Continuous Blue Light Treatment Enhances the Nutritional Value of Hydroponically Grown Eruca vesicaria L. by Improving Ascorbic Acid Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Light Treatment
2.3. Plant Measurements and Compositional Analysis
2.4. Ascorbic Acid Assay
2.5. Non-Structural Carbohydrates Assay
2.6. Pigments Assay
2.7. Total Anthocyanins and Total Phenolic Assay
2.8. Anions Assay
2.9. Ascorbic Acid Biosynthetic and Recycling Enzyme Activity Assay
2.10. Statistical Analysis
3. Results
3.1. Biomass Assay
3.2. Ascorbic Acid Content
3.3. Non-Structural Carbohydrates Content
3.4. Pigments, Total Anthocyanin, and Total Phenolic Content
3.5. Inorganic Anions and Organic Acids Content
3.6. Ascorbic Acid Biosynthetic and Recycling Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-Emitting Diodes in Horticulture. Hortic. Rev. 2015, 43, 1–88. [Google Scholar] [CrossRef]
- Baghalian, K.; Hajirezaei, M.-R.; Lawson, T. Editorial: Current and future perspectives for controlled environment agriculture (CEA) in the 21st century. Front. Plant Sci. 2023, 14, 1334641. [Google Scholar] [CrossRef] [PubMed]
- Morrow, R.C. LED Lighting in Horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Hytönen, T.; Pinho, P.; Rantanen, M.; Kariluoto, S.; Lampi, A.; Edelmann, M.; Joensuu, K.; Kauste, K.; Mouhu, K.; Piironen, V.; et al. Effects of LED light spectra on lettuce growth and nutritional composition. Light. Res. Technol. 2018, 50, 880–893. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous Lighting Promotes Plant Growth, Light Conversion Efficiency, and Nutritional Quality of Eruca vesicaria (L.) Cav. in Controlled Environment with Minor Effects Due to Light Quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of Pre-Harvest Supplemental UV-A/Blue and Red/Blue LED Lighting on Lettuce Growth and Nutritional Quality. Horticulturae 2021, 7, 80. [Google Scholar] [CrossRef]
- Wanlai, Z.; Wenke, L.; Qichang, Y. Reducing Nitrate Content in Lettuce by Pre-Harvest Continuous Light Delivered by Red and Blue Light-Emitting Diodes. J. Plant Nutr. 2013, 36, 481–490. [Google Scholar] [CrossRef]
- Liu, L.; Zabaras, D.; Bennett, L.; Aguas, P.; Woonton, B. Effects of UV-C, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chem. 2009, 115, 495–500. [Google Scholar] [CrossRef]
- Perera, W.P.T.D.; Navaratne, S.; Wickramasinghe, I. Impact of spectral composition of light from light-emitting diodes (LEDs) on postharvest quality of vegetables: A review. Postharvest Biol. Technol. 2022, 191, 111955. [Google Scholar] [CrossRef]
- Sysoeva, M.I.; Markovskaya, E.F.; Shibaeva, T.G. Plants under Continuous Light: A Review. Plant Stress 2010, 4, 5–17. [Google Scholar]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, I.; Terskov, I.; Kovrov, B.; Sidko, F.; Lisovsky, G.; Okladnikov, Y.; Belyanin, V.; Trubachov, I.; Rerberg, M. Life support system with autonomous control employing plant photosynthesis. Acta Astronaut. 1976, 3, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED Light Proportion Affects Vegetative Parameters, Pigment Content, and Oxidative Status of Einkorn (Triticum monococcum L. ssp. monococcum) Wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Goto, E. Effects of Light Quality on Growth of Crop Plants under Artificial Lighting. Environ. Control Biol. 2003, 41, 121–132. [Google Scholar] [CrossRef]
