Extraction of Bioactive Compounds from Wine Lees: A Systematic and Bibliometric Review
Abstract
1. Introduction
2. Results and Discussion
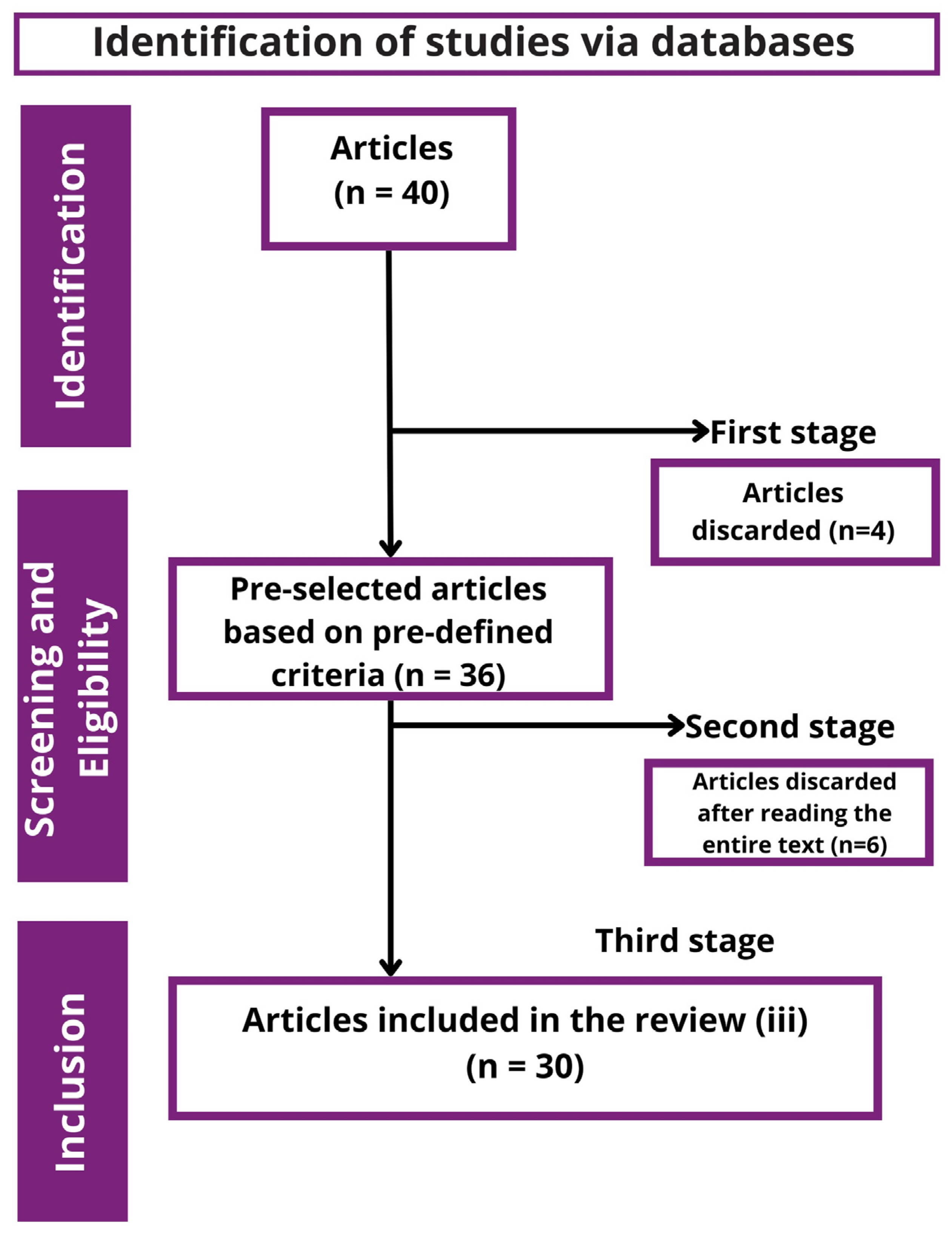
2.1. Study Selection and Flowchart
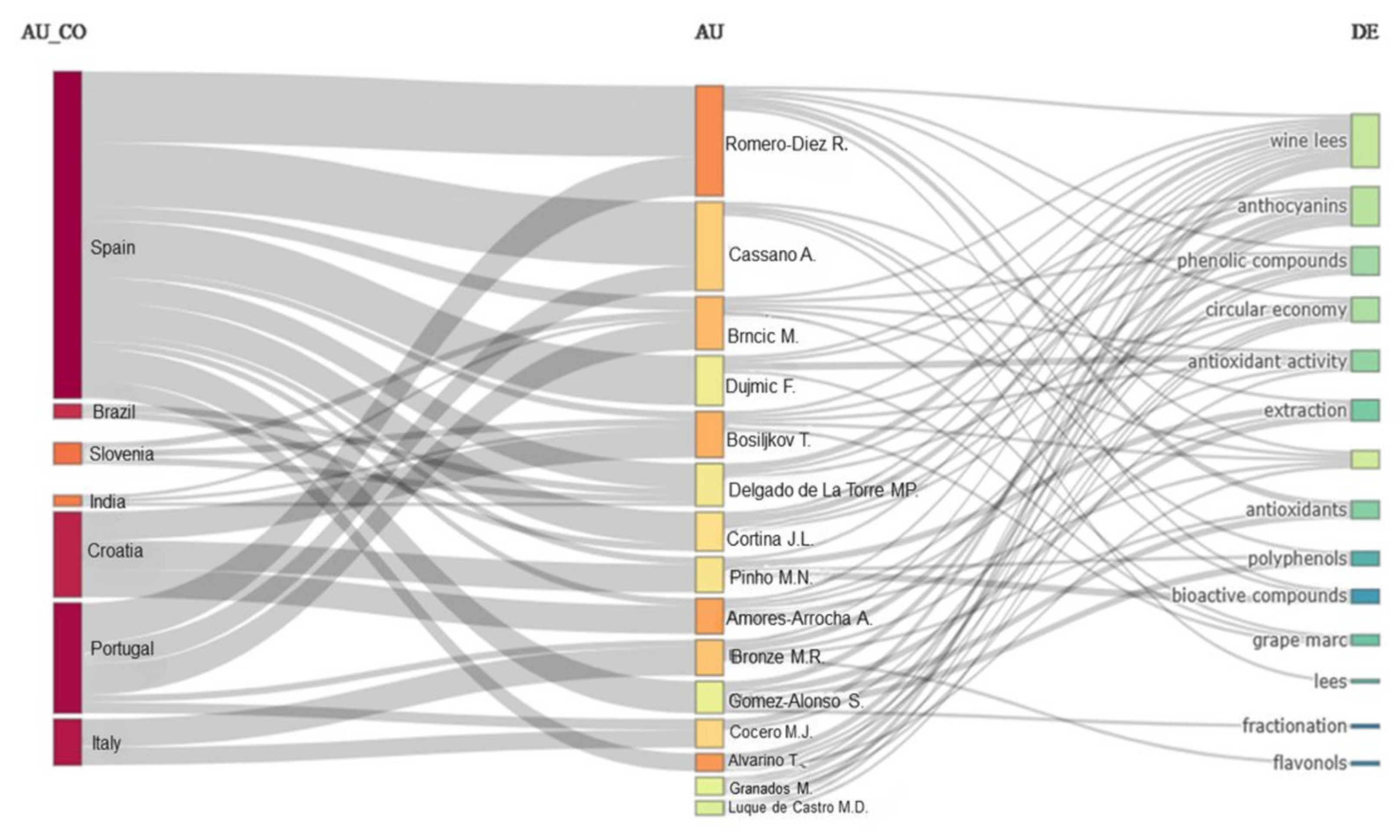
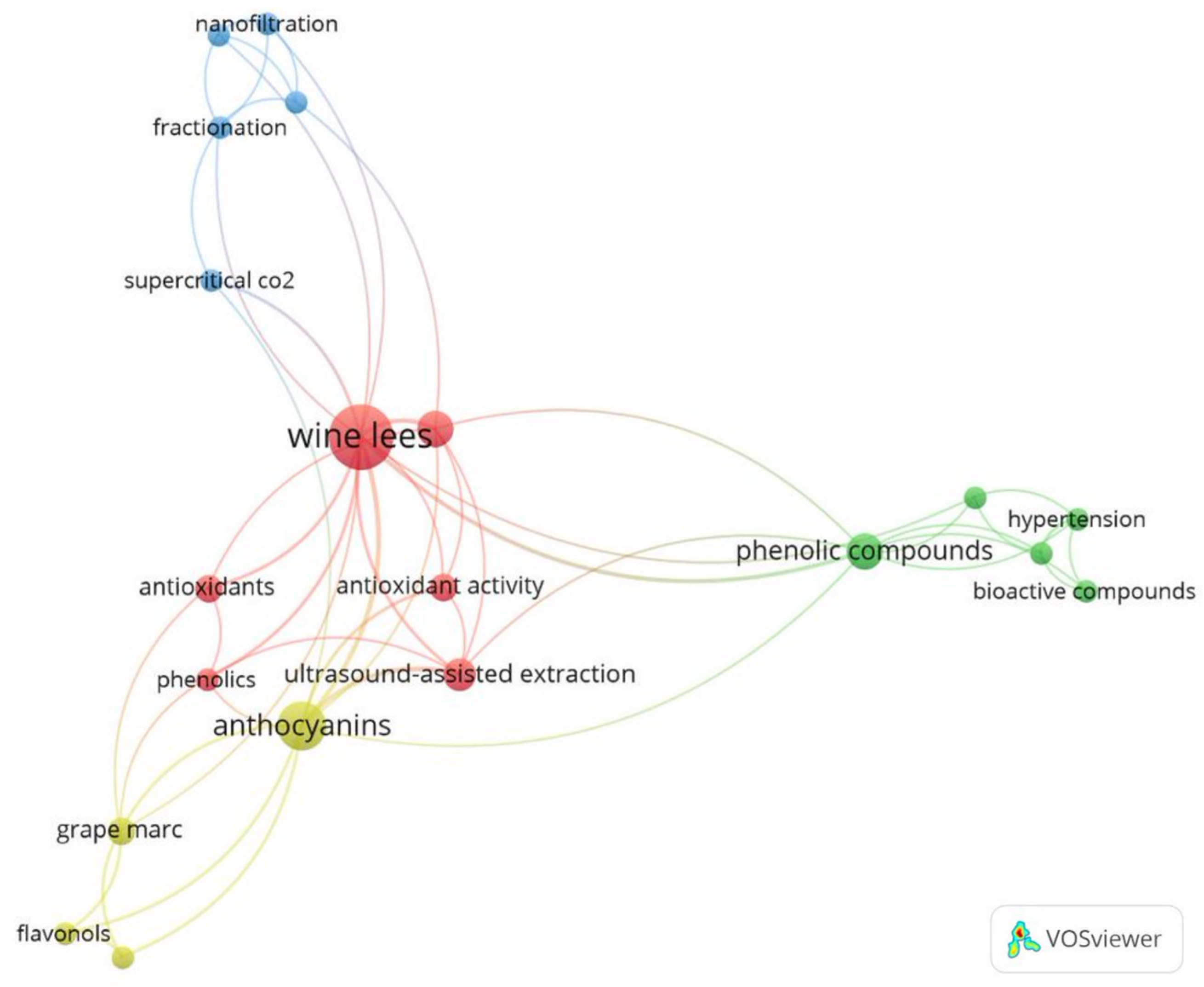
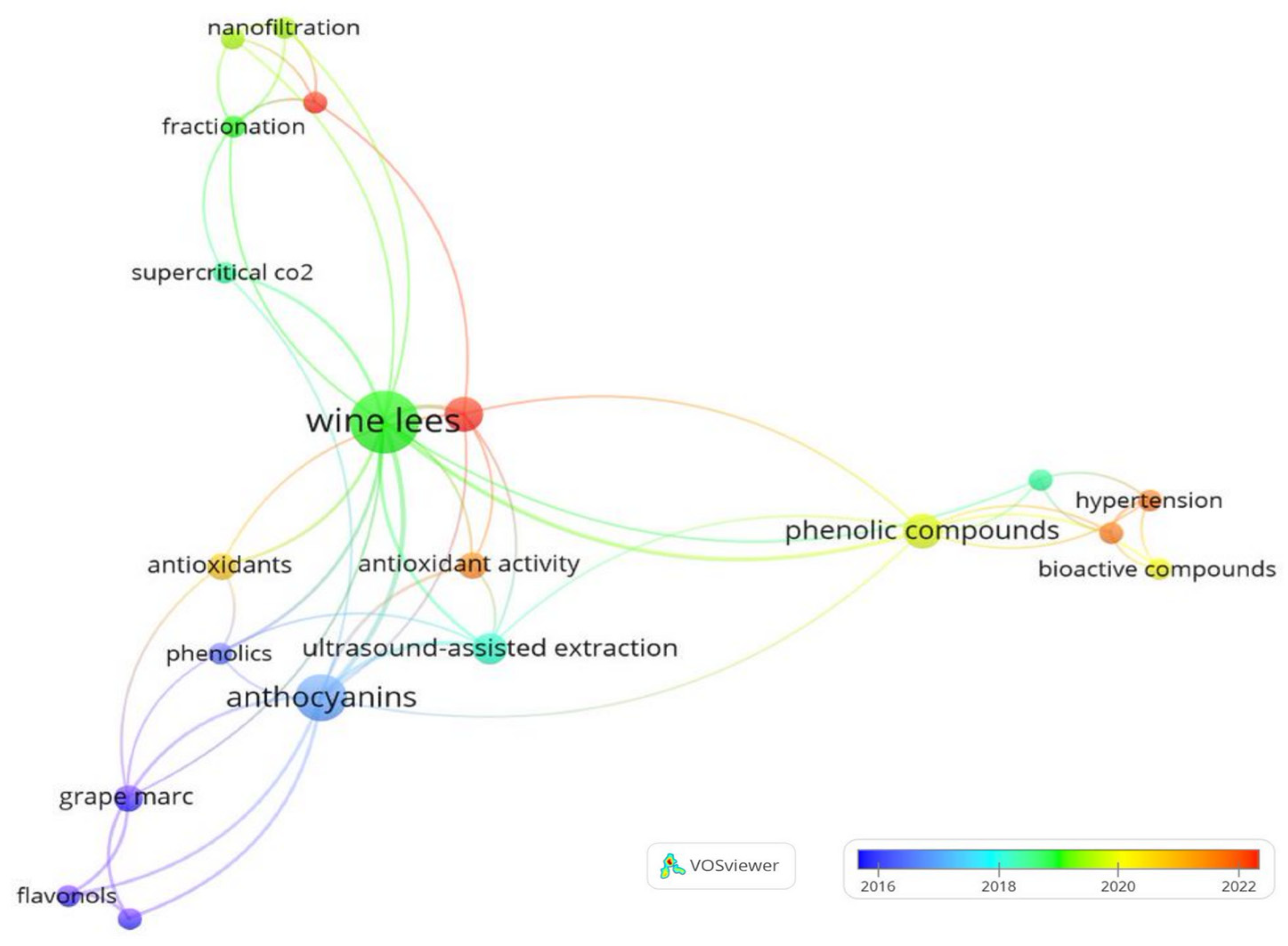
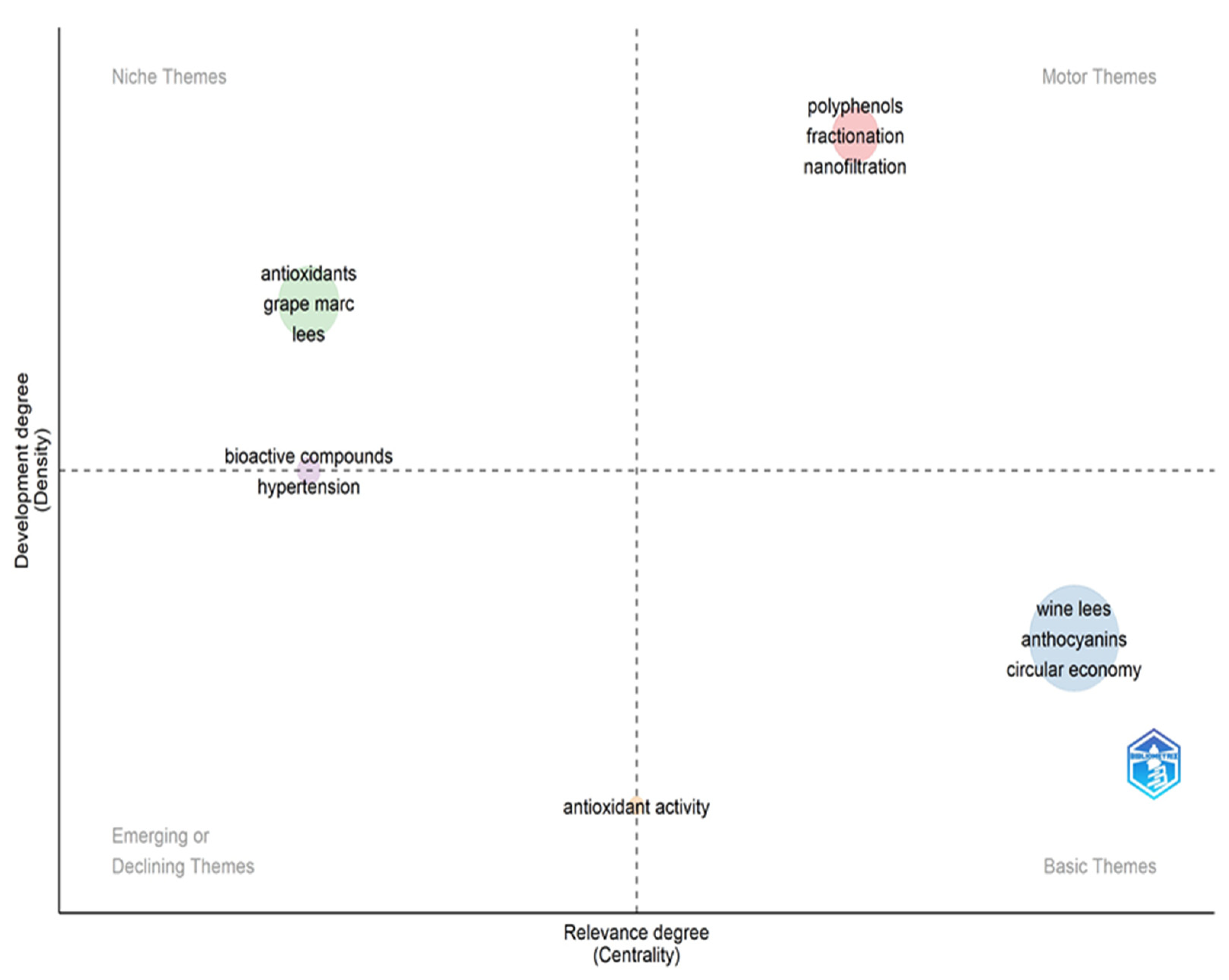
2.2. Bibliometric Review
2.3. Systematic Review
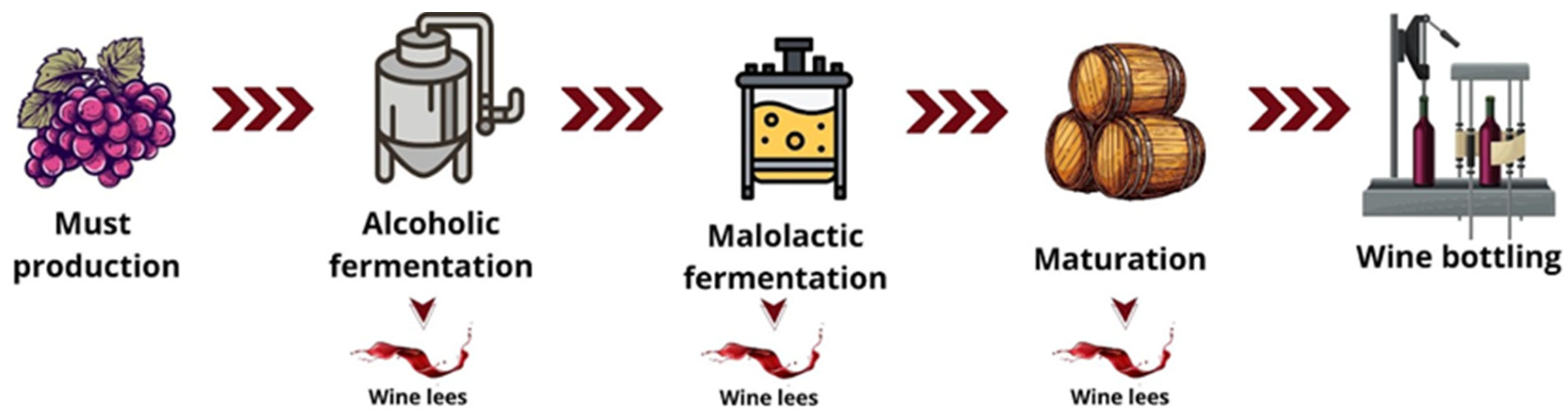
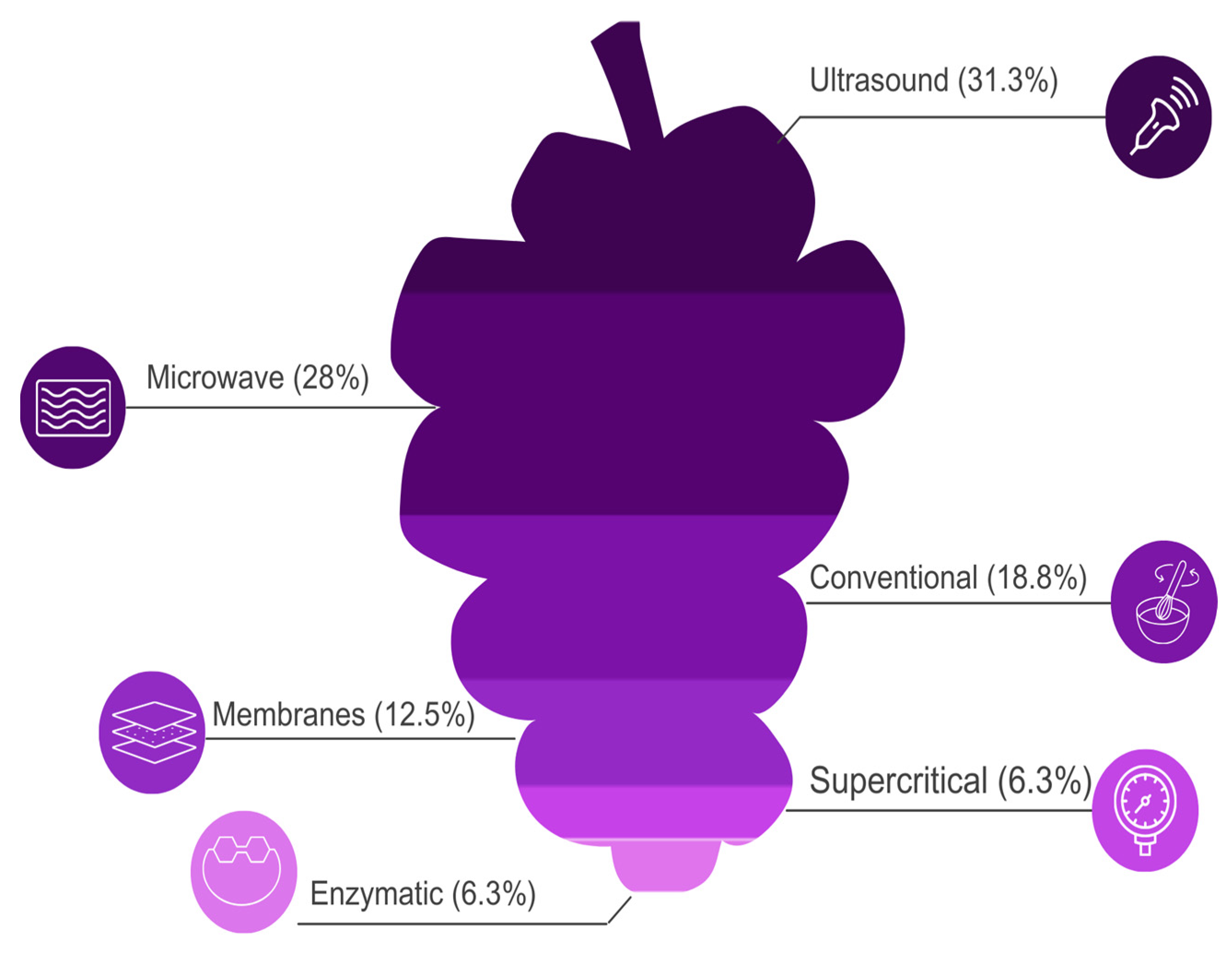
2.4. Methods of Extracting Bioactive from Wine Lees
2.4.1. Conventional Extraction (Maceration)
2.4.2. Ultrasound-Assisted Extraction
2.4.3. Microwave-Assisted Extraction
2.4.4. Enzyme-Facilitated Extraction
2.4.5. Membrane Application
2.4.6. Supercritical and Pressurized Fluid Extraction
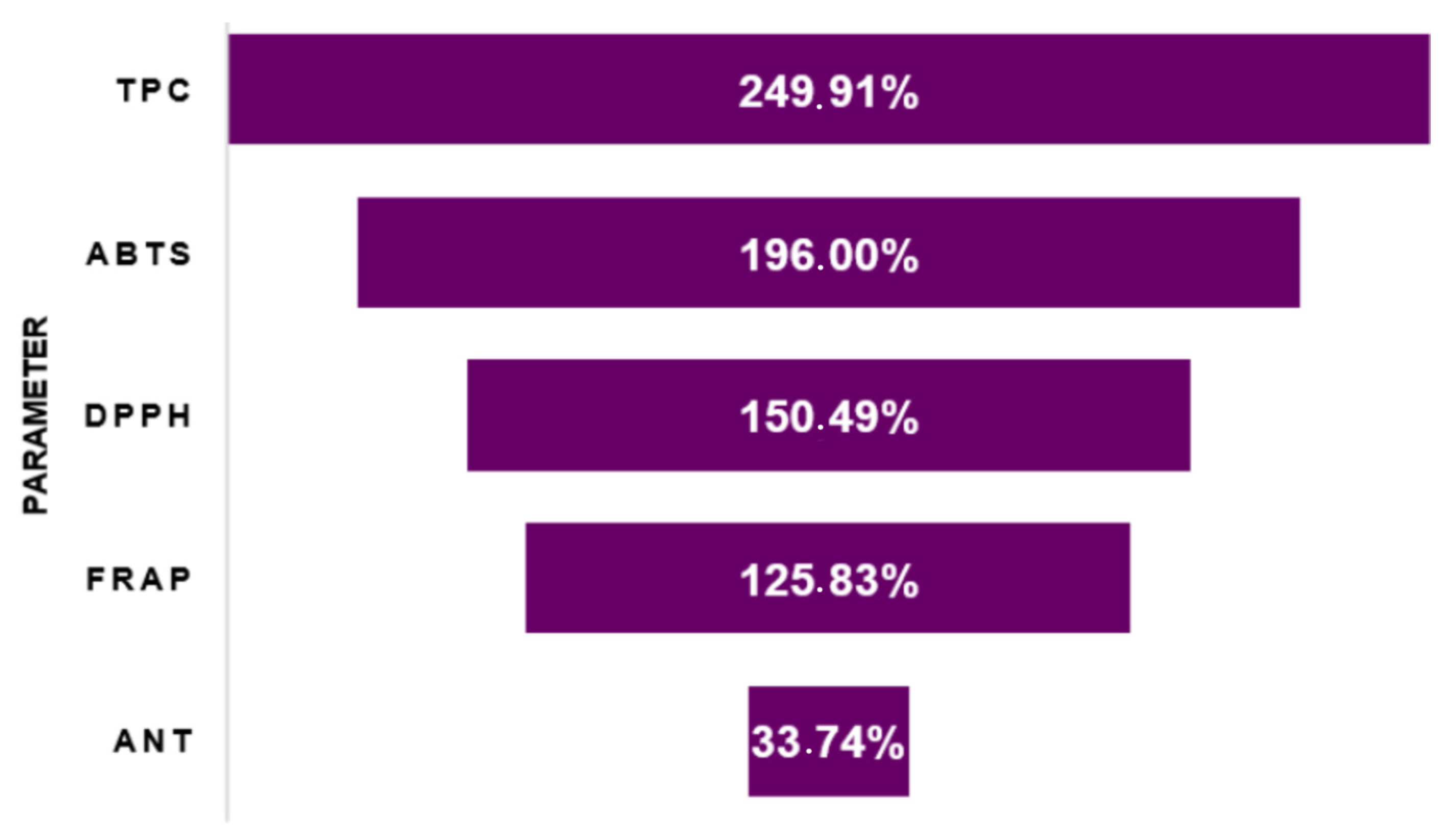
2.4.7. Average Extraction Yield of Bioactive Compounds
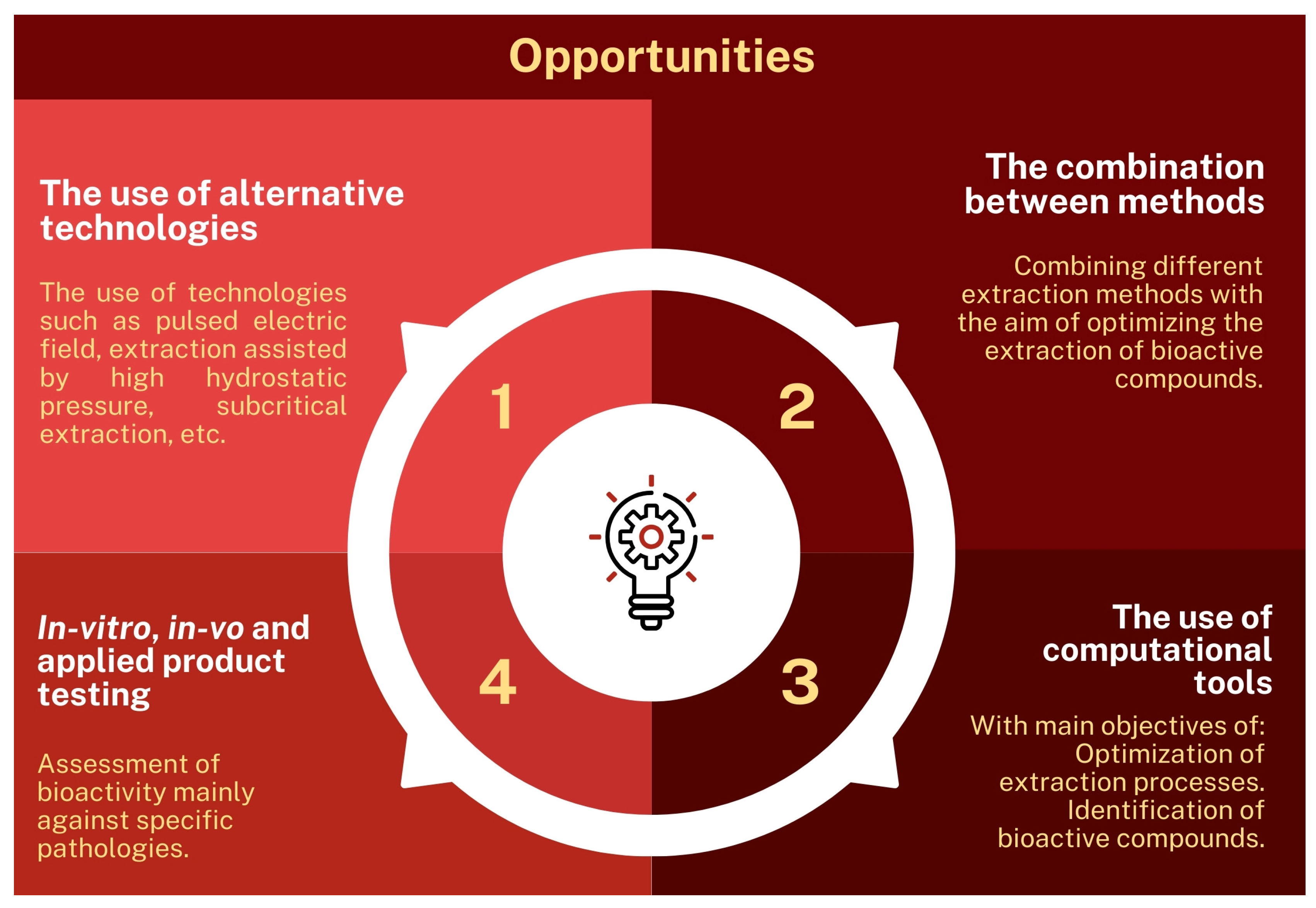
2.4.8. Insights into New Research
3. Materials and Methods
3.1. Database
3.2. Search and Identification Strategies
3.3. Data Organization and Information Grouping
3.4. Evaluation of the Average Yields of Extraction Processes
3.5. Bibliometric Characterization of the Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavelli, V.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery of winemaking by-products for innovative food applications—A review. Ital. J. Food Sci. 2016, 28, 542–564. [Google Scholar]
