Maximizing Antioxidant Potential in Picual Virgin Olive Oil: Tailoring Agronomic and Technological Factors with Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Oil Extraction
2.3. Chemicals
2.4. Virgin Olive Oil Analysis
2.4.1. Determination of Total Polyphenols
2.4.2. Determination of Orthodiphenols
2.4.3. Determination of Photosynthetic Pigments (Chlorophylls and Carotenoids)
2.4.4. Determination of Tocopherols
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Olive Characterization
3.2. Malaxation Conditions
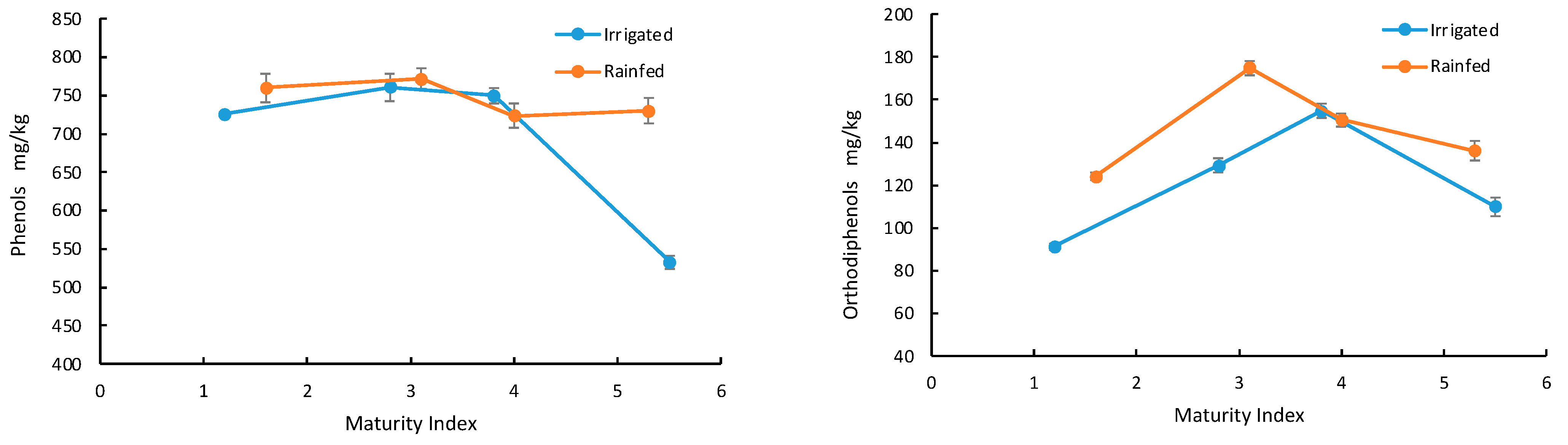
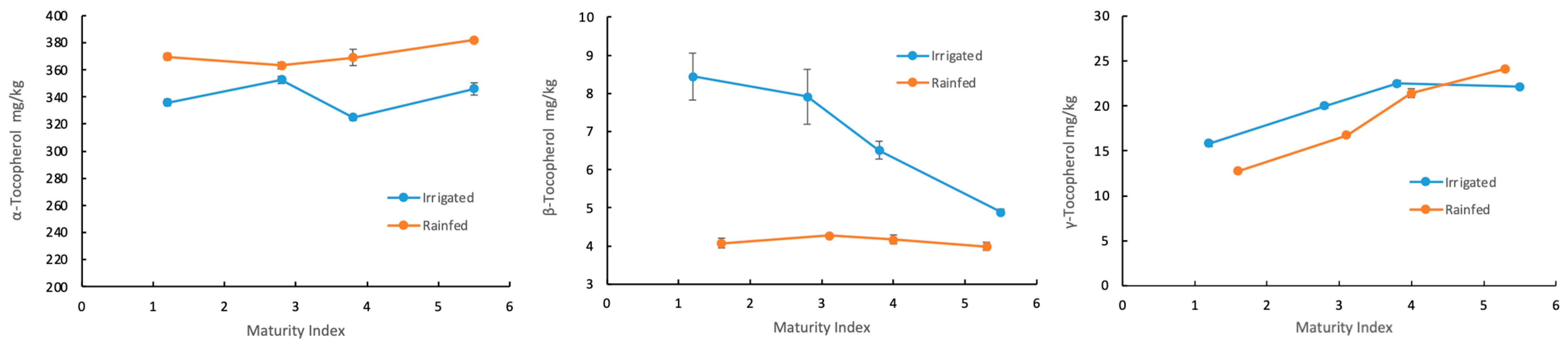
3.3. Fruit Maturation
3.4. Irrigation Management
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Maio, I.D.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Vera, F.; Ruiz-Fernández, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; González-Gay, M.A.; Mobasheri, A.; Gómez, R.; Scotece, M.; et al. Natural molecules for healthy lifestyles: Oleocanthal from extra virgin olive oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutíerrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New phenolic compounds hydrothermally extracted from the olive oil byproduct alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef]
- Hashim, Y.Z.Y.; Worthington, J.; Allsopp, P.; Ternan, N.G.; Brown, E.M.; McCann, M.J.; Rowland, I.R.; Esposto, S.; Servili, M.; Gill, C.I. Virgin olive oil phenolics extract inhibit invasion of HT115 human colon cancer cells in vitro and in vivo. Food Funct. 2014, 5, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Mosca, L.; Puca, R.; Mangino, G.; Rossi, A.; Lendaro, E. Extra-virgin olive oil phenols block cell cycle progression and modulate chemotherapeutic toxicity in bladder cancer cells. Oncol. Rep. 2016, 36, 3095–3104. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D. Discrimination of olive oils and fruits into cultivars and maturity stages bases on phernolic and volatile compounds. J. Agric. Food Chem. 2005, 53, 8054–8062. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of malaxation conditions on phenolic and volatile profiles in olive paste and the corresponding virgin olive oils. (Olea europaea, L. cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and Volatile Compounds of Extra Virgin Olive Oil (Olea europaea L. Cv. Cornicabra) with Regard to Fruit Ripening and Irrigation Management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef]
