Euglena gracilis Protein: Effects of Different Acidic and Alkaline Environments on Structural Characteristics and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Euglena gracilis Protein
2.2. Molecular Weight Distribution (MWD)
2.3. Amino Acid Composition
2.4. Fourier Transform Infrared Spectroscopy (FTIR) of EGP
2.5. Foaming Properties
2.6. Emulsifying Properties
2.7. Solubility
2.8. Free and Total Sulfhydryl
2.9. Water-Holding Capacity (WHC)
2.10. Fat Absorption Capacity (FAC)
2.11. Particle Size Distributions
2.12. Zeta Potential
2.13. Exogenous Fluorescence Spectroscopy
2.14. Intrinsic Fluorescence Spectroscopy
2.15. X-ray Diffraction (XRD)
2.16. Thermal Properties
2.17. Statistical Analysis
3. Results
3.1. Molecular Weight Distribution
3.2. Amino Acid Composition
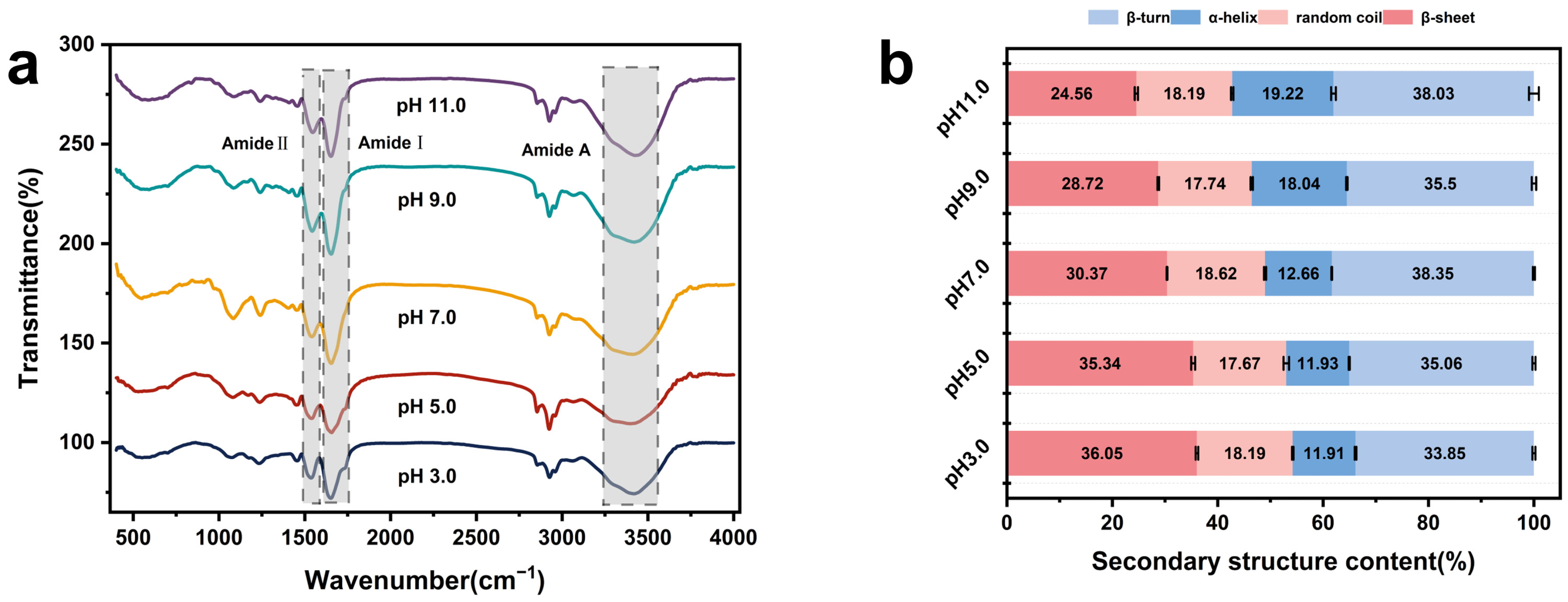
3.3. Fourier Transform Infrared Spectroscopic Analysis (FTIR)
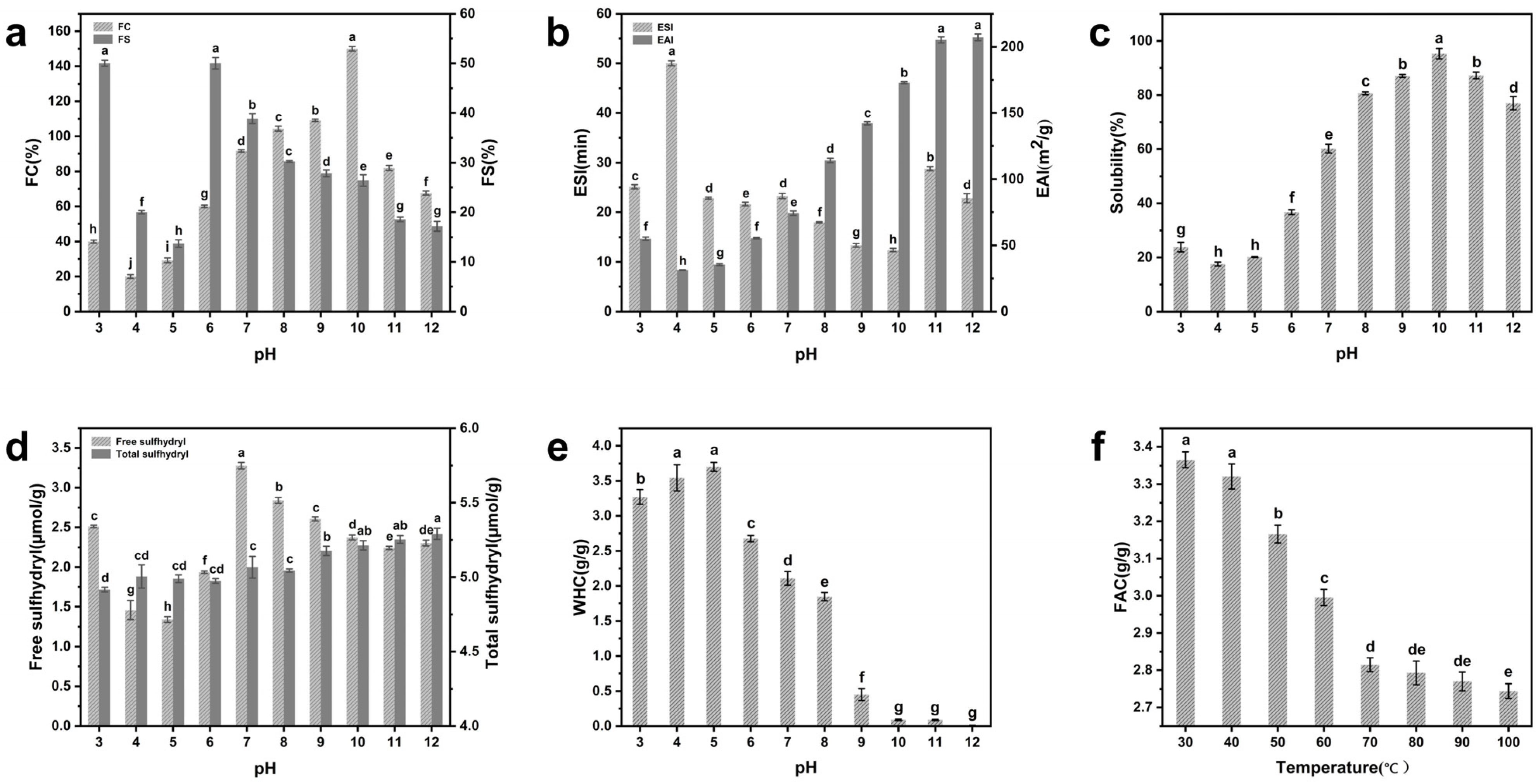
3.4. Foaming Properties
3.5. Emulsifying Properties
3.6. Solubility
3.7. Free Sulfhydryl and Total Sulfhydryl