- Bonamente, E.; Scrucca, F.; Asdrubali, F.; Cotana, F.; Presciutti, A. The Water Footprint of the Wine Industry: Implementation of an Assessment Methodology and Application to a Case Study. Sustainability 2015, 7, 12190–12208. [Google Scholar] [CrossRef]
- de Andrade Bulos, R.B.; da Gama Paz, F.; Machado, C.G.; Tavares, P.P.L.G.; de Souza, C.O.; Umsza-Guez, M.A. Scientific and Technological Research on the Use of Wine Lees. Food Prod. Process. Nutr. 2023, 5, 25. [Google Scholar] [CrossRef]
- De Iseppi, A.; Marangon, M.; Vincenzi, S.; Lomolino, G.; Curioni, A.; Divol, B. A Novel Approach for the Valorization of Wine Lees as a Source of Compounds Able to Modify Wine Properties. LWT 2021, 136, 110274. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical Characterisation of the Solid By-Products and Residues from the Winery and Distillery Industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Delteil, D. Working with Lees: Key Elements to Wine Maturing. Aust. N. Z. Grapegrow. Winemak. 2002, 461, 104–108. [Google Scholar]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Cabezas, M.; Pavani, A.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Concentration of Phenolic Compounds from Olive Washing Wastewater by Forward Osmosis Using Table Olive Fermentation Brine as Draw Solution. Environ. Technol. Innov. 2023, 30, 103054. [Google Scholar] [CrossRef]
- Hapani, U.; Highland, H.; George, L.-B. Phenolic Compounds: A Significant Threat to Agricultural Soils. In Bioremediation of Emerging Contaminants from Soils; Elsevier: Amsterdam, The Netherlands, 2024; pp. 97–106. [Google Scholar]
- Cui, W.; Xu, B.; Chen, F.; Shen, W.; Wan, F.; Cheng, A. Effects of Grape Peel Phenolics on Lipid Accumulation in Sodium Palmitate-Treated HepG2 Cells. J. Funct. Foods 2024, 112, 105923. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Shah, I.M.; Surek, E.; Barile, D. Uncovering the Promising Role of Grape Pomace as a Modulator of the Gut Microbiome: An in-Depth Review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef]
- de Brito Alves, J.L.; Alves Brasil, J.M.; Maia, L.A.; Lima, M.d.C.; Sampaio, K.B.; de Souza, E.L. Phenolic Compounds in Hypertension: Targeting Gut-Brain Interactions and Endothelial Dysfunction. J. Funct. Foods 2023, 104, 105531. [Google Scholar] [CrossRef]
