Effect of Tea Tree Essential Oil on the Quality, Antioxidant Activity, and Microbiological Safety of Lightly Processed Lily (Lilium brownii var. viridulum) during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Tree Essential Oil and Reagents
2.2. Plant Material and Pretreatment
2.3. Sensory Evaluation and Appearance Quality Assessment
2.4. Determination of Respiration Rate, Weight Loss Rate, and Firmness
2.5. Determination of Nutrient Content
2.5.1. Determination of Starch and Reducing Sugar Content
2.5.2. Determination of Soluble Protein Content
2.5.3. Determination of Vitamin C (Vc) Content
2.5.4. Determination of Total Phenolic Content
2.6. Determination of ROS Content
2.7. Determination of Electrolyte Leakage
2.8. Determination of Malondialdehyde (MDA) Content and Lipoxygenase (LOX) Activity
2.9. Determination of Antioxidant Enzyme Activity
2.10. Determination of Antioxidant Activity
2.11. Total Plate Counts, Molds, and Yeasts Content Determination
2.12. Data Analysis
3. Results and Discussion
3.1. Quality-Related Parameters of Lightly Processed Lily
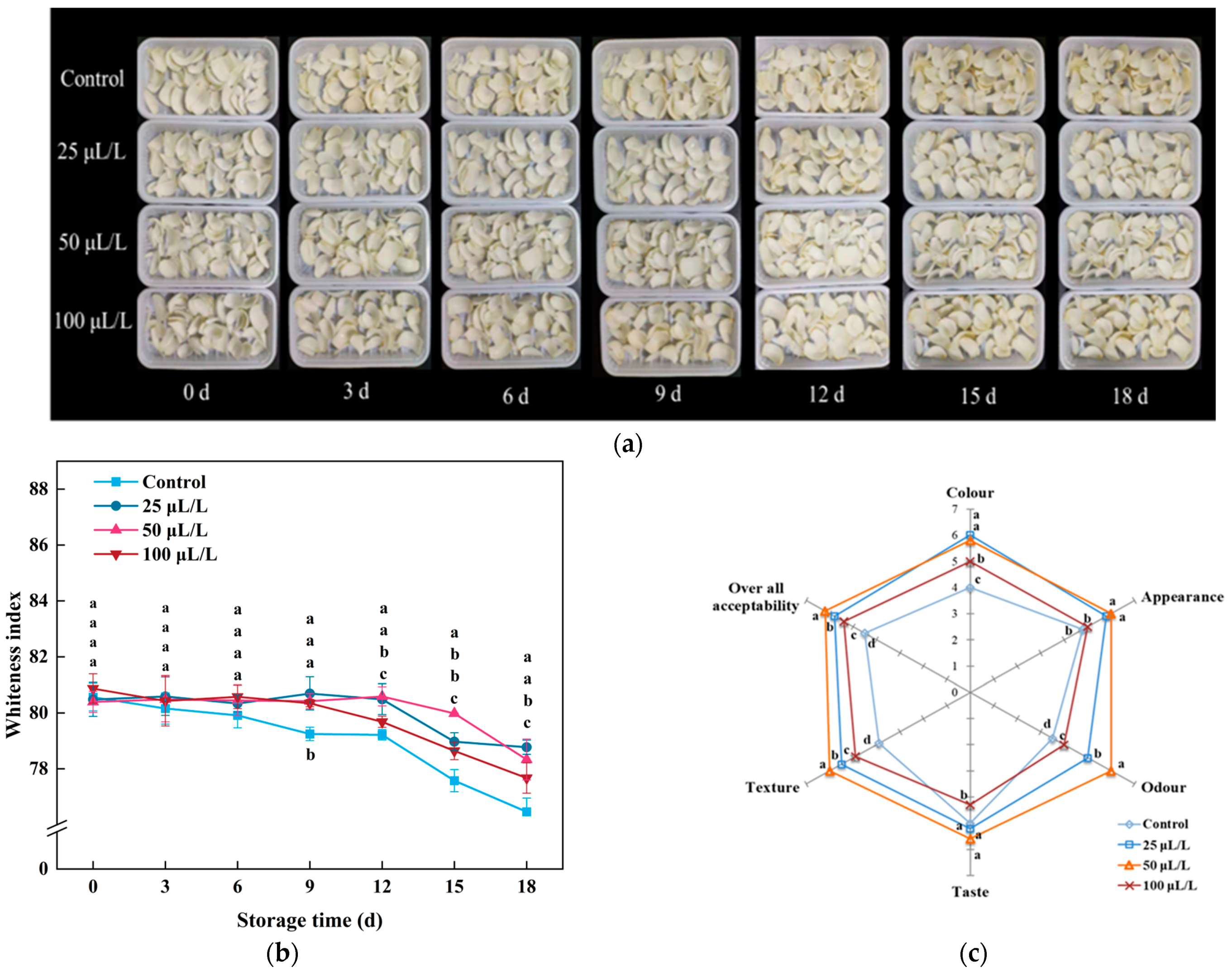
3.1.1. Appearance and Sensory Evaluation
3.1.2. Respiration Rate, Weight Loss Rate, Firmness
3.1.3. Nutrient Content
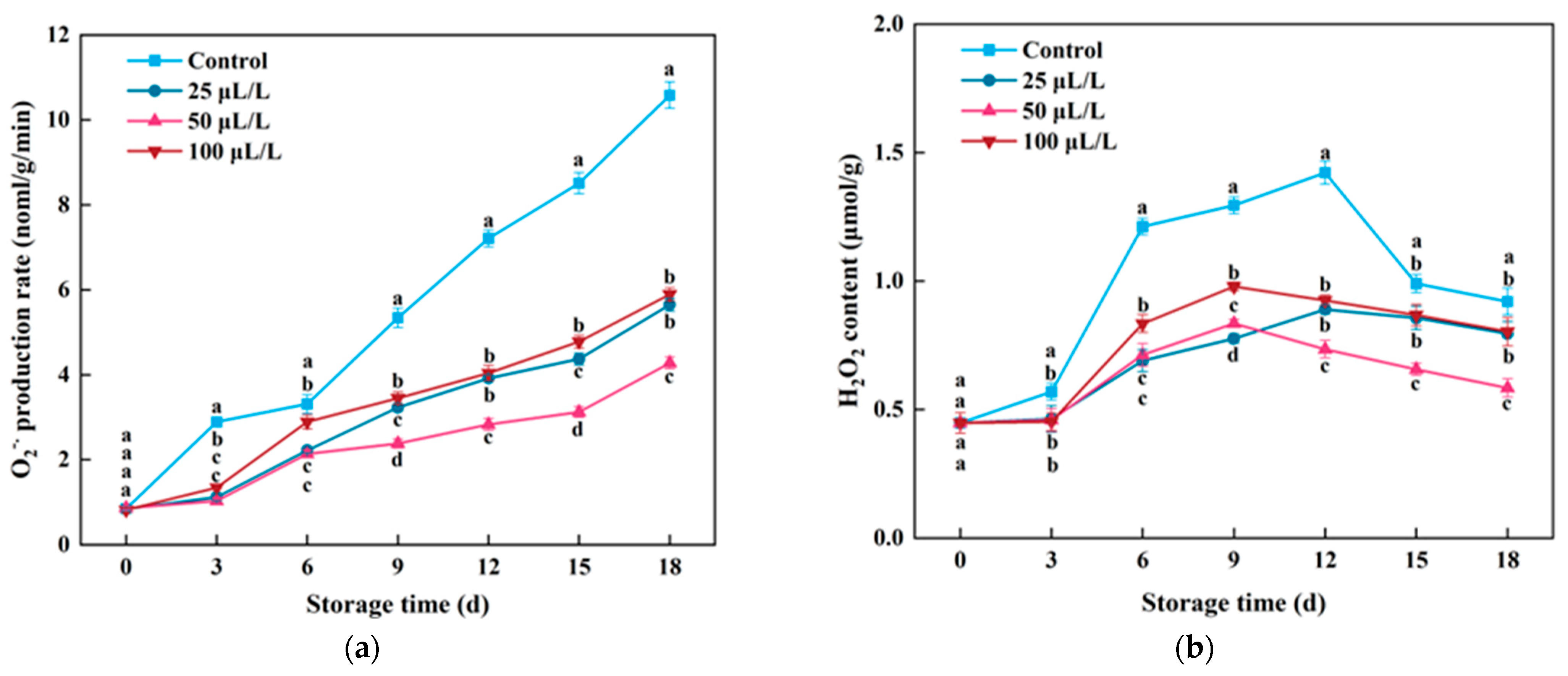
3.2. ROS Content
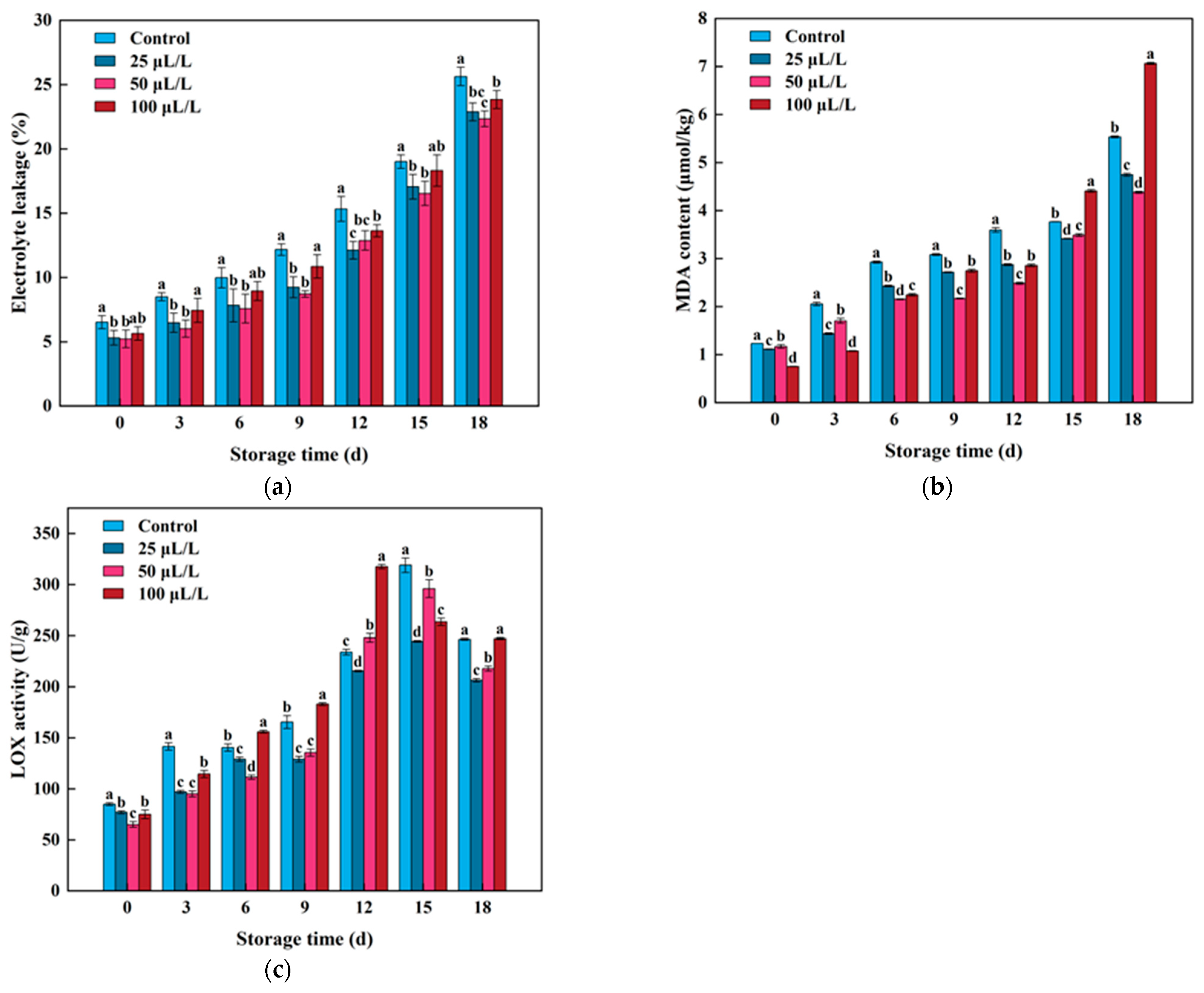
3.3. Key Metabolites and Enzyme Activity in the Pathway of Membrane Lipid Peroxidation
3.4. Vitamin C Content and APX Activity
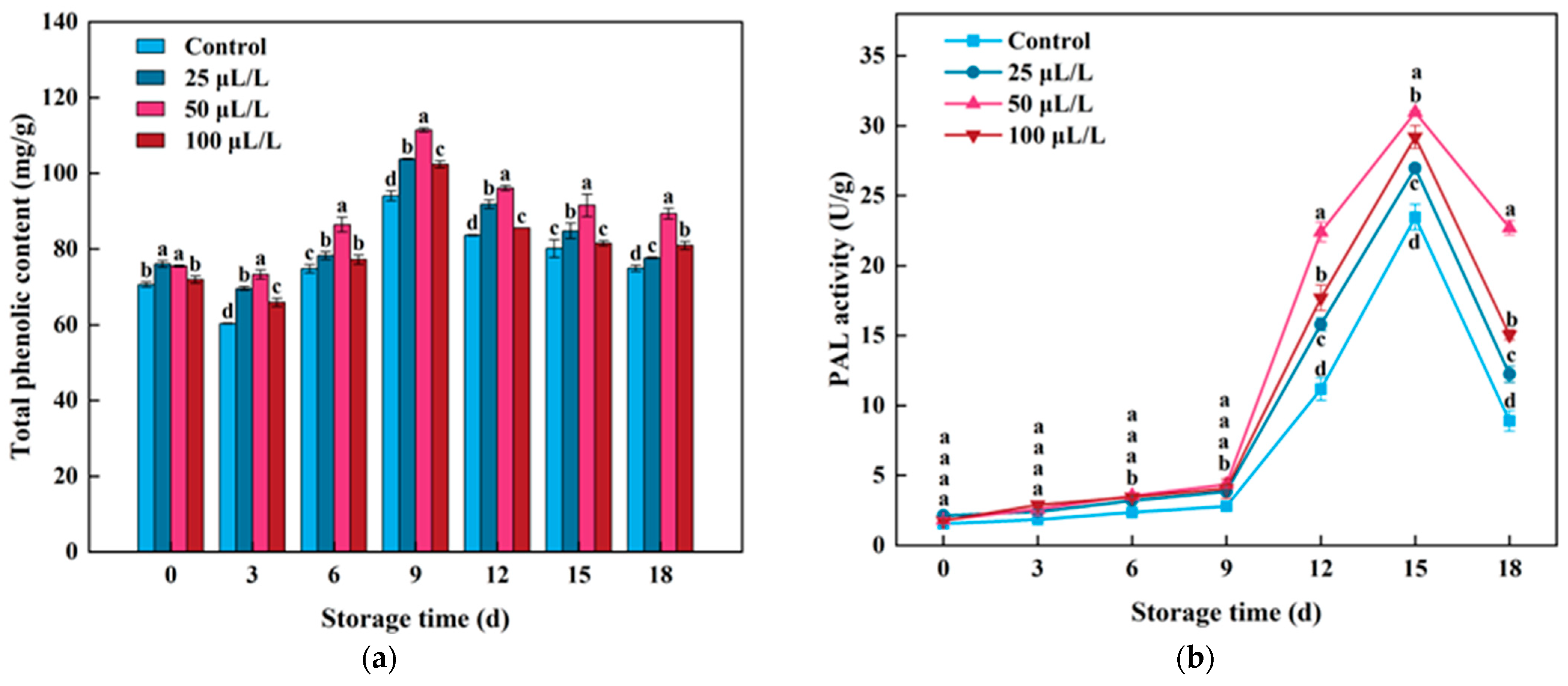
3.5. Total Phenolic Content and PAL Enzyme Activity
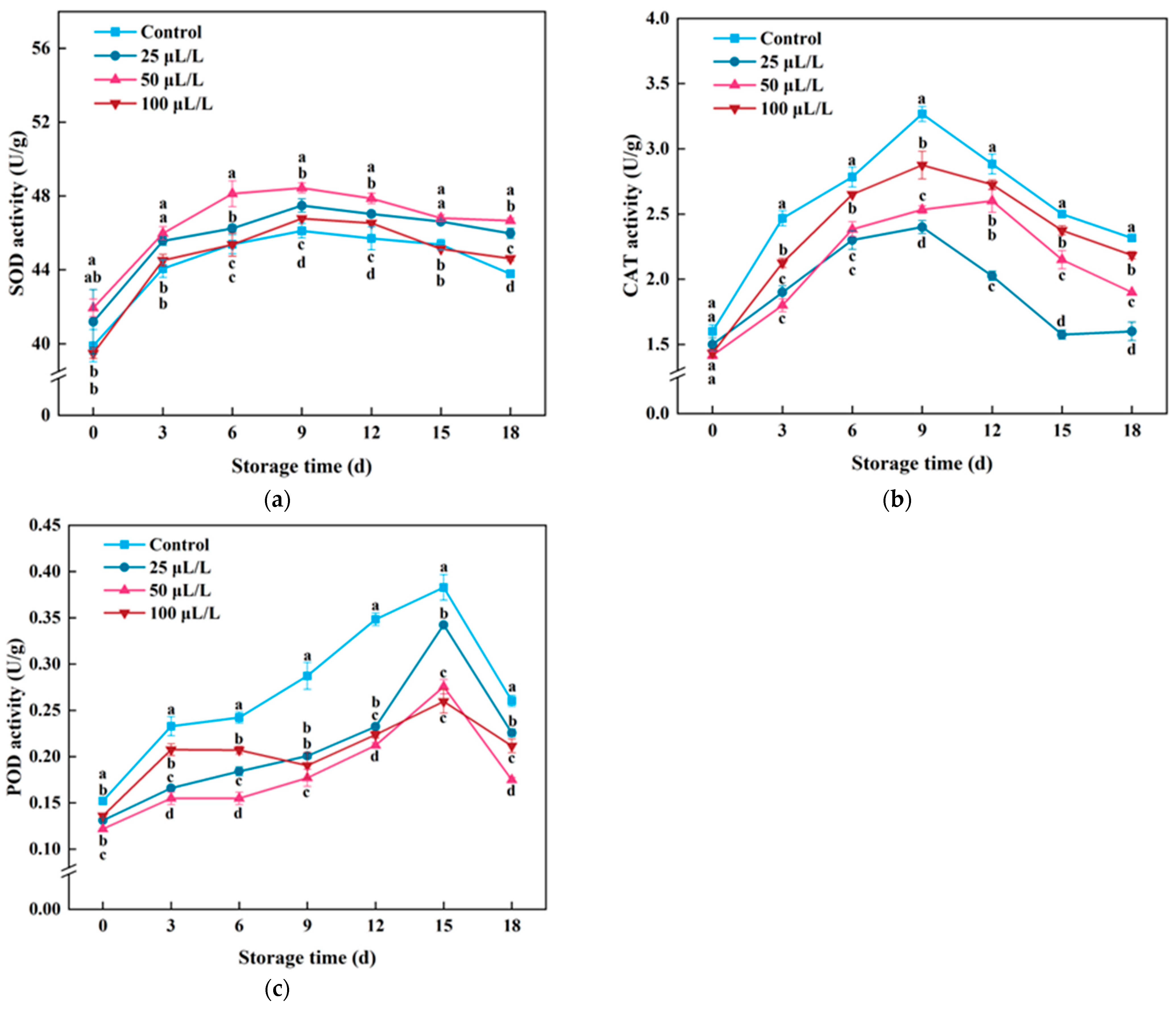
3.6. Antioxidant Enzyme Activity
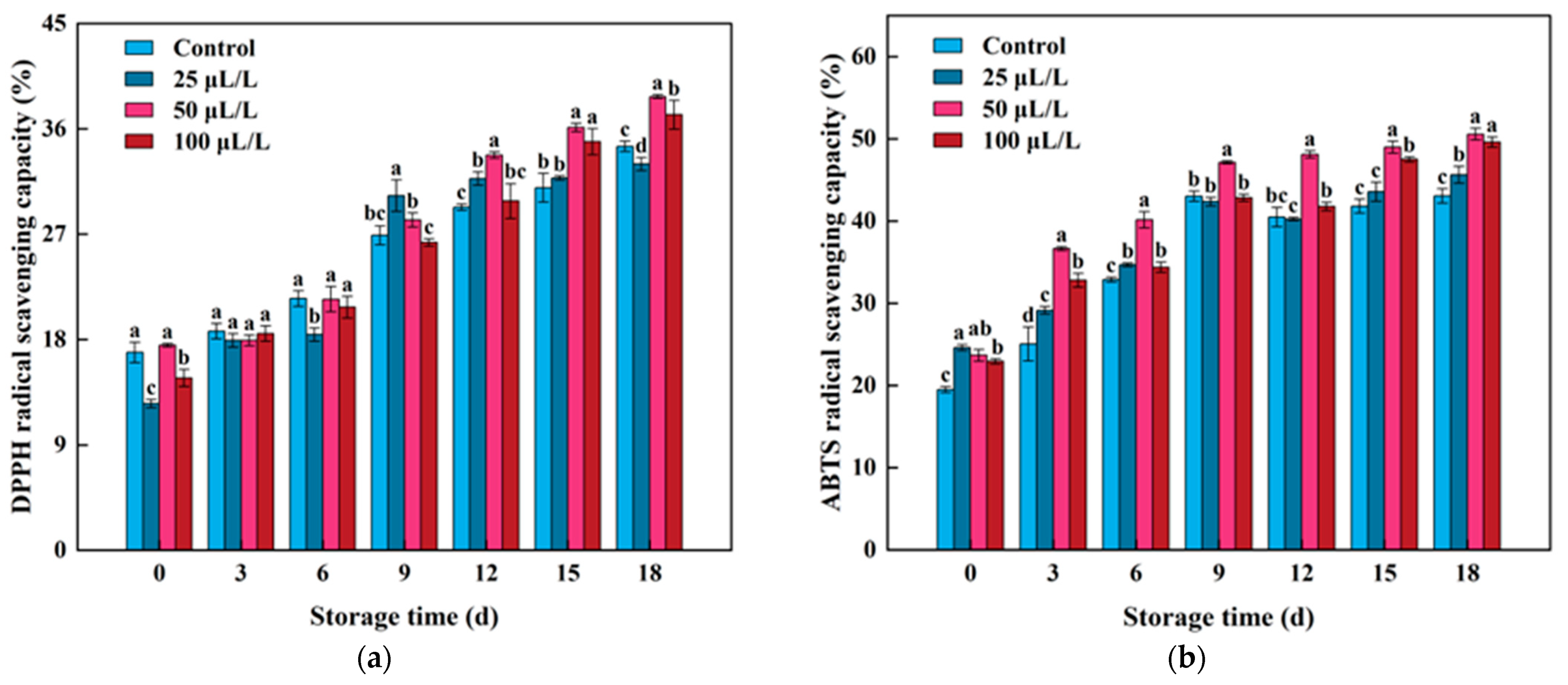
3.7. Antioxidant Capacity
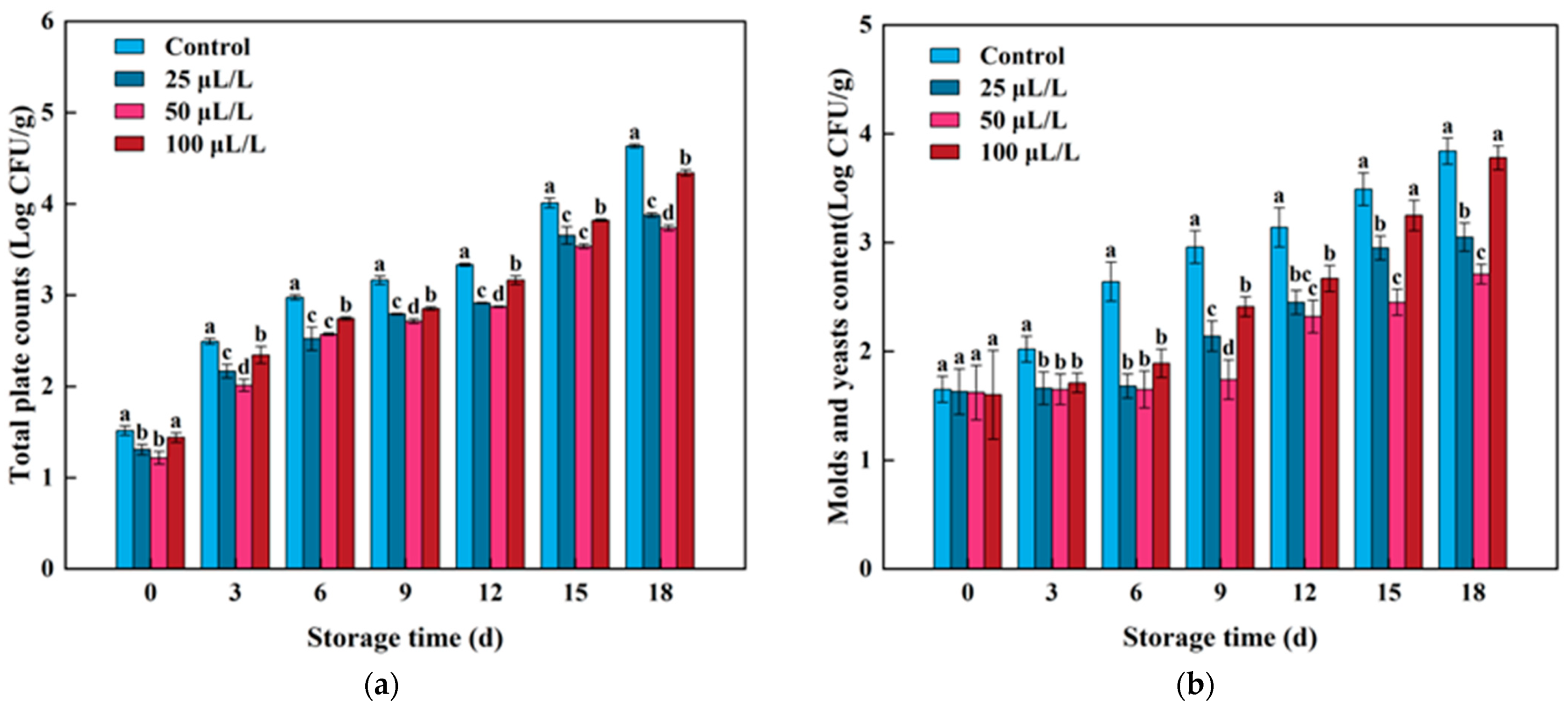
3.8. Microbial Survival
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Ge, Z.; Limwachiranon, J.; Li, L.; Li, W.R.; Luo, Z.S. UV-C treatment affects browning and starch metabolism of minimally processed lily bulb. Postharvest Biol. Technol. 2017, 128, 105–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xie, Z.K.; Yang, G.; Guo, Z.H.; Wang, L. The occurrence and distribution of viruses infecting Lanzhou lily in northwest. China Crop Prot. 2018, 110, 73–76. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, Y.H.; Sridhar, K.; Tsai, P.J. Synergies of modified atmosphere packaging and high-voltage electrostatic field to extend the shelf-life of fresh-cut cabbage and baby corn. LWT Food Sci. Technol. 2021, 138, 110559. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.G.; Feng, K. Biosynthesis of phenolic compounds and antioxidant activity in fresh-cut fruits and vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef] [PubMed]