- Fakourelis, N.; Lee, E.C.; Min, D.B. Effects of chlorophyll and β-carotene on the oxidation stability of olive oil. J. Food Sci. 1987, 28, 495–509. [Google Scholar] [CrossRef]
- Hong, W.K.; Itri, L.M. Retinoids and human cancer. In The Retinoids; Sporn, I.M., Roberts, A.B., Goodman, D.S., Eds.; Raven Press: New York, NY, USA, 1994; pp. 597–630. [Google Scholar]
- Basu, H.N.; Del Vecchio, A.J.; Flider, F.; Orthoefer, F.T. Nutritional and potential disease prevention properties of carotenoids. JOCS 2001, 78, 665–675. [Google Scholar] [CrossRef]
- Gimeno, E.; Calero, E.; Castellote, A.I.; Lamuela-Raventos, R.M.; De la Torre, M.C.; López-Sabater, M.C. Simultaneous determination of α-tocopherol and β-carotene in olive oil by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2000, 881, 255–259. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.) Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Rastrelli, L.; Passi, S.; Ippolito, F.; Vacca, G.; De Simone, F. Rate of degradation of α-tocopherol, squalene, phenolics, and polyunsaturated fatty acids in olive oil during different storage conditions. J. Agric. Food Chem. 2002, 50, 5566–5570. [Google Scholar] [CrossRef]
- Wiseman, S.A.; Tijburg, I.B.; Van de Put, F.H. Olive oil phenolic protect LDL damage and spare ViT E in the hamster. Lipids 2002, 37, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Uceda, M.; Hermoso, M.; Aguilera, M.P. La calidad del aceite de olive. In El Cultivo Del Olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 2004; pp. 656–684. [Google Scholar]
- Beltrán, G.; Aguilera, M.P.; Del Rio, C.; Sánchez, S.; Martinez, L. Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem. 2005, 89, 207–215. [Google Scholar] [CrossRef]
- Facua Report. Available online: https://facua.org/noticias/el-aceite-de-oliva-virgen-extra-ha-subido-este-ano-hasta-257-euros-mas-en-los-super-que-en-origen/ (accessed on 10 May 2024).