- Benincasa, P.; Tosti, G.; Farneselli, M.; Maranghi, S.; Bravi, E.; Marconi, O.; Falcinelli, B.; Guiducci, M. Phenolic content and antioxidant activity of einkorn and emmer sprouts and wheatgrass obtained under different radiation wavelengths. Ann. Agric. Sci. 2020, 65, 68–76. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė-Kairienė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P.; et al. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of Light Quality on Growth and Vegetable Quality in Leaf Lettuce, Spinach and Komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Marques, N.C.; Semedo, J.N.; Matos, M.C.; Quartin, V.L. Photosynthetic Performance and Pigment Composition of Leaves from two Tropical Species is Determined by Light Quality. Plant Biol. 2002, 4, 112–120. [Google Scholar] [CrossRef]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of Vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar] [CrossRef]
- Amitrano, C.; Paglialunga, G.; Battistelli, A.; De Micco, V.; Del Bianco, M.; Liuzzi, G.; Moscatello, S.; Paradiso, R.; Proietti, S.; Rouphael, Y.; et al. Defining growth requirements of microgreens in space cultivation via biomass production, morpho-anatomical and nutritional traits analysis. Front. Plant Sci. 2023, 14, 1190945. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; El Nakhel, C.; Rouphael, Y.; Proietti, S.; Paglialunga, G.; Moscatello, S.; Battistelli, A.; Iovane, M.; Romano, L.E.; De Pascale, S.; et al. Applying productivity and phytonutrient profile criteria in modelling species selection of microgreens as Space crops for astronaut consumption. Front. Plant Sci. 2023, 14, 1210566. [Google Scholar] [CrossRef] [PubMed]
- Paglialunga, G.; El Nakhel, C.; Proietti, S.; Moscatello, S.; Battistelli, A.; Formisano, L.; Ciriello, M.; Del Bianco, M.; De Pascale, S.; Rouphael, Y. Substrate and fertigation management modulate microgreens production, quality and resource efficiency. Front. Sustain. Food Syst. 2023, 7, 1222914. [Google Scholar] [CrossRef]
- Liu, W.; Zha, L.; Zhang, Y. Growth and Nutrient Element Content of Hydroponic Lettuce are Modified by LED Continuous Lighting of Different Intensities and Spectral Qualities. Agronomy 2020, 10, 1678. [Google Scholar] [CrossRef]
- Kang, C.H.; Yoon, E.K.; Muthusamy, M.; Kim, J.A.; Jeong, M.-J.; Lee, S.I. Blue LED light irradiation enhances L-ascorbic acid content while reducing reactive oxygen species accumulation in Chinese cabbage seedlings. Sci. Hortic. 2020, 261, 108924. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Z.; Liu, W.; Bian, Z.; Lu, W.; Zhou, B.; Wang, S.; Li, Q.; Yang, Q. High-Proportion Blue Light Irradiation at the End-of-Production Stage Promotes the Biosynthesis and Recycling of Ascorbate in Lettuce. Int. J. Mol. Sci. 2023, 24, 16524. [Google Scholar] [CrossRef] [PubMed]
- Mainos, D.; Bantis, F.; Ntinas, G.K.; Koukounaras, A. Yield, Quality, and Resources Use Efficiency of Wild Rocket Baby Leaves Grown under Different Controlled Environment Systems and Various Growing Seasons. Horticulturae 2023, 9, 661. [Google Scholar] [CrossRef]
- Zabel, P.; Zeidler, C.; Vrakking, V.; Dorn, M.; Schubert, D. Biomass Production of the EDEN ISS Space Greenhouse in Antarctica During the 2018 Experiment Phase. Front. Plant Sci. 2020, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. 1997–2018. n.d. Available online: https://imagej.net/ij/ (accessed on 10 June 2024).