- Dassamiour, S.; Boujouraf, O.; Sraoui, L.; Bensaad, M.S.; Derardja, A.E.; Alsufyani, S.J.; Sami, R.; Algarni, E.; Aljumayi, H.; Aljahani, A.H. Effect of postharvest UV-C radiation on nutritional quality, oxidation and enzymatic browning of stored mature date. Appl. Sci. 2022, 12, 4947. [Google Scholar] [CrossRef]
- Martinez-Hernandez, G.B.; Artés-Hernández, F.; Gómez, P.A.; Francisco, A.C.; Artés, F. Combination of electrolysed water, UV-C and superatmospheric O2 packaging for improving fresh-cut broccoli quality. Postharvest Biol. Technol. 2013, 76, 125–134. [Google Scholar] [CrossRef]
- Li, X.; Long, Q.; Gao, F.; Han, C.; Jin, P.; Zheng, Y.H. Effect of cutting styles on quality and antioxidant activity in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Chai, Z.; Zhang, F.; Liu, B.J.; Chen, X.F.; Meng, X.H. Antibacterial mechanism and preservation effect of curcumin-based photodynamic extends the shelf life of fresh-cut pears. LWT Food Sci. Technol. 2021, 142, 110941. [Google Scholar] [CrossRef]
- Ali, M.R.; Parmar, A.; Niedbała, G.; Wojciechowski, T.; El-Yazied, A.A.; El-Gawad, H.G.A.; Nahhas, N.A.; Ibrahim, M.F.M.; El-Mogy, M.M. Improved shelf-life and consumer acceptance of fresh-cut and fried potato strips by an edible coating of garden cress seed mucilage. Foods 2021, 10, 1536. [Google Scholar] [CrossRef]
- Kan, J.; Xie, W.; Wan, B.; Huo, T.B.; Lin, X.P.; Liu, J. Heat-induced tolerance to browning of fresh-cut lily bulbs (Lilium lancifolium Thunb.) under cold storage. J. Food Biochem. 2019, 43, e12816. [Google Scholar] [CrossRef]
- Huang, D.J.; Li, W.T.; Dawuda, M.M.; Huo, J.Q.; Li, C.X.; Wang, C.L.; Liao, W.B. Hydrogen sulfide reduced colour change in Lanzhou lily-bulb scales. Postharvest Biol. Technol. 2021, 176, 111520. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.Y.; Wang, X.Q.; Liu, X.X.; Zhu, X.L.; Zhang, J. Integration of metabolome and transcriptome profiling reveals the effect of modified atmosphere packaging (MAP) on the browning of fresh-Cut lanzhou lily (Lilium davidii var. unicolor) bulbs during storage. Foods 2023, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.H.; Zhou, L.; Zhou, W.; Zhong, Z.W.; Yu, K.B.; Xu, J.; Zou, L.Q.; Liu, W. Effect of modified atmosphere packaging combined with plant essential oils on preservation of fresh-cut lily bulbs. LWT Food Sci. 2022, 162, 113513. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Shi, Q.; Sun, T.; Xie, X.; Song, B.; Huang, X. The dynamics and mechanism of the antimicrobial activity of tea tree oil against bacteria and fungi. Appl. Microbiol. Biot. 2016, 100, 8865–8875. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, H.; Xu, F.; Cheng, S. Effects and possible mechanisms of tea tree oil vapor treatment on the main disease in postharvest strawberry fruit. Postharvest Biol. Technol. 2013, 77, 94–101. [Google Scholar] [CrossRef]