- Inarejos-García, A.M.; Gómez-Rico, A.; Salvador, M.D.; Fragapane, G. Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur. Food Res. Technol. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- De Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Espínola, F.; Moya, M.; Fernández, D.G.; Castro, E. Improved extraction of virgin olive oil using calcium carbonate as coadjuvant extractant. J. Food Eng. 2009, 92, 112–118. [Google Scholar] [CrossRef]
- Vázquez, A.; Janer, C.; Jane, M.L. Determinación de polifenoles totales del aceite de olive. Grasas Aceites 1973, 24, 350–357. [Google Scholar]
- Gómez-Alonso, S.; Salvador, M.D.; Fregapane, G. Phenolic compounds profile of Cornicabra virgin olive oil. J. Agric. Food Chem. 2002, 50, 6812–6817. [Google Scholar] [CrossRef]
- Mínguez, M.I.; Rejano, L.; Gandul, B.; Sánchez, A.H.; Garrido, J. Colour pigment correlation in virgin olive oil. JAOCS 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Lozano, E.J.; Blázquez, G.; Calero, M.; Martín-Lara, M.Á.; Pérez-Huertas, S.; Pérez, A. Optimizing the Extraction Process of Value-Added Products from Olive Cake Using Neuro-Fuzzy Models. Processes 2024, 12, 317. [Google Scholar] [CrossRef]
- Jiménez-García, E.A.; Pérez-Huertas, S.; Pérez, A.; Calero, M.; Blázquez, G. Recycling PVC Waste into CO2 Adsorbents: Optimizing Pyrolysis Valorization with Neuro-Fuzzy Models. Processes 2024, 12, 431. [Google Scholar] [CrossRef]
- Beltrán, G.; Jiménez, A.; Del Rio, C.; Sánchez, S.; Martínez, L.; Uceda, M.; Aguilera, M.P. Variability of vitamin E in virgin olive oil by agronomical and genetic factors. J. Food Compos. Anal. 2010, 23, 633–639. [Google Scholar] [CrossRef]
- Franco, M.N.; Galdeano-Díaz, T.; Sánchez, J.; de Miguel, C.; Martín-Vertedor, D. Total Phenolic Compounds and Tocopherols Profiles of Seven Olive Oil Varieties Grown in the South-West of Spain. J. Oleo Sci. 2014, 63, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Parenti, A.; Spugnoli, P.; Masella, P.; Calamai, L. The effect of malaxation temperature on the virgin olive oil phenolic profile under laboratory-scale conditions. Eur. J. Lipids Sci. Technol. 2008, 110, 735–741. [Google Scholar] [CrossRef]