- Scartazza, A.; Moscatello, S.; Gavrichkova, O.; Buia, M.C.; Lauteri, M.; Battistelli, A.; Lorenti, M.; Garrard, S.L.; Calfapietra, C.; Brugnoli, E. Carbon and nitrogen allocation strategy in Posidonia oceanica is altered by seawater acidification. Sci. Total Environ. 2017, 607–608, 954–964. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Long-term Continuous Light Provided by Red and Blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Engler, N.; Krarti, M. Review of energy efficiency in controlled environment agriculture. Renew. Sustain. Energy Rev. 2021, 141, 110786. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of Ascorbate Accumulation and Metabolism in Lettuce by the Red:Blue Ratio of Continuous Light Using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Gallie, D.R. Increasing Vitamin C Content in Plant Foods to Improve Their Nutritional Value—Successes and Challenges. Nutrients 2013, 5, 3424–3446. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic Acid in Plants: Biosynthesis and Function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Kitaya, Y.; Niu, G.; Kozai, T.; Ohashi, M. Photosynthetic Photon Flux, Photoperiod, and CO2 Concentration Affect Growth and Morphology of Lettuce Plug Transplants. HortScience 1998, 33, 988–991. [Google Scholar] [CrossRef]
- Globig, S.; Rosen, I.; Janes, H.W. Continuous Light Effects on Photosynthesis and Carbon Metabolism in Tomato. Acta Hortic. 1997, 418, 141–152. [Google Scholar] [CrossRef]
- Wheeler, R.M.; Tibbitts, T.W. Growth and Tuberization of Potato (Solanum tuberosum L.) under Continuous Light. Plant Physiol. 1986, 80, 801–804. [Google Scholar] [CrossRef]