- Ce, S.; Zhang, X.; Guo, N. The antimicrobial activities and action-mechanism of tea tree oil against food-borne bacteria in fresh cucumber juice. Microb. Pathog. 2018, 125, 262–271. [Google Scholar] [CrossRef]
- Wei, Y.; Shao, X.; Wei, Y.; Xu, F.; Wang, H.F. Effect of preharvest application of tea tree oil on strawberry fruit quality parameters and possible disease resistance mechanisms. Sci. Hortic. 2018, 241, 18–28. [Google Scholar] [CrossRef]
- Harich, M.; Maherani, B.; Salmieri, S.; Lacroix, M. Evaluation of antibacterial activity of two natural bio-preservatives for mulations on freshness and sensory quality of ready to eat (RTE) foods. Food Control 2018, 85, 29–41. [Google Scholar] [CrossRef]
- Zhou, F.H.; Xu, D.Y.; Liu, C.H.; Chen, C.; Tian, M.X.; Jiang, A.L. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Wu, S. Extending shelf-life of fresh-cut potato with cactus Opuntia dillenii polysaccharide-based edible coatings. Int. J. Biol. Macromol. 2019, 130, 640–644. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, A.; Feng, K.; Gu, S.T.; Xu, D.Y.; Hu, W.Z. Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biol. Technol. 2019, 157, 110958. [Google Scholar] [CrossRef]
- Feng, S.M.; Luo, Z.S.; Xie, J.W.; Zeng, F.F. Gamma radiation control quality and lignification of bamboo shoots (Phyllostachys praecox f. prevernalis.) stored at low temperature. Postharvest Biol. Technol. 2015, 102, 17–24. [Google Scholar] [CrossRef]
- Guan, Y.G.; Lu, X.H.; Cheng, J.Y.; Lu, S.N.; Yin, L.Q.; Cheng, Q.F.; Yang, M.Y.; Chen, Y.J.; Sun, J.; Lu, G.Q.; et al. Montmorillonite-based edible coating enhances the postharvest quality of sweetpotato by regulating ROS and membrane lipid metabolism. Food Control 2024, 158, 110259. [Google Scholar] [CrossRef]
- Huang, H.; Guo, L.F.; Wang, L.; Wang, H.; Ma, S.M.; Jiang, Y.M.; Qu, H.X. 1-Methylcyclopropene (1-MCP) slows ripening of kiwifruit and affects energy status, membrane fatty acid contents and cell membrane integrity. Postharvest Biol. Technol. 2019, 156, 110941. [Google Scholar] [CrossRef]
- Guan, Y.; Ji, Y.; Yang, X.Z.; Pang, L.J.; Cheng, J.Y.; Lu, X.H.; Zheng, J.; Yin, L.Q.; Hu, W.Z. Antioxidant activity and microbial safety of fresh-cut red cabbage stored in different packaging films. LWT Food Sci. Technol. 2023, 17, 114478. [Google Scholar] [CrossRef]
- Dai, Y.; Hong, X.; Zhao, X.; Zheng, Y. The effect of sodium nitroprusside treatment on storage ability of fresh-cut potato. Foods 2023, 12, 221. [Google Scholar] [CrossRef]