- Trombetta, D.; Smariglio, A.; Marcoccia, D.; Vincenzo Giofrè, S.; Toscano, G.; Mazzotti, F.; Giovanazzi, A.; Lorenzetti, S. Analytical Evaluation and Antioxidant Properties of Some Secondary Metabolites in Northern Italian Mono-and Multi-Varietal Extra Virgin Olive Oils (EVOOs) from Early and Late Harvested Olives. Int. J. Mol. Sci. 2017, 18, 797. [Google Scholar] [CrossRef]
- Veneziani, G.; Nucciarelli, D.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Tomasone, R.; Pagano, M.; Servili, M. Application of low temperature during the malaxation phase of Virgin Olive Oil mechanical extraction processes of three different Italian cultivars. Foods 2021, 10, 1578. [Google Scholar] [CrossRef] [PubMed]
- Espínola, F.; Moya, M.; Fernández, D.G.; Castro, E. Modelling of virgin olive oil extraction using response surface methodology. Int. J. Food Sci. Technol. 2011, 46, 2576–2583. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; De Torres, A.; Moya, M.; Espínola, F. Industrial production of a balanced virgin olive oil. LWT 2018, 97, 588–596. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Ferreira-Dias, S. Bioactive Compounds of Portuguese Virgin Olive Oils Discriminate Cultivar and Ripening Stage. J. Am. Oil Chem. Soc. 2016, 93, 1137–1147. [Google Scholar] [CrossRef]
- Tang, F.; Li, C.; Yang, X.; Lei, J.; Chen, H.; Zhang, C.; Wang, C. Effect of Variety and Maturity Index on the Physicochemical Parameters Related to Virgin Olive Oil from Wudu (China). Foods 2022, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.N.; Motilva, M.J.; Goñi, M.; Romero, M.P. Comparative study of the effect of maturation process of the olive fruit on chlorophyll and carotenoid fractions of drupes and virgin oils from Arbequina and Farga cultivars. Food Chem. 2007, 100, 748–755. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; Moriana, A.; Pérez, D.; Olmedilla, N.; Ribas, F.; Fregapane, G. Influence of different irrigation strategies in a traditional Cornicabra cv. Olive orchard on virgin olive oil composition and quality. Food Chem. 2007, 100, 568–578. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation effects on quality phenolic composition and selected volatiles of virgin olive oils Cv Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef]