- Yeh, N.; Chung, J.-P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.; Samy, P.; Muneer, S. Light Emitting Diodes (LEDs) as Agricultural Lighting: Impact and Its Potential on Improving Physiology, Flowering, and Secondary Metabolites of Crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Roelfsema, M.R.G.; Hedrich, R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Brown, C.S. Root-Shoot Interaction in the Greening of Wheat Seedlings Grown under Red Light. Plant Physiol. 1995, 107, 407–411. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.; Tripathy, B.C. Photoregulation of the Greening Process of Wheat Seedlings Grown in Red Light*. Plant Mol. Biol. 2005, 59, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, W.; Pan, Q.; Zeng, Q.; Yan, C.; Bai, X.; Liu, Y.; Zhang, L.; Li, B. Effects of long-term blue light irradiation on carotenoid biosynthesis and antioxidant activities in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food Res. Int. 2023, 174, 113661. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Stutte, G.W.; Edney, S.; Skerritt, T. Photoregulation of Bioprotectant Content of Red Leaf Lettuce with Light-emitting Diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef]
- Dougher, T.A.; Bugbee, B. Long-term Blue Light Effects on the Histology of Lettuce and Soybean Leaves and Stems. J. Am. Soc. Hortic. Sci. 2004, 129, 467–472. [Google Scholar] [CrossRef]