- Shi, J.Y.; Zuo, J.H.; Zhou, F.H.; Gao, L.P.; Wang, Q.; Jiang, A.L. Low-temperature conditioning enhances chilling tolerance and reduces damage in cold-stored eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2018, 141, 33–38. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol. Bioch. 2019, 141, 466–476. [Google Scholar] [CrossRef]
- Pei, F.; Han, P.; Zhou, Z.C.; Fang, D.L.; Mariga, A.M.; Yang, W.J.; Ma, N.; Hu, Q.H. The characteristics of the film assembled by caffeic acid-grafted-chitosan/polylactic acid and its effect on the postharvest quality of Agaricus bisporus. Food Packag. Shelf. 2022, 32, 100828. [Google Scholar] [CrossRef]
- Gao, H.; Chai, H.K.; Cheng, N.; Cao, W. Effects of 24-epibrassinolide on enzymatic browning and antioxidant activity of fresh-cut lotus root slices. Food Chem. 2017, 217, 45–51. [Google Scholar] [CrossRef]
- Li, J.Q.; Chen, J.Y.; Xiao, G.S.; Chen, L.; Guo, X.B. Impact of kernel development on phenolic profiles and antioxidant activity in Castanea henryi. Int. J. Food Sci. Technol. 2022, 57, 5801–5810. [Google Scholar] [CrossRef]
- Quan, H.; Cai, Y.X.; Lu, Y.Z.; Shi, C.F.; Han, X.H.; Liu, L.L.; Yin, X.; Lan, X.Z.; Guo, X.B. Effect of microwave treatments combined with hot-air drying on phytochemical profiles and antioxidant activities in lily bulbs (Lilium lancifolium). Foods. 2023, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Sarengaowa; Hu, W.Z.; Jiang, A.L.; Xiu, Z.L.; Feng, K. Effect of thyme oil–alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef]
- Xu, Y.H.; Bao, Y.Q.; Chen, J.H.; Yi, Y.; Ai, Y.W.; Hou, W.F.; Wang, L.M.; Wang, H.X.; Min, T. Mechanisms of ethanol treatment on controlling browning in fresh-cut lotus roots. Sci. Hortic. 2023, 310, 111708. [Google Scholar] [CrossRef]
- Chen, J.H.; Xu, Y.H.; Yang, Y.; Hou, W.F.; Wang, L.M.; Ai, Y.W.; Wang, H.X.; Min, T. Regulations and mechanisms of 1-methylcyclopropene treatment on browning and quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root slices. Postharvest Biol. Technol. 2022, 185, 111782. [Google Scholar] [CrossRef]
- Xu, J.G.; Huang, X.N.; Meng, J.; Chen, J.J.; Han, B.Z. Characterization and comparison of the bacterial community on environmental surfaces through a fresh- cut vegetables processing line in China. Food Res. Int. 2022, 155, 111075. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Li, M.; Fu, X.; Zhao, X.; Min, D. Hot air pretreatment alleviates browning of fresh-cut pitaya fruit by regulating phenylpropanoid pathway and ascorbate-glutathione cycle. Postharvest Bio. Technol. 2022, 190, 111954. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wei, H.L.; Zhang, Y.B.; Guo, Z.L.; Qiu, Y.; Wen, L.G.; Xie, Z.K. Structural characterization of Lanzhou lily (Lilium davidii var. unicolor) polysaccharides and determination of their associated antioxidant activity. J. Sci. Food Agric. 2020, 100, 5603–5616. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Yang, H.; Tian, T.; Gu, H.; Li, X.M.; Lu, J. Analysis of factors related to browning of Dangshan pear (Pyrus spp.) wine. Food Chem. 2020, 308, 125665. [Google Scholar] [CrossRef]