- De Torres, A.; Espínola, F.; Moya, M.; Castro, E. Composition of secoiridoid derivatives from Picual virgin olive oil using response surface methodology with regard to malaxation conditions, fruit ripening and irrigation management. Eur. Food Res. Technol. 2016, 242, 1709–1718. [Google Scholar] [CrossRef]
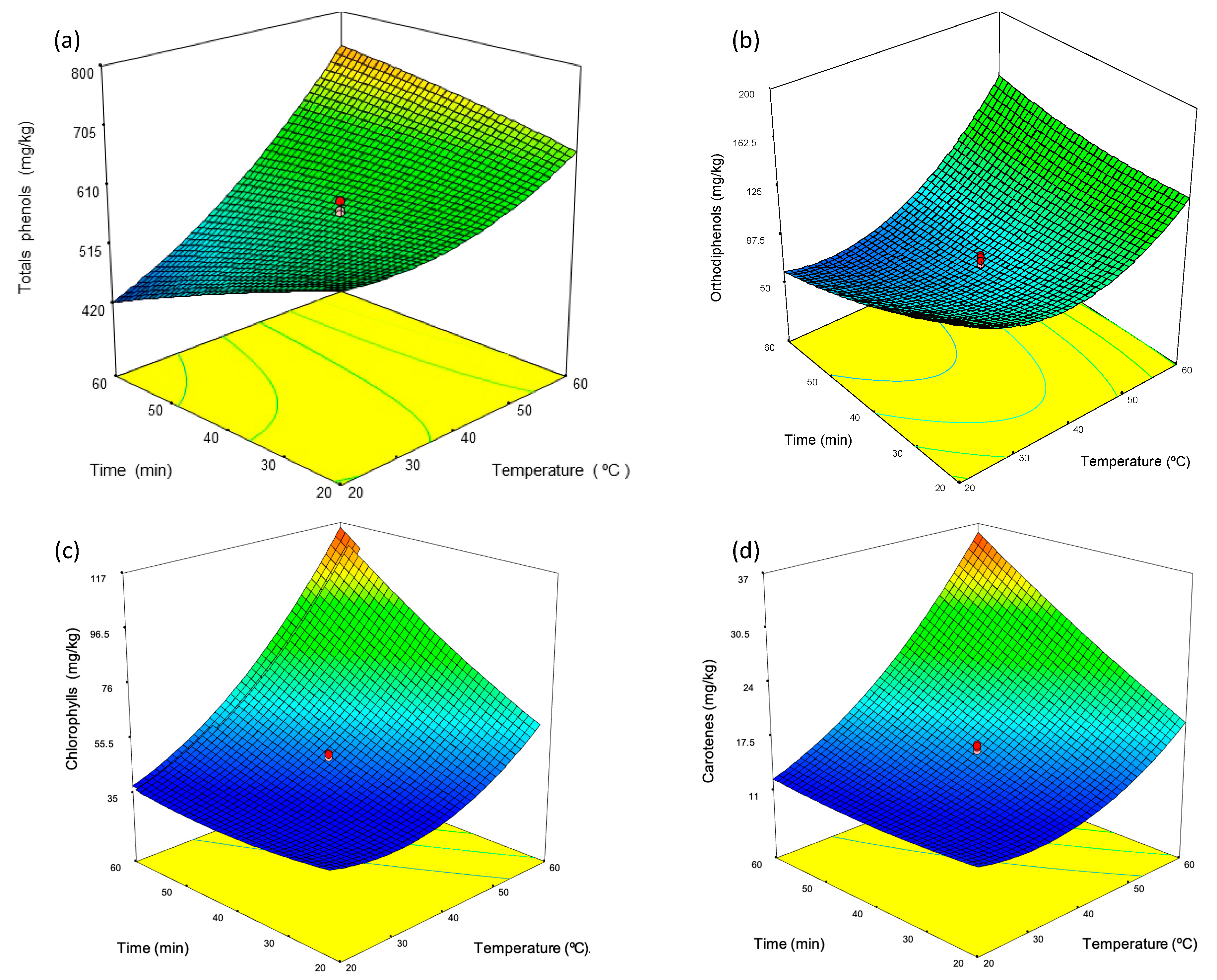


| Run | Temperature | Time | Talc Dosage | |||
|---|---|---|---|---|---|---|
| Coded Values | Actual Values (°C) | Coded Values | Actual Values (min) | Coded Values | Actual Values (%) | |
| 11 | −1.68 * | 6.4 | 0 | 40 | 0 | 1.25 |
| 6 | −1 | 20 | −1 | 20 | −1 | 0.5 |
| 9 | −1 | 20 | −1 | 20 | +1 | 2 |
| 2 | −1 | 20 | +1 | 60 | −1 | 0.5 |
| 5 | −1 | 20 | +1 | 60 | +1 | 2 |
| 17 | 0 | 40 | −1.68 * | 6.4 | 0 | 1.25 |
| 10 | 0 | 40 | 0 | 40 | −1.68 * | 0 |
| 3 | 0 | 40 | 0 | 40 | 0 | 1.25 |
| 7 | 0 | 40 | 0 | 40 | 0 | 1.25 |
| 8 | 0 | 40 | 0 | 40 | 0 | 1.25 |
| 15 | 0 | 40 | 0 | 40 | 0 | 1.25 |
| 13 | 0 | 40 | 0 | 40 | +1.68 * | 2.5 |
| 1 | 0 | 40 | +1.68 * | 73.6 | 0 | 1.25 |
| 16 | +1 | 60 | −1 | 20 | −1 | 0.5 |
| 12 | +1 | 60 | −1 | 20 | +1 | 2 |
| 14 | +1 | 60 | +1 | 60 | −1 | 0.5 |
| 18 | +1 | 60 | +1 | 60 | +1 | 2 |
| 4 | +1.68 * | 70 | 0 | 40 | 0 | 1.25 |
| Harvest Dates | Irrigation Management | Maturity Index | Moisture (g/kg) | Oil (g/kg) | Solids (g/kg) |
|---|---|---|---|---|---|
| October | Rainfed | 1.6 | 552 ± 4 | 175 ± 2 | 273 ± 3 |
| October | Rainfed | 3.1 | 493 ± 3 | 215 ± 3 | 292 ± 6 |
| November | Rainfed | 4.0 | 461 ± 11 | 250 ± 7 | 289 ± 7 |
| December | Rainfed | 5.3 | 426 ± 8 | 256 ± 10 | 319 ± 11 |
| October | Irrigated | 1.2 | 563 ± 15 | 175 ± 3 | 262 ±14 |
| October | Irrigated | 2.8 | 538 ± 8 | 207 ± 4 | 256 ± 7 |
| November | Irrigated | 3.8 | 507 ± 10 | 233 ± 5 | 260 ± 7 |
| December | Irrigated | 5.5 | 438 ± 11 | 272 ± 4 | 290 ± 8 |
| Run | Phenolic Compounds (mg/kg) | Pigments (mg/kg) | Tocopherols (mg/kg) | ||||
|---|---|---|---|---|---|---|---|
