannan Oligosaccharides on Diphenoxylate-Induced Constipation in Mice. J. Funct. Foods 2019, 57, 399–407. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, B.; Sun, G.; Zheng, J.; Hu, H.; Yang, H.; Cheng, X.; Lin, A.; Liu, H. Plasma Metabolomic Profiles Reveal Regulatory Effect of Chitosan Oligosaccharides on Loperamide-Induced Constipation in Mice. J. Pharm. Biomed. 2022, 211, 114590. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary Synbiotic Ameliorates Constipation through the Modulation of Gut Microbiota and Its Metabolic Function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.; Nuche-Berenguer, B.; Moreno, P.; Mantey, S.A.; Iordanskaia, T.; Jensen, R.T. Presence of the P21-activated kinase, PAK4, in pancreatic acinar cells and its activation by numerous gastrointestinal hormones and growth factors. Gastroenterology 2017, 152, S902. [Google Scholar] [CrossRef]
- Kendig, D.M.; Grider, J.R. Serotonin and Colonic Motility. Neurogastroenterol. Motil. 2015, 27, 899–905. [Google Scholar] [CrossRef] [PubMed]
| Name | Glucosidic Linkage | Monosaccharides | DP | Preparation | Natural Sources | References |
|---|---|---|---|---|---|---|
| FOS | β-1,2 | Fructose Glucose | 2–5 | Enzyme degradation, microbial Fermentation | Onions, chicory, wheat | [55,56] |
| IMO | α-1,3 α-1,4 α-1,6 | Glucose | 2–5 | Enzyme synthesis, enzymatic hydrolysis, enzymatic conversion | Starch, corn, milk, wheat, bran | [57,58] |
| XOS | β-1,4 | Xylose | 2–7 | Enzymatic conversion | Shoots, corn cob, wheat straw | [59,60] |
| MOS | α-1,2 α-1,6 | Mannose Glucose | 2–10 | Enzymatic hydrolysis | Carrageenan konjac | [57,61] |
| GOS | β-1,4 β-1,6 | Galactose Glucose | 3–6 | Enzymatic conversion | Lactose | [55,62] |
| SOS | α-1,2 α-1,6 | Galactose, Glucose Fructose | 2–5 | Extracted | Soybean | [57] |
| COS | β-1,4 | D-glucosamine | — | Enzymatic hydrolysis | Shell of crustaceans | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Zhang, Z.; Liu, T.; Feng, H.; Liu, Y.; Chen, K. The Role of Nondigestible Oligosaccharides in Alleviating Human Chronic Diseases by Regulating the Gut Microbiota: A Review. Foods 2024, 13, 2157. https://doi.org/10.3390/foods13132157
Yuan M, Zhang Z, Liu T, Feng H, Liu Y, Chen K. The Role of Nondigestible Oligosaccharides in Alleviating Human Chronic Diseases by Regulating the Gut Microbiota: A Review. Foods. 2024; 13(13):2157. https://doi.org/10.3390/foods13132157
Chicago/Turabian StyleYuan, Meiyu, Zhongwei Zhang, Tongying Liu, Hua Feng, Yuhuan Liu, and Kai Chen. 2024. "The Role of Nondigestible Oligosaccharides in Alleviating Human Chronic Diseases by Regulating the Gut Microbiota: A Review" Foods 13, no. 13: 2157. https://doi.org/10.3390/foods13132157
APA StyleYuan, M., Zhang, Z., Liu, T., Feng, H., Liu, Y., & Chen, K. (2024). The Role of Nondigestible Oligosaccharides in Alleviating Human Chronic Diseases by Regulating the Gut Microbiota: A Review. Foods, 13(13), 2157. https://doi.org/10.3390/foods13132157