- Nida, F.; Farid, M.; Fatima, F.; Maryam, D. Advances in formulation, functionality, and application of edible coatings on fresh produce and fresh-cut products: A review. Food Chem. 2023, 407, 135186. [Google Scholar] [CrossRef]
- Hassan, H.S.; EL-Hefny, M.; Ghoneim, I.M.; El-Lahot, M.S.R.A.; Akrami, M.; Al-Huqail, A.A.; Ali, H.M.; Abd-Elkader, D.Y. Assessing the use of aloe vera gel alone and in combination with lemongrass essential oil as a coating material for strawberry fruits: HPLC and EDX analyses. Coatings 2022, 12, 489. [Google Scholar] [CrossRef]
- Huang, L.; Wang, C.; Zhang, Y.Z.; Chen, X.H.; Huang, Z.H.; Xing, G.L.; Dong, M.S. Degradation of anti-nutritional factors and reduction of immunoreactivity of tempeh by cofermentation with Rhizopus oligosporus RT-3 and Actinomucor elegans DCY-1. Int. J. Food Sci. Technol. 2019, 54, 1836–1848. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.F.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Yao, W.R. Effects of double layer membrane loading eugenol on postharvest quality of cucumber. LWT Food Sci. Technol. 2021, 145, 111310. [Google Scholar] [CrossRef]
- Hu, W.Z.; Guan, Y.G.; Ji, Y.R.; Yang, X.Z. Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes. Food Biol. 2021, 44, 101435. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Vel’azquez, D.A.; Martínez-Hernandez, G.B.; Silvia, D.C.R.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Chotikakham, S.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Exogenous methyl salicylate alleviates senescent spotting by enhancing the activity of antioxidative ascorbate-glutathione cycle in harvested ‘Sucrier’ bananas. Sci. Hortic. 2022, 267, 109324. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Breusegem, F.V. Reactive oxygen species signaling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 22, 663–679. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.Y.; Wang, X.Y.; Zhang, X.H. Short-term O2/CO2 controlled atmosphere altered the water status and thus promoted phenolic biosynthesis during wound healing of fresh-cut white mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2022, 188, 111879. [Google Scholar] [CrossRef]