| Phenols | Orthodiphenols | Chlorophylls | Carotenes | α-Tocopherol | β-Tocopherol | γ-Tocopherol | |
| 1 | 524 | 62.9 | 39.3 | 13.1 | 325.9 | 5.60 | 22.9 |
| 2 | 417 | 60.2 | 33.9 | 11.1 | 323.1 | 3.15 | 21.6 |
| 3 | 571 | 91.6 | 41.3 | 14.3 | 330.7 | 3.79 | 20.1 |
| 4 | 800 | 197.5 | 66.1 | 21.3 | 318.0 | 6.45 | 18.8 |
| 5 | 420 | 61.8 | 35.5 | 11.6 | 328.2 | 2.84 | 19.8 |
| 6 | 603 | 94.1 | 35.7 | 11.2 | 323.8 | 2.05 | 18.3 |
| 7 | 586 | 99.1 | 42.2 | 14.8 | 321.4 | 3.77 | 18.5 |
| 8 | 565 | 91.4 | 41.5 | 13.9 | 312.6 | 3.34 | 19.1 |
| 9 | 572 | 86.3 | 34.3 | 11.4 | 317.0 | 3.71 | 19.5 |
| 10 | 575 | 86.5 | 36.2 | 12.3 | 315.5 | 4.14 | 19.3 |
| 11 | 394 | 84.6 | 32.1 | 10.1 | 319.1 | 3.32 | 18.6 |
| 12 | 686 | 149.2 | 57.4 | 19.4 | 333.6 | 5.40 | 19.2 |
| 13 | 562 | 79.0 | 40.4 | 13.6 | 315.2 | 3.92 | 18.9 |
| 14 | 734 | 139.6 | 59.4 | 21.3 | 314.3 | 6.12 | 18.9 |
| 15 | 571 | 94.1 | 39.7 | 13.5 | 310.4 | 3.87 | 18.8 |
| 16 | 658 | 118.5 | 48.6 | 16.6 | 311.5 | 5.20 | 18.8 |
| 17 | 588 | 86.5 | 35.5 | 11.7 | 317.1 | 3.57 | 19.0 |
| 18 | 749 | 145.2 | 70.2 | 23.0 | 324.4 | 6.51 | 18.5 |
| Run | Phenolic Compounds (mg/kg) | Pigments (mg/kg) | Tocopherols (mg/kg) | ||||
|---|---|---|---|---|---|---|---|
| Phenols | Orthodiphenols | Chlorophylls | Carotenes | α-Tocopherol | β-Tocopherol | γ-Tocopherol | |
| 1 | 551 | 83.1 | 55.2 | 20.3 | 369.1 | 5.15 | 19.8 |
| 2 | 459 | 56.6 | 34.6 | 11.3 | 323.7 | 3.53 | 19.9 |
| 3 | 551 | 93.3 | 51.1 | 18.8 | 353.6 | 3.92 | 18.1 |
| 4 | 696 | 183.9 | 68.3 | 23.1 | 344.0 | 3.87 | 21.4 |
| 5 | 471 | 68.7 | 34.0 | 11.0 | 322.1 | 3.90 | 20.3 |
| 6 | 524 | 78.9 | 34.3 | 11.1 | 287.8 | 5.21 | 19.6 |
| 7 | 548 | 88.2 | 44.8 | 16.9 | 345.8 | 3.86 | 18.9 |
| 8 | 541 | 88.8 | 46.5 | 17.7 | 335.8 | 3.86 | 18.9 |
| 9 | 575 | 85.5 | 33.0 | 10.3 | 319.1 | 3.73 | 19.8 |
| 10 | 506 | 94.8 | 48.7 | 18.5 | 360.1 | 4.27 | 18.5 |
| 11 | 393 | 69.5 | 30.7 | 10.3 | 326.6 | 4.10 | 20.2 |
| 12 | 734 | 128.0 | 54.5 | 19.1 | 344.9 | 4.08 | 20.4 |
| 13 | 509 | 91.3 | 44.9 | 16.2 | 340.2 | 3.91 | 19.9 |
| 14 | 651 | 146.4 | 68.1 | 23.1 | 340.5 | 3.83 | 20.5 |
| 15 | 524 | 81.2 | 45.3 | 17.2 | 341.9 | 3.46 | 19.9 |