- Martín-Garcia, A.; Riu-Aumatell, M.; López-Tamames, E. By-Product Revalorization: Cava Lees Can Improve the Fermentation Process and Change the Volatile Profile of Bread. Foods 2022, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Miolla, R.; Vacca, M.; Difonzo, G.; De Angelis, M. Wine Lees as Functional Ingredient to Produce Biscuits Fortified with Polyphenols and Dietary Fibre. LWT 2024, 198, 115943. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Shyu, Y.-S.; Hsu, C.-K. Grape Wine Lees Improves the Rheological and Adds Antioxidant Properties to Ice Cream. LWT—Food Sci. Technol. 2009, 42, 312–318. [Google Scholar] [CrossRef]
- Borges, M.S.; Biz, A.P.; Bertolo, A.P.; Bagatini, L.; Rigo, E.; Cavalheiro, D. Enriched Cereal Bars with Wine Fermentation Biomass. J. Sci. Food Agric. 2021, 101, 542–547. [Google Scholar] [CrossRef]
- Berk, Z. Food Process Engineering and Technology; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780123736604. [Google Scholar]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Feitosa, B.F.; Decker, B.L.A.; Brito, E.S.d.; Rodrigues, S.; Mariutti, L.R.B. Microencapsulation of Anthocyanins as Natural Dye Extracted from Fruits—A Systematic Review. Food Chem. 2023, 424, 136361. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine World Wine Production Outlook 2023. Available online: https://www.oiv.int/sites/default/files/documents/OIV_World_Wine_Production_Outlook_2023_2.pdf (accessed on 15 June 2024).