- Folta, K.M.; Lieg, E.J.; Durham, T.; Spalding, E.P. Primary Inhibition of Hypocotyl Growth and Phototropism Depend Differently on Phototropin-Mediated Increases in Cytoplasmic Calcium Induced by Blue Light. Plant Physiol. 2003, 133, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Appelgren, M. Effects of light quality on stem elongation of Pelargonium in vitro. Sci. Hortic. 1991, 45, 345–351. [Google Scholar] [CrossRef]
- Maruta, T. How does light facilitate vitamin C biosynthesis in leaves? Biosci. Biotechnol. Biochem. 2022, 86, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.G.; Yu, J.; Gómez, F.; Fernández, L.; McIntosh, L.; Foyer, C.H. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Bournonville, C.; Mori, K.; Deslous, P.; Decros, G.; Blomeier, T.; Mauxion, J.-P.; Jorly, J.; Gadin, S.; Cassan, C.; Maucourt, M.; et al. Blue light promotes ascorbate synthesis by deactivating the PAS/LOV photoreceptor that inhibits GDP-L-galactose phosphorylase. Plant Cell 2023, 35, 2615–2634. [Google Scholar] [CrossRef]
- Miyake, C.; Asada, K. Ferredoxin-Dependent Photoreduction of the Monodehydroascorbate Radical in Spinach Thylakoids. Plant Cell Physiol. 1994, 35, 539–549. [Google Scholar] [CrossRef]
- Morell, S.; Follmann, H.; De Tullio, M.; Häberlein, I. Dehydroascorbate and dehydroascorbate reductase are phantom indicators of oxidative stress in plants. FEBS Lett. 1997, 414, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Lester, G.E. Environmental Regulation of Human Health Nutrients (Ascorbic Acid, β-Carotene, and Folic Acid) in Fruits and Vegetables. HortScience 2006, 41, 59–64. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.S.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K.-I. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Talbott, L.D.; Zeiger, E. Sugar and Organic Acid Accumulation in Guard Cells of Vicia faba in Response to Red and Blue Light. Plant Physiol. 1993, 102, 1163–1169. [Google Scholar] [CrossRef]