| 16 | 684 | 133.4 | 54.9 | 19.7 | 338.7 | 3.87 | 20.5 |
| 17 | 676 | 105.3 | 43.4 | 15.7 | 362.8 | 3.46 | 18.4 |
| 18 | 624 | 154.5 | 74.1 | 25.4 | 354.1 | 3.82 | 22.2 |
| Phenols | Orthodiphenols | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Irrigation Management | MI | Max. Value (mg/kg) | Temperature (°C) | Time (min) | Talc (%) | Max. Value (mg/kg) | Temperature (°C) | Time (min) | Talc (%) |
| Irrigated | 1.2 | 725.70 ± 1.67 | 60 | 30.41 | 0.50 | 91.22 ± 1.67 | 60 | 32.59 | 1.22 |
| 2.8 | 760.90 ± 18.2 | 60 | 60 | 0.96 | 129.28 ± 3.31 | 60 | 60 | 0.50 | |
| 3.8 | 749.87 ± 9.91 | 60 | 60 | 2 | 154.83 ± 3.24 | 60 | 38.23 | 2 | |
| 5.5 | 533.04 ± 8.77 | 46.99 | 29.56 | 1.39 | 109.99 ± 4.43 | 60 | 37.78 | 0.51 | |
| Rainfed | 1.6 | 760.02 ± 18.9 | 60 | 20 | 0.54 | 124.06 ± 3.61 | 60 | 60 | 0.5 |
| 3.1 | 771.72 ± 13.7 | 60 | 59.6 | 2 | 175.02 ± 7.95 | 60 | 60 | 2 | |
| 4.0 | 723.47 ± 15.6 | 60 | 20.04 | 2 | 150.55 ± 5.54 | 60 | 60 | 1.36 | |
| 5.3 | 730.12 ± 16.5 | 60 | 60 | 2 | 136.14 ± 3.62 | 60 | 48.76 | 1.11 | |
| Chlorophylls | Carotenes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Irrigation Management | MI | Max. Value (mg/kg) | Temperature (°C) | Time (min) | Talc (%) | Max. Value (mg/kg) | Temperature (°C) | Time (min) | Talc (%) |
| Irrigated | 1.2 | 112.02 ± 3.87 | 60 | 60 | 1.50 | 28.53 ± 0.73 | 60 | 60 | 1.23 |
| 2.8 | 83.32 ± 1.96 | 60 | 60 | 0.82 | 22.75 ± 0.52 | 60 | 60 | 2 | |
| 3.8 | 66.77 ± 2.80 | 60 | 60 | 2 | 22.89 ± 0.23 | 60 | 60 | 2 | |
| 5.5 | 38.40 ± 0.77 | 59.8 | 20.11 | 2 | 13.70 ± 0.29 | 60 | 60 | 2 | |
| Rainfed | 1.6 | 117.16 ± 2.96 | 60 | 60 | 1.75 | 36.63 ± 0.74 | 60 | 60 | 2 |
| 3.1 | 85.64 ± 1.95 | 60 | 60 | 0.51 | 26.42 ± 1.56 | 60 | 60 | 0.59 | |
| 4.0 | 73.42 ± 2.20 | 60 | 60 | 2 | 24.30 ± 0.84 | 60 | 60 | 1.69 | |
| 5.3 | 47.26 ± 1.46 | 60 | 60 | 0.5 | 17.74 ± 0.46 | 60 | 60 | 0.50 | |
| α-Tocopherol | β-Tocopherol | γ-Tocopherol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation Management | MI | Max. Value (mg/kg) | T (°C) | Time (min) | Talc (%) | Max. Value (mg/kg) | T (°C) | Time (min) | Talco (%) | Max. Value (mg/kg) | T (°C) | Time (min) | Talco (%) |