- Chen, C.; Hu, W.Z.; He, Y.B.; Jiang, A.L.; Zhang, R.D. Effect of citric acid combined with UV-C on the quality of fresh-cut apples. Postharvest Biol. Technol. 2016, 111, 126–131. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The combined and single effect of marjoram essential oil, ascorbic acid, and chitosan on fresh-cut lettuce preservation. Foods 2021, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Pal, A.K.; Sharma, V.; Kalia, S.; Kumar, S.; Ahuja, P.S.; Singh, A.K. Transgenic potato plants overexpressing SOD and APX exhibit enhanced lignification and starch biosynthesis with improved salt stress tolerance. Plant Mol. Biol. Rep. 2017, 35, 504–518. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jimenez, A.; Munoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Hu, W.Z.; Jiang, A.L.; Tian, M.X.; Liu, C.H.; Wang, Y.Y. Effect of ethanol treatment on physiological and quality attributes of fresh-cut eggplant. J. Sci. Food Agric. 2010, 90, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest wounding stress in horticultural crops as a tool for designing novel functional foods and beverages with enhanced nutraceutical content: Carrot juice as a case study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Li, X.A.; Li, M.L.; Wang, L.; Wang, J.; Jin, P.; Zheng, Y.H. Methyl jasmonate primes defense responses against wounding stress and enhances phenolic accumulation in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2018, 145, 101–107. [Google Scholar] [CrossRef]
- Xu, D.Y.; Gu, S.T.; Zhou, F.H.; Hu, W.Z.; Feng, K.; Chen, C.; Jiang, A.L. Mechanism underlying sodium isoascorbate inhibition of browning of fresh-cut mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2020, 173, 111357. [Google Scholar] [CrossRef]
- Guan, Y.G.; Hu, W.Z.; Jiang, A.L.; Xu, Y.P.; Zhao, M.R.; Ji, Y.R.; Sa, R.G.W.; Yang, X.Z.; Feng, K. The effect of cutting style on the biosynthesis of phenolics and cellular antioxidant capacity in wounded broccoli. Food Res. Int. 2020, 137, 109565. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme stresses and the impact on nutraceuticals and quality during pre- and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Chen, J.; Lin, Q.; Zhong, Y.G.; Duan, Y.Q.; Bi, J.F. Phenolic profiling reveals the metabolite basis of flesh colour and fresh-cut browning in apple fruit. Int. J. Food Sci. Technol. 2022, 57, 2257–2266. [Google Scholar] [CrossRef]
- Valerga, L.; González, R.E.; Pérez, M.B.; Concellón, A.; Cavagnaro, P.F. Differential and cultivar-dependent antioxidant response of whole and fresh-cut carrots of different root colors to postharvest UV-C radiation. Plants 2023, 12, 1297. [Google Scholar] [CrossRef]
- Fan, M.C.; Li, W.X.; Hu, X.L.; Sun, Y.N.; Yu, G.; Zhang, X. Effect of micro-vacuum storage on active oxygen metabolism, internal browning and related enzyme activities in Laiyang pear (Pyrus bretschneideri Reld). LWT Food Sci. Technol. 2016, 72, 467–474. [Google Scholar] [CrossRef]