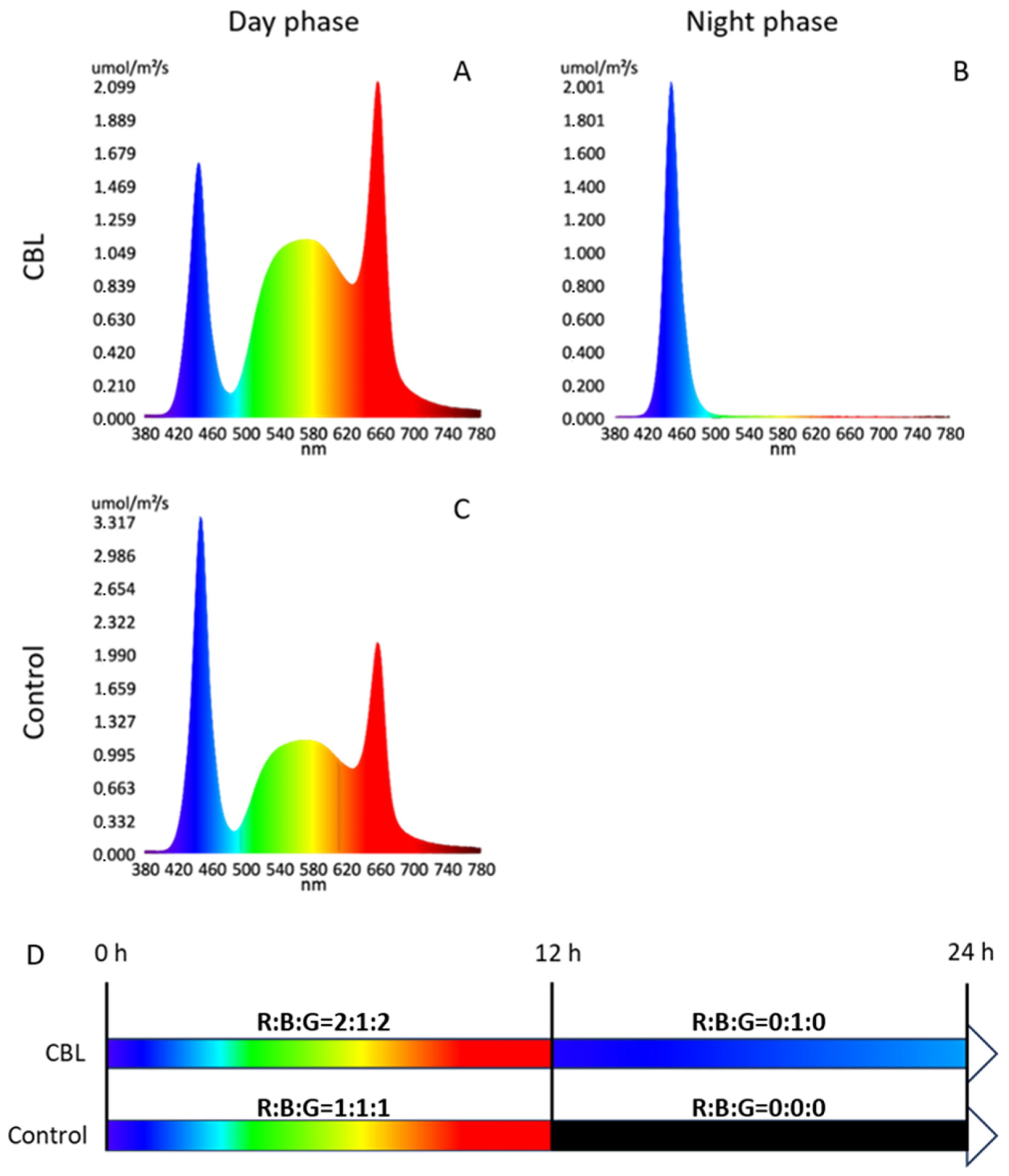

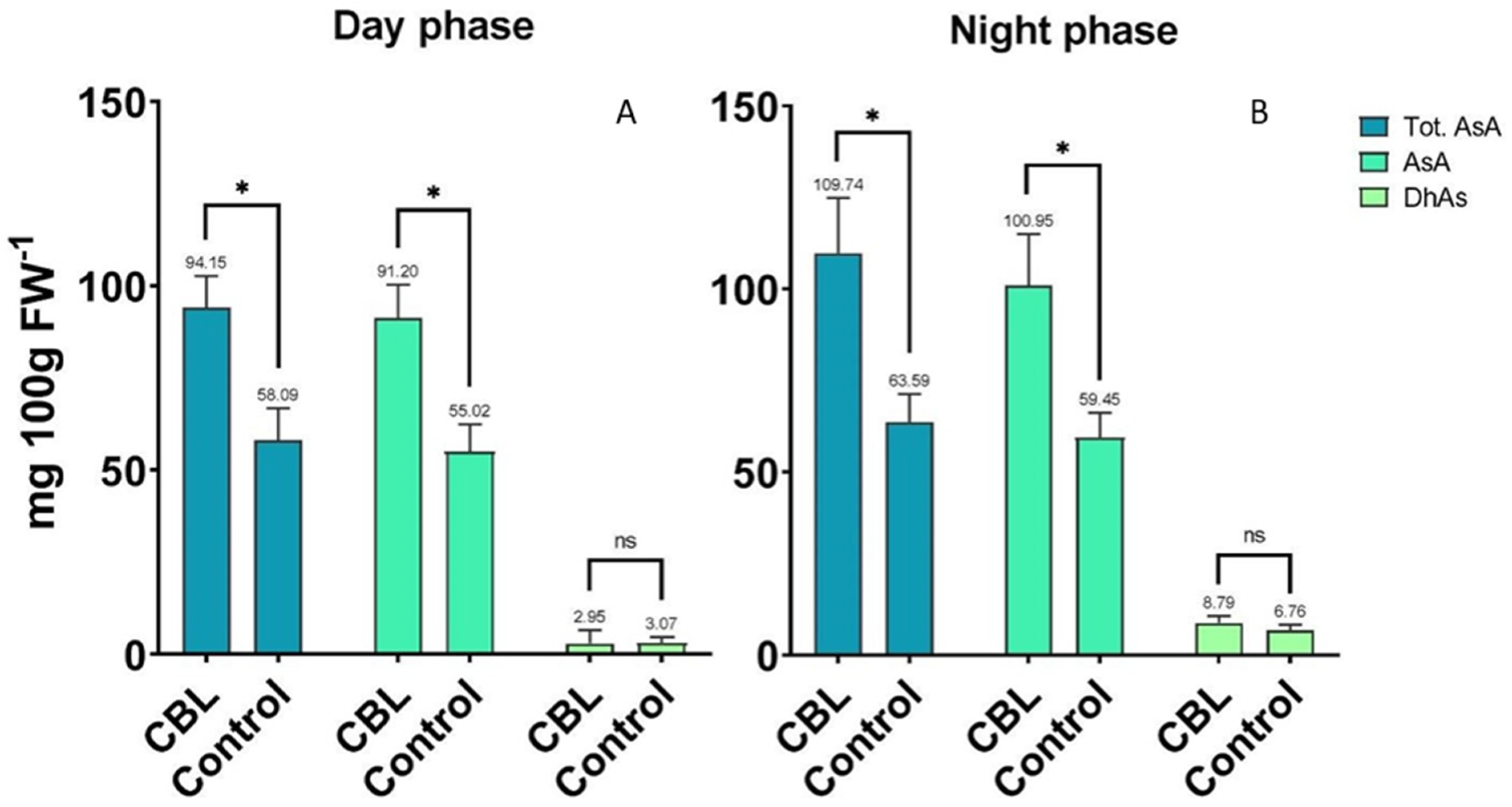
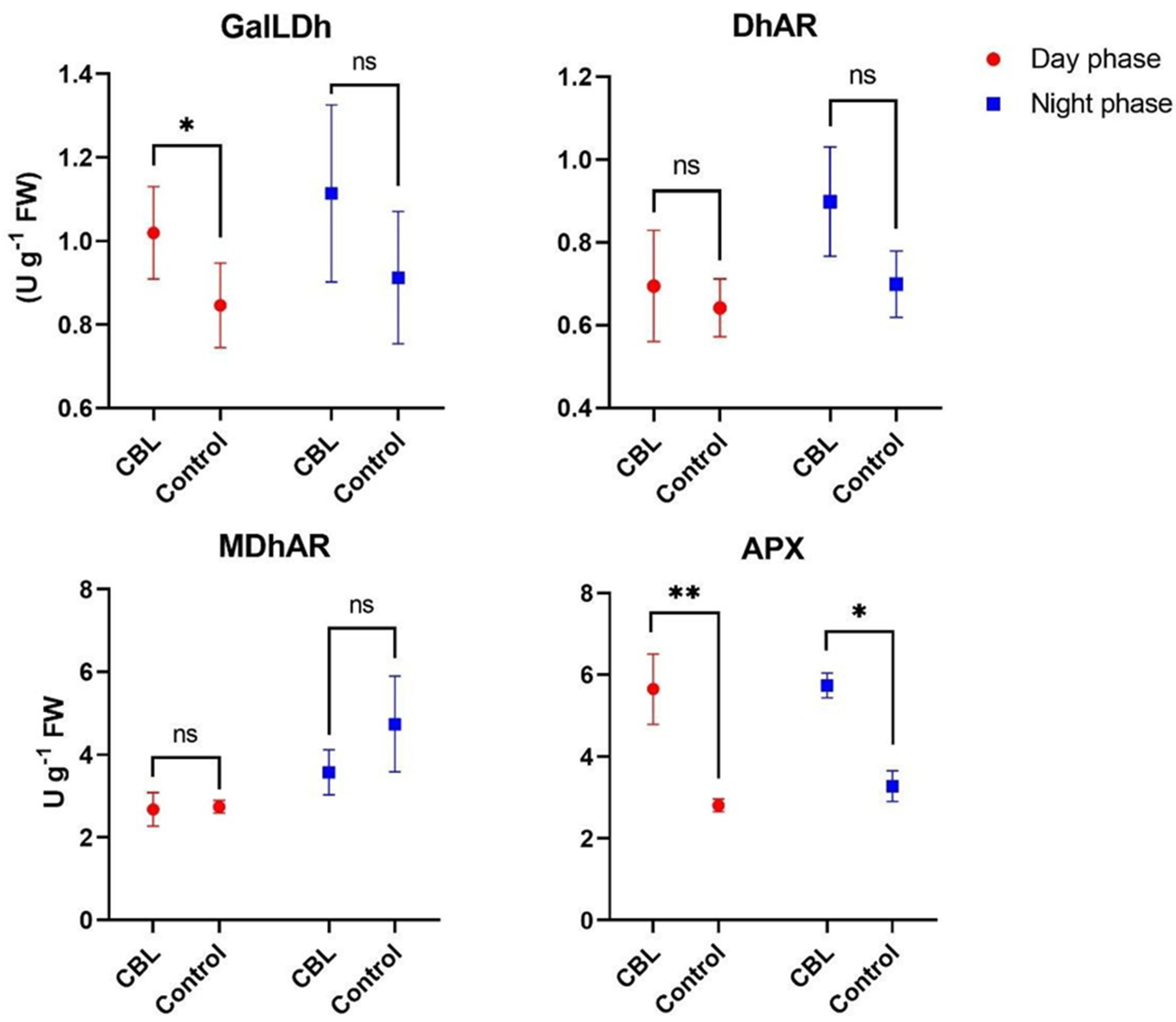
| Fresh Weight | Dry Weight | Dry Matter | SLDW | Ash | LAI | |
|---|---|---|---|---|---|---|
| (g/m2) | (g/m2) | (%) | (g/m2) | (% DW) | ||
| CBL | 3471.06 ± 302 | 239.17 ± 22.2 | 6.86 ± 0.09 | 40.00 ± 2.6 | 7.07 ± 0.39 | 7.43 ± 0.70 |
| Control | 3111.47 ± 208 | 193.51 ± 11.9 | 6.24 ± 0.05 | 33.36 ± 1.3 | 7.80 ± 1.16 | 6.53 ± 0.59 |
| Sign. | ns | ns | *** | * | ns | ns |
| Glu | Fru | Suc | Starch | Tot. sol | Tot. NSC | |
|---|---|---|---|---|---|---|
| (% DW) | ||||||
| CBL | 12.48 ± 2.6 | 1.85 ± 0.52 | 7.65 ± 0.43 | 5.56 ± 0.38 | 21.98 ± 3.4 | 27.53 ± 3.7 |
| Control | 10.45 ± 2.9 | 1.52 ± 0.64 | 6.73 ± 0.51 | 5.83 ± 0.32 | 18.70 ± 3.6 | 24.53 ± 3.8 |
| Sign. | ns | ns | ns | ns | ns | ns |
| Chl a | Chl b | Tot. Chl | Chla/Chlb | β-Car | Lut | Neo | Vio | Tot. Ant | TPC | |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg 100 g−1 FW) | ||||||||||
| CBL | 93.0 ± 5.0 | 33.8 ± 1.9 | 126.9 ± 7.0 | 2.7 ± 0.16 | 6.6 ± 0.66 | 9.0 ± 0.44 | 3.0 ± 0.24 | 102.4 ± 7.3 | 0.89 ± 0.08 | 105 ± 7.9 |
| Control | 77.9 ± 3.0 | 28.6 ± 1.1 | 106.5 ± 4.1 | 2.7 ± 0.16 | 6.0 ± 0.24 | 7.6 ± 0.33 | 2.6 ± 0.13 | 93.7 ± 6.6 | 0.77 ± 0.05 | 88.2 ± 4.3 |
| Sign. | * | * | * | ns | ns | * | ns | ns | ns | ns |
| Nitrate | Sulfate | Phosphate | Malate | Citrate | |