- Zhijing, Y.; Shavandi, A.; Harrison, R.; Bekhit, A.E.-D. Characterization of Phenolic Compounds in Wine Lees. Antioxidants 2018, 7, 48. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Physicochemical and Nutritional Characterization of Winemaking Lees: A New Food Ingredient. Agronomy 2020, 10, 996. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M.; Reig, M. A Green Approach to Phenolic Compounds Recovery from Olive Mill and Winery Wastes. Sci. Total Environ. 2022, 835, 155552. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, G.P.S.; Magro, C.D.; Carvalho, G.O.; Santos, A.E.; Lanza, M.; Oliveira, J.V. Co-Precipitation of Anthocyanin in PHBV by the SEDS Technique. J. Food Sci. Technol. 2021, 58, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Schieber, A. Preparative Fractionation of Phenolic Compounds and Isolation of an Enriched Flavonol Fraction from Winemaking Industry By-Products by High-Performance Counter-Current Chromatography. Plants 2023, 12, 2242. [Google Scholar] [CrossRef]
- Costa, R.D.; Domínguez-Perles, R.; Abraão, A.; Gomes, V.; Gouvinhas, I.; Barros, A.N. Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction. Molecules 2023, 28, 6660. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural Deep Eutectic Solvents and Ultrasound-Assisted Extraction: Green Approaches for Extraction of Wine Lees Anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Dujmić, F.; Kovačević Ganić, K.; Ćurić, D.; Karlović, S.; Bosiljkov, T.; Ježek, D.; Vidrih, R.; Hribar, J.; Zlatić, E.; Prusina, T.; et al. Non-Thermal Ultrasonic Extraction of Polyphenolic Compounds from Red Wine Lees. Foods 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.N.; Taofiq, O.; Dias, M.I.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Amaral, J.S. Chemical Characterization and Bioactive Properties of Wine Lees and Diatomaceous Earth towards the Valorization of Underexploited Residues as Potential Cosmeceuticals. Cosmetics 2023, 10, 58. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic Characterization of Aging Wine Lees: Correlation with Antioxidant Activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Barcia, M.T.; Pertuzatti, P.B.; Rodrigues, D.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; Godoy, H.T. Occurrence of Low Molecular Weight Phenolics in Vitis Vinifera Red Grape Cultivars and Their Winemaking By-Products from São Paulo (Brazil). Food Res. Int. 2014, 62, 500–513. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.-A.; Sun, D.-W. Ultrasound-Assisted Extraction of Phenolics from Wine Lees: Modeling, Optimization and Stability of Extracts during Storage. Ultrason. Sonochem 2014, 21, 706–715. [Google Scholar] [CrossRef]
- De Luca, M.; Restuccia, D.; Spizzirri, U.G.; Crupi, P.; Ioele, G.; Gorelli, B.; Clodoveo, M.L.; Saponara, S.; Aiello, F. Wine Lees as Source of Antioxidant Molecules: Green Extraction Procedure and Biological Activity. Antioxidants 2023, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Caro, M.; Sansone, A.; Amezaga, J.; Navarro, V.; Ferreri, C.; Tueros, I. Wine Lees Modulate Lipid Metabolism and Induce Fatty Acid Remodelling in Zebrafish. Food Funct. 2017, 8, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Umsza-Guez, M.A.; Vázquez-Espinosa, M.; Chinchilla, N.; Aliaño-González, M.J.; Oliveira de Souza, C.; Ayena, K.; Fernández Barbero, G.; Palma, M.; Carrera, C. Enhancing Anthocyanin Extraction from Wine Lees: A Comprehensive Ultrasound-Assisted Optimization Study. Antioxidants 2023, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- Tagkouli, D.; Tsiaka, T.; Kritsi, E.; Soković, M.; Sinanoglou, V.J.; Lantzouraki, D.Z.; Zoumpoulakis, P. Towards the Optimization of Microwave-Assisted Extraction and the Assessment of Chemical Profile, Antioxidant and Antimicrobial Activity of Wine Lees Extracts. Molecules 2022, 27, 2189. [Google Scholar] [CrossRef] [PubMed]
- Delgado de la Torre, M.P.; Priego-Capote, F.; Luque de Castro, M.D. Tentative Identification of Polar and Mid-polar Compounds in Extracts from Wine Lees by Liquid Chromatography–Tandem Mass Spectrometry in High-resolution Mode. J. Mass Spectrom. 2015, 50, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and Ultrasound Pre-Treatments to Enhance Anthocyanins Extraction from Different Wine Lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Delgado de la Torre, M.P.; Priego-Capote, F.; Luque de Castro, M.D. Characterization and Comparison of Wine Lees by Liquid Chromatography–Mass Spectrometry in High-Resolution Mode. J. Agric. Food Chem. 2015, 63, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, M.G.; Francavilla, M.; Albenzio, M.; Inghese, C.; Santillo, A.; Sevi, A.; Caroprese, M. Green Extraction of Bioactive Compounds from Wine Lees and Their Bio-Responses on Immune Modulation Using in Vitro Sheep Model. J. Dairy. Sci. 2022, 105, 4335–4353. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef]
- Delgado de la Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Luque de Castro, M.D. Anthocyanidins, Proanthocyanidins, and Anthocyanins Profiling in Wine Lees by Solid-Phase Extraction–Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry with Data-Dependent Methods. J. Agric. Food Chem. 2013, 61, 12539–12548. [Google Scholar] [CrossRef]
- López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats. Antioxidants 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Bravo, F.I.; Mas-Capdevila, A.; López-Fernández-Sobrino, R.; Torres-Fuentes, C.; Mulero, M.; Alcaide-Hidalgo, J.M.; Muguerza, B. Identification of Novel Antihypertensive Peptides from Wine Lees Hydrolysate. Food Chem. 2022, 366, 130690. [Google Scholar] [CrossRef]
- Mejia, J.A.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; de Pinho, M.N.; Parpinello, G.P. Membrane-Based Operations for the Fractionation of Polyphenols and Polysaccharides From Winery Sludges. Food Bioproc. Tech. 2022, 15, 933–948. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Carretero, I.; Coves, J.R.; Pedrouso, A.; Castro-Barros, C.M.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M.; Sentellas, S. Recovery of Phenolic Compounds from Wine Lees Using Green Processing: Identifying Target Molecules and Assessing Membrane Ultrafiltration Performance. Sci. Total Environ. 2023, 857, 159623. [Google Scholar] [CrossRef] [PubMed]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential Pressure-Driven Membrane Operations to Recover and Fractionate Polyphenols and Polysaccharides from Second Racking Wine Lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Arboleda Meija, J.A.; Parpinello, G.P.; Versari, A.; Conidi, C.; Cassano, A. Microwave-Assisted Extraction and Membrane-Based Separation of Biophenols from Red Wine Lees. Food Bioprod. Process. 2019, 117, 74–83. [Google Scholar] [CrossRef]
- Naziri, E.; Glisic, S.B.; Mantzouridou, F.T.; Tsimidou, M.Z.; Nedovic, V.; Bugarski, B. Advantages of Supercritical Fluid Extraction for Recovery of Squalene from Wine Lees. J. Supercrit. Fluids 2016, 107, 560–565. [Google Scholar] [CrossRef]
- Giacobbo, A.; Dias, B.B.; Onorevoli, B.; Bernardes, A.M.; de Pinho, M.N.; Caramão, E.B.; Rodrigues, E.; Jacques, R.A. Wine Lees from the 1st and 2nd Rackings: Valuable by-Products. J. Food Sci. Technol. 2019, 56, 1559–1566. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods 2022, 11, 284. [Google Scholar] [CrossRef]
- Ivanović, M.; Albreht, A.; Krajnc, P.; Vovk, I.; Razboršek, M.I. Sustainable Ultrasound-Assisted Extraction of Valuable Phenolics from Inflorescences of Helichrysum arenarium L. Using Natural Deep Eutectic Solvents. Ind. Crops Prod. 2021, 160, 113102. [Google Scholar] [CrossRef]
- Sittek, L.-M.; Schmidts, T.M.; Schlupp, P. Potential Application of a Wine Extract in Skin Care: How to Benefit from the Antibacterial, Antioxidant and Elastase Inhibiting Properties. J. Cosmet. Dermatol. Sci. Appl. 2023, 13, 136–155. [Google Scholar] [CrossRef]
- Emmulo, E.; Ceccantoni, B.; Bellincontro, A.; Mencarelli, F. Use of Water and Ethanol Extracts from Wine Grape Seed Pomace to Prepare an Antioxidant Toothpaste. J. Sci. Food Agric. 2021, 101, 5813–5818. [Google Scholar] [CrossRef] [PubMed]
- Carpes, S.T.; Pereira, D.; Moura, C.d.; dos Reis, A.S.; da Silva, L.D.; Oldoni, T.L.C.; Almeida, J.F.; Plata-Oviedo, M.V.S. Lyophilized and Microencapsulated Extracts of Grape Pomace from Winemaking Industry to Prevent Lipid Oxidation in Chicken Pâté. Braz. J. Food Technol. 2020, 23, e2019112. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; Garcia, A.L.; Beitum, L.R.; Zitei-Baptista, L.F.; Aguilar, P.F. Use of Biobased Materials from Agro-Industrial Residues in Food Packaging. In Advanced Applications of Biobased Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 173–229. [Google Scholar]
- Queiroz, V.A.V.; Alves, M.P.; de Oliveira, K.G.; Rocha, M.C.; Conceição, R.R.P.d.; Miguel, R.d.A. Substituição de Metanol Por Água Na Extração de Antocianinas Totais de Glumas de Sorgo Para Uso Como Corante Alimentício. In Boletim de Pesquisa e Desenvolvimento—Embrapa Milho e Sorgo; Sete Lagoas, Brazil, 2014; Volume 1, p. 18. Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/1019952/substituicao-de-metanol-por-agua-na-extracao-de-antocianinas-totais-de-glumas-de-sorgo-para-uso-como-corante-alimenticio (accessed on 15 June 2024).