- León, J.; Rojo, E.; Sanchez-Serrano, J.J. Wound signaling in plants. J. Exp. Bot. 2001, 354, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sarengaowa; Hu, W.; Feng, K.; Xiu, Z.L.; Jiang, A.L.; Lao, Y. Thyme oil alginate-based edible coatings inhibit growth of pathogenic microorganisms spoiling fresh-cut cantaloupe. Food Biosci. 2019, 32, 100467. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.; Faleiro, M.L.; Miguel, M.G.; Antunes, M.D. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Chen, W.Z.; Ma, S.B.; Wang, Q.K.; Mcclements, D.J.; Liu, X.B.; Ngai, T.; Liu, F.G. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. 2022, 62, 5029–5055. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]

| Storage (d) | Treatment | Respiration Rate (mg/(kg·h)) | Weight Loss Rate (%) | Firmness (N) | Starch Content (%) | Reducing Sugar Content (%) | Soluble Protein Content (mg/g) |
|---|---|---|---|---|---|---|---|
| 0 | Control | 36.92 ± 1.01 b | 0.00 | 3.11 ± 0.16 a | 23.51 ± 0.32 a | 8.20 ± 0.05 a | 0.75 ± 0.03 a |
| 25 μL/L | 38.66 ± 0.38 a | 0.00 | 3.16 ± 0.06 a | 23.32 ± 0.19 a | 8.19 ± 0.07 a | 0.72 ± 0.04 a | |
| 50 μL/L | 36.66 ± 0.28 b | 0.00 | 3.22 ± 0.07 a | 23.44 ± 0.21 a | 8.23 ± 0.06 a | 0.75 ± 0.02 a | |
| 100 μL/L | 34.23 ± 0.03 c | 0.00 | 3.23 ± 0.03 a | 23.50 ± 0.17 a | 8.16 ± 0.10 a | 0.73 ± 0.03 a | |
| 3 | Control | 31.83 ± 0.41 b | 1.03 ± 0.16 b | 2.87 ± 0.08 b | 21.97 ± 0.16 b | 8.26 ± 0.11 a | 0.70 ± 0.22 ab |
| 25 μL/L | 35.23 ± 2.02 a | 1.03 ± 0.09 b | 2.87 ± 0.04 b | 23.21 ± 0.09 a | 8.18 ± 0.04 a | 0.71 ± 0.03 ab | |
| 50 μL/L | 34.36 ± 1.20 a | 1.05 ± 0.18 b | 3.01 ± 0.07 a | 23.42 ± 0.23 a | 8.15 ± 0.07 a | 0.73 ± 0.02 a | |
| 100 μL/L | 35.03 ± 0.72 a | 1.60 ± 0.14 a | 3.06 ± 0.03 a | 23.09 ± 0.17 a | 8.12 ± 0.05 a | 0.68 ± 0.01 b | |
| 6 | Control | 31.54 ± 1.05 b | 2.60 ± 0.12 a | 2.78 ± 0.01 b | 19.11 ± 0.15 b | 8.65 ± 0.13 a | 0.78 ± 0.02 b |
| 25 μL/L | 33.15 ± 0.53 a | 2.08 ± 0.01 b | 2.78 ± 0.05 b | 23.12 ± 0.16 a | 8.07 ± 0.09 b | 0.84 ± 0.03 a | |
| 50 μL/L | 31.11 ± 0.53 b | 1.96 ± 0.24 b | 2.93 ± 0.04 a | 23.09 ± 0.23 a | 8.02 ± 0.12 b | 0.85 ± 0.02 a | |
| 100 μL/L | 32.07 ± 0.72 a | 2.53 ± 0.27 a | 2.96 ± 0.03 a | 22.73 ± 0.29 a | 8.10 ± 0.11 b | 0.72 ± 0.01 c | |
| 9 | Control | 27.35 ± 1.15 a | 3.23 ± 0.22 a | 2.63 ± 0.04 c | 18.87 ± 0.09 c | 9.58 ± 0.12 a | 0.86 ± 0.01 b |
| 25 μL/L | 27.03 ± 1.28 a | 2.73 ± 0.02 b | 2.77 ± 0.03 b | 23.11 ± 0.12 a | 7.65 ± 0.06 c | 0.87 ± 0.02 b | |
| 50 μL/L | 26.91 ± 1.72 a | 2.66 ± 0.33 b | 2.90 ± 0.05 a | 22.98 ± 0.14 a | 7.73 ± 0.05 bc | 0.93 ± 0.02 a | |