|---|---|---|---|---|---|
| (ppm) | (ppm) | (ppm) | (mg 100 g FW−1) | (mg 100 g FW−1) | |
| CBL | 4929.7 ± 293.1 | 1010.6 ± 74.2 | 1047.4 ± 74.3 | 470.6 ± 47.5 | 71.3 ± 8.7 |
| Control | 4502.0 ± 247.4 | 1102.2 ± 77.0 | 1316.8 ± 325.8 | 228.0 ± 16.9 | 124.2 ± 19.1 |
| Sign. | ns | ns | ns | *** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paglialunga, G.; Moscatello, S.; Battistelli, A.; Mattioni, M.; Del Bianco, M.; Proietti, S. Continuous Blue Light Treatment Enhances the Nutritional Value of Hydroponically Grown Eruca vesicaria L. by Improving Ascorbic Acid Biosynthesis. Foods 2024, 13, 2141. https://doi.org/10.3390/foods13132141
Paglialunga G, Moscatello S, Battistelli A, Mattioni M, Del Bianco M, Proietti S. Continuous Blue Light Treatment Enhances the Nutritional Value of Hydroponically Grown Eruca vesicaria L. by Improving Ascorbic Acid Biosynthesis. Foods. 2024; 13(13):2141. https://doi.org/10.3390/foods13132141
Chicago/Turabian StylePaglialunga, Gabriele, Stefano Moscatello, Alberto Battistelli, Michele Mattioni, Marta Del Bianco, and Simona Proietti. 2024. "Continuous Blue Light Treatment Enhances the Nutritional Value of Hydroponically Grown Eruca vesicaria L. by Improving Ascorbic Acid Biosynthesis" Foods 13, no. 13: 2141. https://doi.org/10.3390/foods13132141
APA StylePaglialunga, G., Moscatello, S., Battistelli, A., Mattioni, M., Del Bianco, M., & Proietti, S. (2024). Continuous Blue Light Treatment Enhances the Nutritional Value of Hydroponically Grown Eruca vesicaria L. by Improving Ascorbic Acid Biosynthesis. Foods, 13(13), 2141. https://doi.org/10.3390/foods13132141