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- He, Q.; Tang, G.; Hu, Y.; Liu, H.; Tang, H.; Zhou, Y.; Deng, X.; Peng, D.; Qian, Y.; Guo, W.; et al. Green and Highly Effective Extraction of Bioactive Flavonoids from Fructus Aurantii Employing Deep Eutectic Solvents-Based Ultrasonic-Assisted Extraction Protocol. Ultrason. Sonochem. 2024, 102, 106761. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3. [Google Scholar] [CrossRef]
- Kamil Hussain, M.; Saquib, M.; Faheem Khan, M. Techniques for Extraction, Isolation, and Standardization of Bio-Active Compounds from Medicinal Plants. In Natural Bio-Active Compounds; Springer: Singapore, 2019; pp. 179–200. [Google Scholar]
- Luque de Castro, M.D.; García-Ayuso, L.E. Soxhlet Extraction of Solid Materials: An Outdated Technique with a Promising Innovative Future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, A.; Kumari, P.; Nishad, J.H.; Gautam, V.S.; Yadav, M.; Bharti, R.; Kumar, D.; Kharwar, R.N. Advances in Extraction Technologies: Isolation and Purification of Bioactive Compounds from Biological Materials. In Natural Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 409–433. [Google Scholar]
- Blanco-Vega, D.; López-Bellido, F.J.; Alía-Robledo, J.M.; Hermosín-Gutiérrez, I. HPLC–DAD–ESI-MS/MS Characterization of Pyranoanthocyanins Pigments Formed in Model Wine. J. Agric. Food Chem. 2011, 59, 9523–9531. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-Assisted Extraction for Food and Environmental Samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Piñeiro, Z.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Development of a Rapid and Accurate UHPLC-PDA-FL Method for the Quantification of Phenolic Compounds in Grapes. Food Chem. 2021, 334, 127569. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent Advances in Extraction of Nutraceuticals from Plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Horžić, D.; Jambrak, A.R.; Belščak-Cvitanović, A.; Komes, D.; Lelas, V. Comparison of Conventional and Ultrasound Assisted Extraction Techniques of Yellow Tea and Bioactive Composition of Obtained Extracts. Food Bioproc. Tech. 2012, 5, 2858–2870. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González de Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic Compounds and Antioxidant Activity of Seed and Skin Extracts of Red Grape (Vitis vinifera and Vitis labrusca) Pomace from Brazilian Winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Boateng, I.D.; Clark, K. Trends in Extracting Agro-Byproducts’ Phenolics Using Non-Thermal Technologies and Their Combinative Effect: Mechanisms, Potentials, Drawbacks, and Safety Evaluation. Food Chem. 2024, 437, 137841. [Google Scholar] [CrossRef] [PubMed]
- José Aliaño González, M.; Carrera, C.; Barbero, G.F.; Palma, M. A Comparison Study between Ultrasound–Assisted and Enzyme–Assisted Extraction of Anthocyanins from Blackcurrant (Ribes nigrum L.). Food Chem. X 2022, 13, 100192. [Google Scholar] [CrossRef] [PubMed]
- Luque García, J.L.; de Castro, M.D.L. Acceleration and Automation of Solid Sample Treatment, 1st ed.; Elsiever: Amsterdam, The Netherlands, 2002; Volume 24. [Google Scholar]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-Assisted Extraction of Phenolic Compounds from Wine Lees and Spray-Drying of the Extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent-Free Microwave Extraction of Essential Oil from Aromatic Herbs: From Laboratory to Pilot and Industrial Scale. Food Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef]
- Tsiaka, T.; Sinanoglou, V.J.; Zoumpoulakis, P. Extracting Bioactive Compounds from Natural Sources Using Green High-Energy Approaches: Trends and Opportunities in Lab- and Large-Scale Applications. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 307–365. [Google Scholar]
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave Extraction. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Chen, H.-J.; Huang, J.-P.; Lee, P.-C.; Tsai, C.-R.; Hsu, T.-F.; Huang, W.-Y. Kinetics of Tyrosinase Inhibitory Activity Using Vitis vinifera Leaf Extracts. BioMed Res. Int. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Álvarez, A.; Poejo, J.; Matias, A.A.; Duarte, C.M.M.; Cocero, M.J.; Mato, R.B. Microwave Pretreatment to Improve Extraction Efficiency and Polyphenol Extract Richness from Grape Pomace. Effect on Antioxidant Bioactivity. Food Bioprod. Process. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of Microwave-Assisted Extraction of Sargassum Thunbergii Polysaccharides and Its Antioxidant and Hypoglycemic Activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- IOVW. Global Economic Vitiviniculture Data. Available online: https://www.oiv.int/ (accessed on 26 June 2024).
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2021, 11, 59. [Google Scholar] [CrossRef]
- PALOMERO, F.; MORATA, A.; BENITO, S.; GONZALEZ, M.; SUAREZLEPE, J. Conventional and Enzyme-Assisted Autolysis during Ageing over Lees in Red Wines: Influence on the Release of Polysaccharides from Yeast Cell Walls and on Wine Monomeric Anthocyanin Content. Food Chem. 2007, 105, 838–846. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Panja, P. Green Extraction Methods of Food Polyphenols from Vegetable Materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Salanță, L.C.; Chiș, M.S.; Pop, C.R.; Borșa, A.; Diaconeasa, Z.; Vodnar, D.C. Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview. Foods 2022, 11, 2454. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A Review on Protein–Phenolic Interactions and Associated Changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-Waste Technology through the Enzymatic Hydrolysis of Agro-Industrial by-Products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Ozón, B.; Cotabarren, J.; Valicenti, T.; Graciela Parisi, M.; David Obregón, W. Chia Expeller: A Promising Source of Antioxidant, Antihypertensive and Antithrombotic Peptides Produced by Enzymatic Hydrolysis with Alcalase and Flavourzyme. Food Chem. 2022, 380, 132185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; Suo, H.; Tan, J.; Zhang, Y.; Song, J. Identification and Molecular Mechanism of Action of Antibacterial Peptides from Flavourzyme-Hydrolyzed Yak Casein against Staphylococcus Aureus. J. Dairy. Sci. 2023, 106, 3779–3790. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Ferracini-Santos, L.; Sato, H.H. Isolamento de Polímeros Da Parede Celular de Saccharomyces Cerevisiae e Avaliação Da Atividade Antioxidante Da Manana-Proteína Isolada. Quim. Nova 2009, 32, 322–326. [Google Scholar] [CrossRef]
- Romero Díez, R.; Rodrigues, L.; Rodríguez Rojo, S. Wine Lees Valorization: Pretreatment Effect on Anthocyanin Extraction Kinetics from Different Wine Lees. In Proceedings of the 13th International Conference on Renewable Resources and Biorefineries, Wroclaw, Poland, 7–9 June 2017. [Google Scholar]
- Castro-Muñoz, R.; Fíla, V.; Barragán-Huerta, B.E.; Yáñez-Fernández, J.; Piña-Rosas, J.A.; Arboleda-Mejía, J. Processing of Xoconostle Fruit (Opuntia joconostle ) Juice for Improving Its Commercialization Using Membrane Filtration. J. Food Process Preserv. 2018, 42, e13394. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Conidi, C.; Cassano, A. A Membrane-Assisted Green Strategy for Purifying Bioactive Compounds from Extracted White Wine Lees. Sep. Purif. Technol. 2024, 336, 126183. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and Fractionation of Different Phenolic Classes from Winery Sludge Using Ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Cerón-Martínez, L.J.; Hurtado-Benavides, A.M.; Ayala-Aponte, A.; Serna-Cock, L.; Tirado, D.F. A Pilot-Scale Supercritical Carbon Dioxide Extraction to Valorize Colombian Mango Seed Kernel. Molecules 2021, 26, 2279. [Google Scholar] [CrossRef]
- de Souza, R.d.C.; Machado, B.A.S.; Barreto, G.d.A.; Leal, I.L.; dos Anjos, J.P.; Umsza-Guez, M.A. Effect of Experimental Parameters on the Extraction of Grape Seed Oil Obtained by Low Pressure and Supercritical Fluid Extraction. Molecules 2020, 25, 1634. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Kundu, A.; Dutta, A.; Mandal, A.; Saha, S. Citrus Peel as a Source for Waste Valorization and Its Greener Processing; American Chemical Society: Washington, DC, USA, 2022; pp. 147–174. ISBN 9780841297753. [Google Scholar]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical Fluid Extraction Using CO2: Main Applications and Future Perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Pereira, G.S.L.; Magalhães, R.d.S.; Fraga, S.; de Souza, P.T.; de Lima, J.P.; Meirelles, A.J.d.A.; Sampaio, K.A. Extraction of Bioactive Compounds from Butia capitata Fruits Using Supercritical Carbon Dioxide and Pressurized Fluids. J. Supercrit. Fluids 2023, 199, 105959. [Google Scholar] [CrossRef]
- Zulkafli, Z.D.; Wang, H.; Miyashita, F.; Utsumi, N.; Tamura, K. Cosolvent-Modified Supercritical Carbon Dioxide Extraction of Phenolic Compounds from Bamboo Leaves (Sasa palmata). J. Supercrit. Fluids 2014, 94, 123–129. [Google Scholar] [CrossRef]
- Berna, A.; Cháfer, A.; Montón, J.B.; Subirats, S. High-Pressure Solubility Data of System Ethanol (1)+catechin (2)+CO2 (3). J. Supercrit. Fluids 2001, 20, 157–162. [Google Scholar] [CrossRef]
- Kitrytė, V.; Bagdonaitė, D.; Rimantas Venskutonis, P. Biorefining of Industrial Hemp (Cannabis sativa L.) Threshing Residues into Cannabinoid and Antioxidant Fractions by Supercritical Carbon Dioxide, Pressurized Liquid and Enzyme-Assisted Extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial Activity and Composition Profile of Grape (Vitis vinifera) Pomace Extracts Obtained by Supercritical Fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Mihalcea, L.; Coman, G.; Constantin, O.E.; Grigore-Gurgu, L.; Dănilă, G.-M.; Cucolea, E.I.; Turturică, M.; Nicoleta, S. Conjugates-Based Design for Microencapsulation of CO2 Supercritical Extract from Red Grape by-Products to Provide Functional Ingredients. LWT 2023, 184, 114996. [Google Scholar] [CrossRef]
- Comuzzo, P.; Marconi, M.; Zanella, G.; Querzè, M. Pulsed Electric Field Processing of White Grapes (Cv. Garganega): Effects on Wine Composition and Volatile Compounds. Food Chem. 2018, 264, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed Electric Field (PEF): Avant-Garde Extraction Escalation Technology in Food Industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Mok, H.-W.; Ko, M.-J.; Choi, H.-J.; Chung, M.-S. Extraction of Chlorogenic Acids from Hibiscus (Hibiscus syriacus L.) by Subcritical-Water. J. Ind. Eng. Chem. 2022, 111, 255–262. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of Phenolic Compounds from Grape Pomace Using Ohmic Heating: Chemical Composition, Bioactivity and Bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery By-Products: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Hernandes, E.; Zamboni, A.; Fabbri, S.; THOMMAZO, A. Di Using GQM and TAM to Evaluate StArt—A Tool That Supports Systematic Review. CLEI Electron. J. 2012, 15, 3. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer 4.6 Retrieved 2022. Available online: https://automeris.io/WebPlotDigitizer (accessed on 15 January 2024).