| 100 μL/L | 25.63 ± 0.46 b | 3.66 ± 0.36 a | 2.80 ± 0.01 b | 22.16 ± 0.17 b | 7.92 ± 0.11 b | 0.82 ± 0.01 c | |
| 12 | Control | 27.51 ± 0.60 a | 4.71 ± 0.10 a | 2.53 ± 0.02 c | 18.53 ± 0.20 c | 13.51 ± 0.09 a | 0.63 ± 0.02 c |
| 25 μL/L | 26.07 ± 0.52 b | 3.66 ± 0.03 b | 2.61 ± 0.03 b | 22.92 ± 0.19 a | 8.09 ± 0.12 b | 0.79 ± 0.04 b | |
| 50 μL/L | 26.03 ± 0.79 b | 3.54 ± 0.32 b | 2.70 ± 0.05 a | 22.73 ± 0.08 a | 8.15 ± 0.14 b | 0.89 ± 0.05 a | |
| 100 μL/L | 26.10 ± 1.27 a | 4.54 ± 0.44 a | 2.67 ± 0.10 a | 21.55 ± 0.12 b | 8.32 ± 0.10 b | 0.57 ± 0.02 d | |
| 15 | Control | 23.80 ± 0.07 b | 5.77 ± 0.07 a | 2.50 ± 0.04 b | 18.01 ± 0.13 c | 11.47 ± 0.14 a | 0.59 ± 0.02 c |
| 25 μL/L | 21.95 ± 0.78 c | 4.05 ± 0.17 b | 2.60 ± 0.09 a | 22.78 ± 0.21 a | 7.99 ± 0.05 c | 0.65 ± 0.03 b | |
| 50 μL/L | 23.24 ± 1.35 b | 4.22 ± 0.29 b | 2.67 ± 0.03 a | 22.59 ± 0.15 a | 7.84 ± 0.06 c | 0.73 ± 0.02 a | |
| 100 μL/L | 28.05 ± 1.35 a | 5.50 ± 0.35 a | 2.63 ± 0.04 a | 21.64 ± 0.11 b | 8.25 ± 0.09 b | 0.51 ± 0.01 d | |
| 18 | Control | 27.29 ± 0.98 a | 7.10 ± 0.23 a | 2.39 ± 0.02 c | 17.12 ± 0.16 c | 13.81 ± 0.15 a | 0.43 ± 0.01 b |
| 25 μL/L | 25.51 ± 0.17 b | 5.31 ± 0.22 b | 2.49 ± 0.01 b | 22.19 ± 0.17 a | 7.76 ± 0.07 c | 0.65 ± 0.02 a | |
| 50 μL/L | 23.64 ± 0.17 c | 5.32 ± 0.19 b | 2.64 ± 0.03 a | 22.57 ± 0.23 a | 7.85 ± 0.05 c | 0.70 ± 0.03 a | |
| 100 μL/L | 28.19 ± 0.12 a | 7.19 ± 0.35 a | 2.62 ± 0.02 a | 21.41 ± 0.19 b | 8.69 ± 0.06 b | 0.39 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Lu, S.; Sun, Y.; Zhang, R.; Lu, X.; Pang, L.; Wang, L. Effect of Tea Tree Essential Oil on the Quality, Antioxidant Activity, and Microbiological Safety of Lightly Processed Lily (Lilium brownii var. viridulum) during Storage. Foods 2024, 13, 2106. https://doi.org/10.3390/foods13132106
Guan Y, Lu S, Sun Y, Zhang R, Lu X, Pang L, Wang L. Effect of Tea Tree Essential Oil on the Quality, Antioxidant Activity, and Microbiological Safety of Lightly Processed Lily (Lilium brownii var. viridulum) during Storage. Foods. 2024; 13(13):2106. https://doi.org/10.3390/foods13132106
Chicago/Turabian StyleGuan, Yuge, Sainan Lu, Yan Sun, Rentao Zhang, Xinghua Lu, Linjiang Pang, and Lei Wang. 2024. "Effect of Tea Tree Essential Oil on the Quality, Antioxidant Activity, and Microbiological Safety of Lightly Processed Lily (Lilium brownii var. viridulum) during Storage" Foods 13, no. 13: 2106. https://doi.org/10.3390/foods13132106
APA StyleGuan, Y., Lu, S., Sun, Y., Zhang, R., Lu, X., Pang, L., & Wang, L. (2024). Effect of Tea Tree Essential Oil on the Quality, Antioxidant Activity, and Microbiological Safety of Lightly Processed Lily (Lilium brownii var. viridulum) during Storage. Foods, 13(13), 2106. https://doi.org/10.3390/foods13132106