| Irrigated | 1.2 | 335.9 ± 2.48 | 46 | 60 | 0.5 | 8.44 ± 0.62 | 60 | 20 | 2 | 15.81 ± 0.31 | 20 | 60 | 0.67 |
| 2.8 | 352.72 ± 2.83 | 59.4 | 60 | 2 | 7.92 ± 0.72 | 20 | 20 | 2 | 20.10 ± 0.23 | 60 | 35.3 | 1.44 | |
| 3.8 | 324.86 ± 2.20 | 20 | 60 | 0.5 | 6.51 ± 0.24 | 59.8 | 60 | 2 | 22.51 ± 0.31 | 60 | 60 | 0.5 | |
| 5.5 | 346.06 ± 4.70 | 59.8 | 60 | 0.5 | 4.89 ± 0.07 | 60 | 53.7 | 2 | 22.14 ± 0.26 | 60 | 60 | 2 | |
| Rainfed | 1.6 | 369.89 ± 2.61 | 38.2 | 46.7 | 1.7 | 4.06 ± 0.13 | 20 | 43.1 | 0.5 | 12.73 ± 0.21 | 20.9 | 34.03 | 2 |
| 3.1 | 363.15 ± 2.65 | 60 | 60 | 2 | 4.27 ± 0.05 | 60 | 60 | 0.5 | 16.74 ± 0.14 | 60 | 46.3 | 0.5 | |
| 4.0 | 369.10 ± 6.01 | 55.9 | 57.9 | 1.9 | 4.17 ± 0.11 | 60 | 60 | 0.5 | 21.42 ± 0.49 | 60 | 20 | 2 | |
| 5.3 | 382.01 ± 0.1 | 52.4 | 60 | 1.4 | 3.99 ± 0.10 | 44.5 | 60 | 0.5 | 24.10 ± 0.20 | 59.4 | 20 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Torres, A.; Espínola, F.; Moya, M.; Cara Corpas, C.; Vidal, A.M.; Pérez-Huertas, S. Maximizing Antioxidant Potential in Picual Virgin Olive Oil: Tailoring Agronomic and Technological Factors with Response Surface Methodology. Foods 2024, 13, 2093. https://doi.org/10.3390/foods13132093
de Torres A, Espínola F, Moya M, Cara Corpas C, Vidal AM, Pérez-Huertas S. Maximizing Antioxidant Potential in Picual Virgin Olive Oil: Tailoring Agronomic and Technological Factors with Response Surface Methodology. Foods. 2024; 13(13):2093. https://doi.org/10.3390/foods13132093
Chicago/Turabian Stylede Torres, Antonia, Francisco Espínola, Manuel Moya, Cristóbal Cara Corpas, Alfonso M. Vidal, and Salvador Pérez-Huertas. 2024. "Maximizing Antioxidant Potential in Picual Virgin Olive Oil: Tailoring Agronomic and Technological Factors with Response Surface Methodology" Foods 13, no. 13: 2093. https://doi.org/10.3390/foods13132093
APA Stylede Torres, A., Espínola, F., Moya, M., Cara Corpas, C., Vidal, A. M., & Pérez-Huertas, S. (2024). Maximizing Antioxidant Potential in Picual Virgin Olive Oil: Tailoring Agronomic and Technological Factors with Response Surface Methodology. Foods, 13(13), 2093. https://doi.org/10.3390/foods13132093