- Wos Web of Science, Clarivate Analytics. Available online: https://www.webofscience.com (accessed on 11 November 2023).
- Clarivate Analytics Ferramentas WoS: Treinamento e Dicas. 2020. Available online: https://clarivate.com/webofsciencegroup/wp-content/uploads/sites/2/2020/06/CAPES_Ferramentas-WoS-treinamento-Dicas-tutorial_2020-002.pdf (accessed on 9 November 2023).
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Ferreira, V.C.; Ampese, L.C.; Sganzerla, W.G.; Colpini, L.M.S.; Forster-Carneiro, T. An Updated Review of Recent Applications and Future Perspectives on the Sustainable Valorization of Pitaya (Hylocereus spp.) by-Products. Sustain. Chem. Pharm. 2023, 33, 101070. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
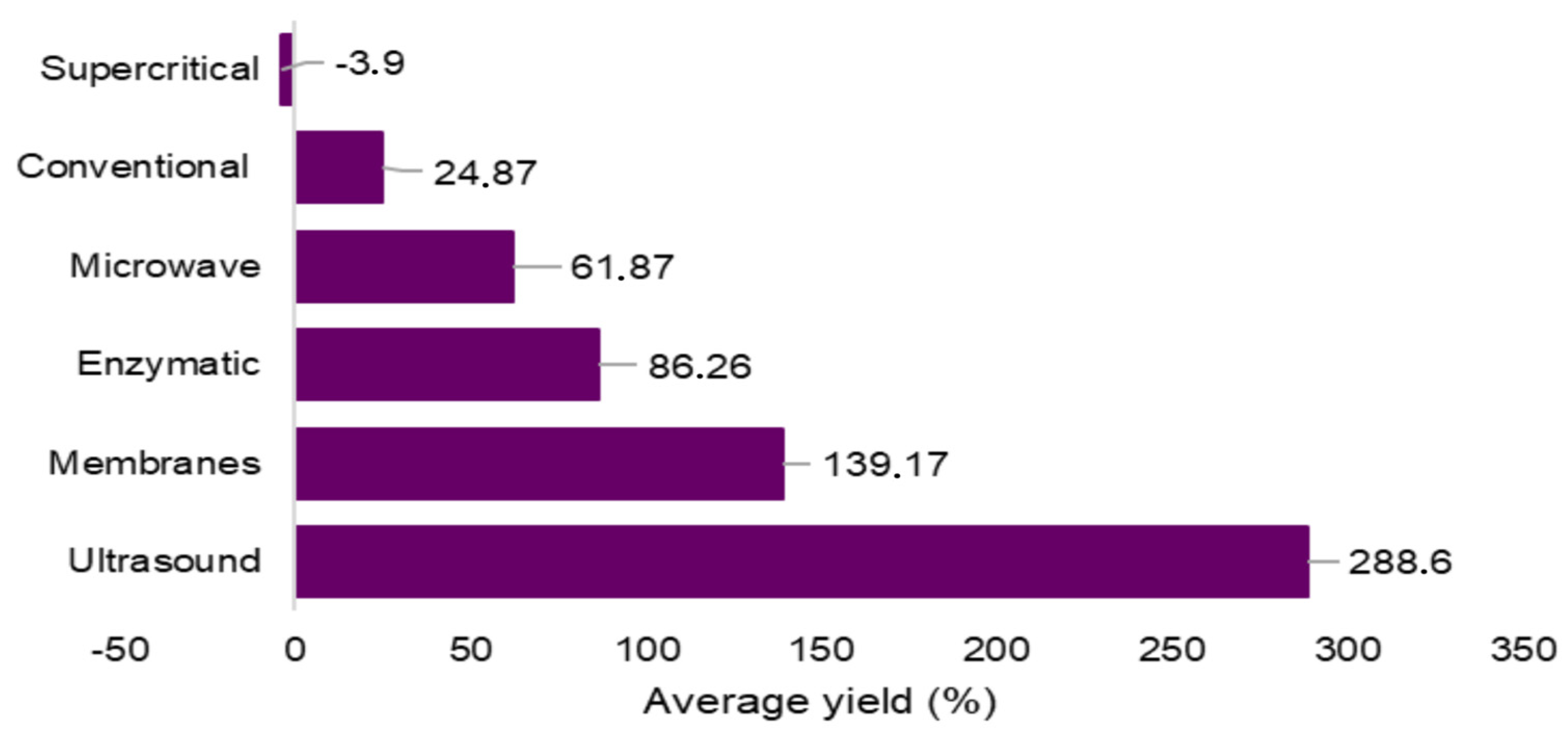
| Order | Extraction Method | Lees Type | Variety | Solvent Employed | Extraction Conditions | Compounds and/or Bioactive Activity Identified | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Conventional | - | Whites (Sauvignon Blanc, Chardonnay, Pinot Gris (PG); reds (Pinot Noir (PN)) | EtOH:H2O (50:50) | Maceration for 60 m | TPC (red wine lees—Pinot Noir—17.3 ± 0.4 and 40.9 ± 1.6 mg/g dry weigh; PN; rosé—9.8 ± 0.2 and 10.5 ± 0.5 mg/g FDM); white wine lees—(3.1 ± 0.2 and 10.3 ± 0.4 mg/g dry weigh). | [22] |
| 2 | Conventional | AF | White (Sauvignon Blanc); red and rosé (Tempranillo) | Methanol | Maceration for 12 h | ANT (rosé—1.147 ± 0.004; red—2.149 ± 0.059) | [23] |
| 3 | Conventional | - | White wine (Albariño); red wine (Tempranillo); rosé wine (Mencía) | Ultrapure water (green solvents) | Proportion 1:100 (lees–solvent Kg/L); temperature: 70 °C; 1 extraction cycle; pH 5.00 | TPC (white wine (Albariño)—Ranging from 84.68 ± 0.47 to 172 ± 0.01; red wine (Tempranillo)—ranging from 341 ± 0.01 to 534 ± 0.20 mg GAE/Kg; rosé wine (Mencía) 48 ± 6 (mg EGA/Kg) | [24] |
| 4 | Conventional | - | - | EtOH:H2O (70:30 v/v) and 1.5 M hydrochloric acid, pH adjusted to 1.5 | Solute–solvent 1:1.5 (m:v) | ANT (316.90 mg per 100 g of extract) | [25] |
| 5 | Conventional | AF | Malbec | EtOH:H2O acidified with HCl (75:25 v/v, pH 4) | Maceration for 60 min. | TPC (57 different types identified); ANT (a wide variety of anthocyanin derivatives, the pyranosanthocyanins) | [26] |
| 6 | Conventional | AF | Braca (Rabigato, Malvasia Fina, Viosinho); Tinta (Touriga Nacional, Touriga Franc, Tinta Roriz) | EtOH:H2O (50:50, v/v) | Maceration for 30 min. | TPC (32.26 mg EAG/g dry weight); TF (20 mg ECAT/g dry weight) | [27] |
| 7 | Ultrasound | AF | Merlot | Eutectic solvent (choline chloride (Ch), acidified with malic acid (Ma)) | Water content in NADES: 35.4% Extraction time: 30.6 min Ultrasonic power: 341.5 W | ANT (6.55 mg malvidin-3-glucoside equivalents (mg/g)) | [28] |
| 8 | Ultrasound | - | Merlot and Vranac | EtOH:H2O (50:50) acidified with 1.5% formic acid | Probe diameter (22 mm); amplitude (90%); extraction time (Vranac—1500 s; Merlot: 921.81 s to 1492.15 s) | TF (trans-resveratrol glucoside (300%), trans-resveratrol (45.75%), quercetin (43.83%), kaempferol (72.73%); ANT (petunidin-3-glucoside (64.95%), malvidin-3-glucoside (89.17%), malvidin-3-(6-O-acetyl)glucoside (49.74%), and malvidin-3-(6-O-p-coumaroyl) glycoside (34.93%)) | [29] |
| 9 | Ultrasound | - | - | EtOH:H2O (80:20, v/v) | Extraction time (5 min); sonication (5 min) | TPC (WL (37.54 mg EAG/g extract); SL (26.66 mg EAG/g extract)) | [30] |
| 10 | Ultrasound | MF | Tempranillo | EtOH:H2O (75:25, v/v) | Sonication (10 min, 700 Hz) | TPC (254 ± 24 mg EAG/g); FT (16 ± 1 to 146 ± 5 mg ECAT/g) | [31] |
| 11 | Ultrasound | AF | Cabernet Sauvignon; Cabernet Franc | Methanol, water, and formic acid (50:48.5:1.5 v/v) | ANT (19 anthocyanins and 9 pyranoanthocyanins were provisionally identified) | [32] | |
| 12 | Ultrasound | MF | Cabernet Sauvignon 60%, Merlot 30%, Cabernet Franc 10% | EtOH:H2O (43.9%) EtOH:H2O (51.5%) | CFT (ultrasound frequency: 40 kHz; extraction time: 25 min and 36.3 min; temperature: 60 °C; ethanol: 43.9%) ANT (extraction time: 36.3 min; temperature: 59.9 °C; solid–solvent: 60:1; ethanol: 51.5%) | TPC (58.76 ± 0.38 mg/g) | [33] |
| 13 | Ultrasound | AF | Nocera Rosso, Magliocco Rosato, Magliocco Canino, Gaglioppo | EtOH:H2O (50:50, v/v) acidified (pH 2) | Frequency: 40 KHz/15 min at 30 °C | TPC (from 4.61 ± 0.09 to 49.56 ± 0.56 mg GAE/g); TF (from 1.54 ± 0.07 to 20.15 ± 0.32 mg ECAT/g) | [34] |
| 14 | Microwave Ultrasound Supercritical Fluid | - | Tempranillo | EtOH:H2O (50:50, v/v) | Ultrasound (30 min; 1:20 g/mL (sample–solvent)); microwave (5 min, 1:20 g/mL, (sample–solvent); temperature: 90 °C) Pressurized liquid (5 min, pressure: 1500 PSI, temperature: 100 °C) | TPC (US—0.27 to 30 mg GAE/g fresh extract; MO—0.31 to 33 mg GAE/g fresh extract; FP—0.32 to 0.37 mg GAE/g fresh extract) | [24] |
| 15 | Ultrasound | MF | Tempranillo | MeOH:H2O:formic acid (75:24.9:0.1; v/v/v) | Sonication 30 °C/30 min | TPC (42,330 ± 2963 µg GAE/g) | [35] |
| 16 | Ultrasound | AF and MF | Syrah and Cabernet Sauvignon | EtOH:H2O (50:50, v/v) | Amplitude of 53%, with cycle 0.30 s−1/ 10 min at 40 °C | ANT (148.03 ± 1.71 mg/100 g) | [36] |
| 17 | Microwave | - | Whites (Kidonitsa, Savvatiano, Chardonnay, Moschofilero); reds (Red Garnet 1, Red Garnet 2, Merlot, Cabernet, Agiorgitiko) | EtOH:H2O (50:50, v/v) | Power: 54 W/35 min; temperature: 85 °C | TPC (red wine—8.0 (±1.4) to 26.0 (±1.1) mg GAE/g of dry sediment); white wine—3.57 (±0.40) to 13.22 (±0.79) mg of ET/g of dry sediment.) | [37] |
| 18 | Microwave | AF | - | EtOH:H2O (60:40) (v/v) adjusted to pH 4 with formic acid | Power: 140 W/10 min | Flavonols (quercetin, quercetin 3-O-glucoside, myricetin, kaempferol and isorhamnetin, catechin, epicatechin, gallocatechin, procyanidin B2, and cinnamtannin); flavones (3,4,5-trimethoxyflavone); Anthocyanins (peonidin 3-O-glucoside, peonidin 3-(6-p—coumarylglucoside), malvidin 3-(6-p—coumarylglucoside)) | [38] |
| 19 | Microwave | FA and MF | Tempranillo | EtOH:H2O (50:50, v/v) | Power: 300 W/90 s | TPC—(23.44 ± 0.11 to 42.04 ± 0.22 mg GAE/g); ANT (2.9 ± 0.2 to 6.2 ± 0.4 mg malvidin-equivalents/g) | [39] |
| 20 | Microwave | MF | Tempranillo; Mazuelo; Garnacha Cabernet | EtOH:H2O (60:40, v/v)—pH 4.0 with formic acid | Power 140 W/10 min | 3,4,5-trimethoxyflavone and malvidin 3-(6-p-coumarylglucoside); two flavanol isomers, catechin and epicatechin; quercetin, myricetin, and conjugates, such as malvidin 3-galactoside; primary amino acid; valine | [40] |
| 21 | Microwave | - | White (Trebbiano); red and rosé (Nero di Troia) | EtOH:H2O (50:50, v/v) | Frequency 2450 MHz/10 min | TPC (white—64.60 (without Na2CO3 at 200 °C)—EtOH; rosé—85.48 ± 10.26 mg EAG/g dry weight (without Na2CO3 at 200 °C)—EtOH:H2O; red—33.62 (with Na2CO3 at 200 °C)—EtOH | [41] |
| 22 | Microwave | - | Tempranillo | EtOH:H2O (60:40, v/v) | Power of 300 W/90 s at 115 °C | TPC (266.0 ± 5.6 mg GAE/g); ANT (29.5 ± 2.3 mg of malv-3-O-gl/g extract) | [42] |
| 23 | Microwave | AF | Tempranillo; Mazuelo; Graciano Garnacha; Syrah; Cabernet Franc Merlot | EtOH:H2O (60:40, v/v) (v/v) adjusted to pH 4 with HCl | Power of 140 W/10 min | ANT (peonidin-3-glucoside, petunidin-3-glucoside, delphinidin-3-glucoside and delphinidin-3-rutinoside) | [43] |
| 24 | Enzymatic | AF | Cabernet | - | Enzyme–substrate ratio, 80 LAPU/g protein 25 °C/2 h, pH 4.0 and 250 rpm | TPC (33.52% of phenolic compounds (24.5 mg/g)) | [44] |
| 25 | Enzymatic | - | Cabernet | - | Enzyme–substrate ratio, 80 LAPU/g protein 25 °C/2 h, pH 4.0 and 250 rpm | 6 new peptides | [45] |
| 26 | Membranes | - | Sangiovese and Cabernet Sauvignon | - | UF (2 bar/25 ± 1 °C/0.55 L/min) | TPC mg GAE/L (UF—feed: 655.4 ± 13.6; permeate: 382.6 ± 25.1; retentate: 715.9 ± 44.5) | [46] |
| 27 | Membranes | MF | Albariño | Water (green solvent) | 40 °C/30 min shaking | Caftaric acid (200 µg/g); transcoutaric acid, cis-coutaric acid, gallic acid, and astilbine with concentrations between 15 and 40 µg/g | [47] |
| 28 | Membranes | - | Merlot | - | Feeding speed 150 L/h | TPC (rejection coefficient—NF270—92.84%) | [48] |
| 29 | Membranes | AF | - | EtOH:H2O (75:25, v/v) with hydrochloric acid | Microwave (power: 350 W, time: 2 min); microfiltration (PVDF membrane, 0.15 μm) and nanofiltration (polyamide membrane, 150 Da) membranes | Microwave—TPC (933 GAE mg/L); membranes—TPC (4662.5 ± 224.8 GAE mg/L) | [49] |
| 30 | Supercritical Fluid | AF | Xynomavro | CO2 | Temperature: 40 °C | Scalene (Total: 16.9 g/kg) | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, F.d.O.; Ferreira, V.C.; Barbero, G.F.; Carrera, C.; Ferreira, E.d.S.; Umsza-Guez, M.A. Extraction of Bioactive Compounds from Wine Lees: A Systematic and Bibliometric Review. Foods 2024, 13, 2060. https://doi.org/10.3390/foods13132060
Melo FdO, Ferreira VC, Barbero GF, Carrera C, Ferreira EdS, Umsza-Guez MA. Extraction of Bioactive Compounds from Wine Lees: A Systematic and Bibliometric Review. Foods. 2024; 13(13):2060. https://doi.org/10.3390/foods13132060
Chicago/Turabian StyleMelo, Filipe de Oliveira, Vanessa Cosme Ferreira, Gerardo Fernandez Barbero, Ceferino Carrera, Ederlan de Souza Ferreira, and Marcelo Andrés Umsza-Guez. 2024. "Extraction of Bioactive Compounds from Wine Lees: A Systematic and Bibliometric Review" Foods 13, no. 13: 2060. https://doi.org/10.3390/foods13132060
APA StyleMelo, F. d. O., Ferreira, V. C., Barbero, G. F., Carrera, C., Ferreira, E. d. S., & Umsza-Guez, M. A. (2024). Extraction of Bioactive Compounds from Wine Lees: A Systematic and Bibliometric Review. Foods, 13(13), 2060. https://doi.org/10.3390/foods13132060