3.8. Water-Holding Capacity
3.9. Fat Absorption Capacity
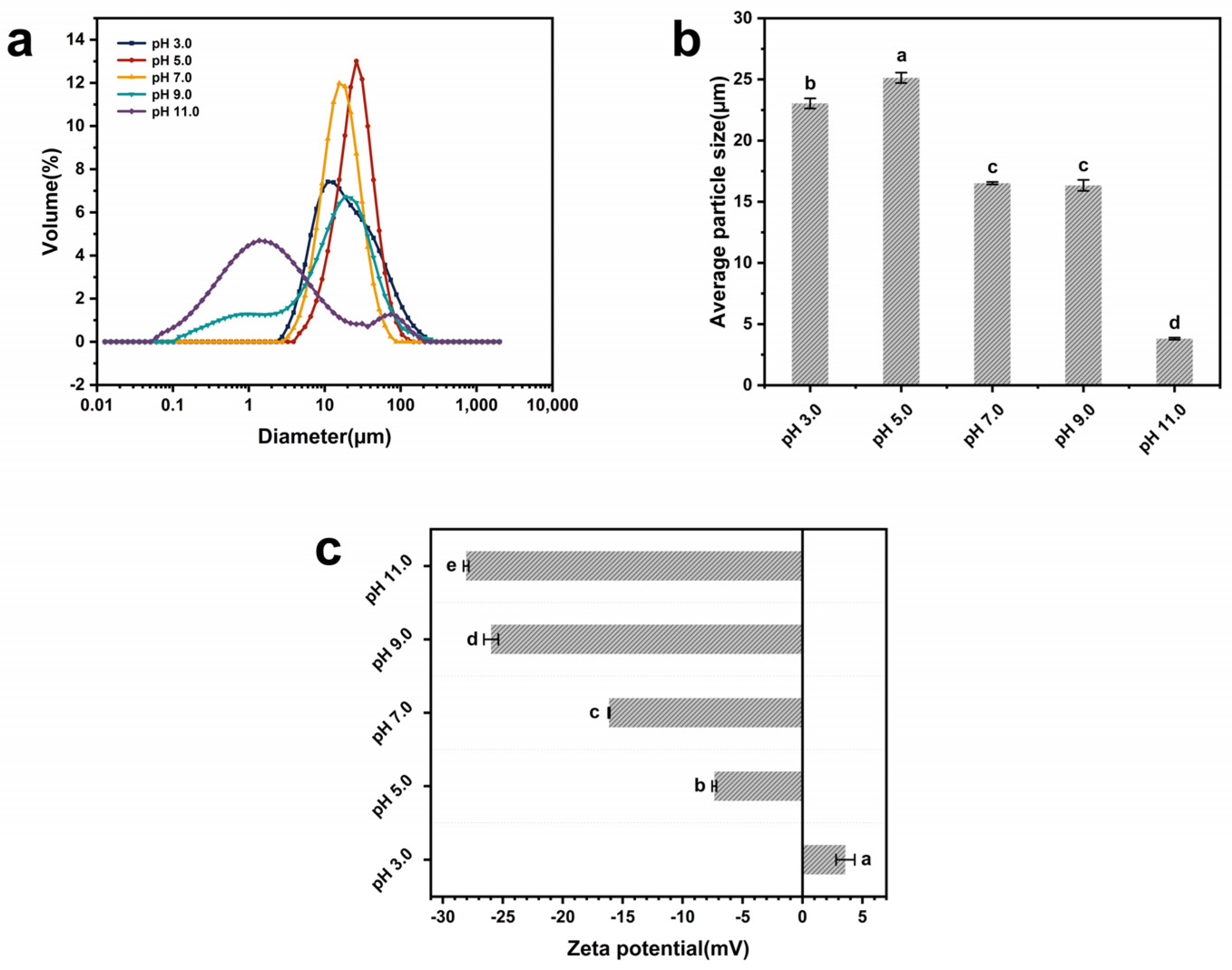
3.10. Particle Size Distribution and Zeta Potential
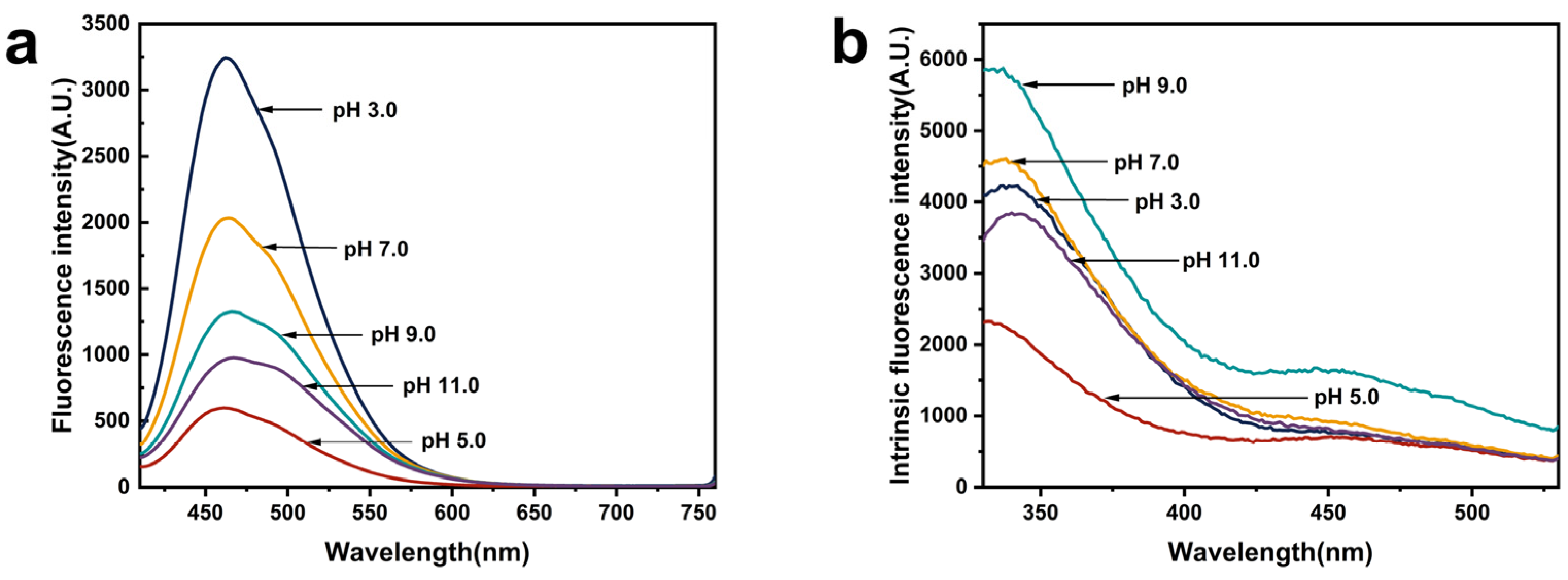
3.11. Exogenous Fluorescence Spectroscopy
3.12. Intrinsic Fluorescence Spectroscopy
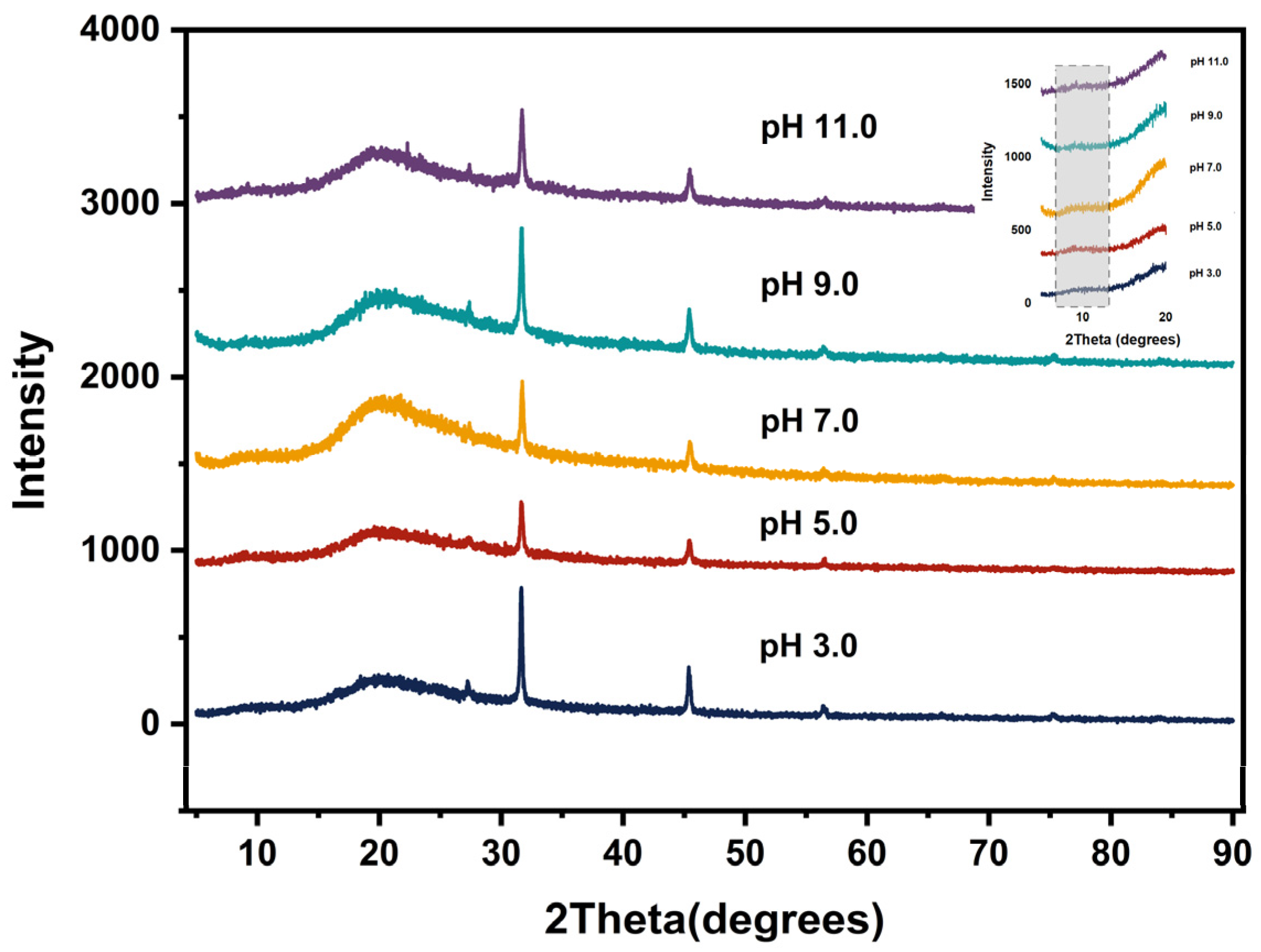
3.13. X-ray Diffraction Analysis
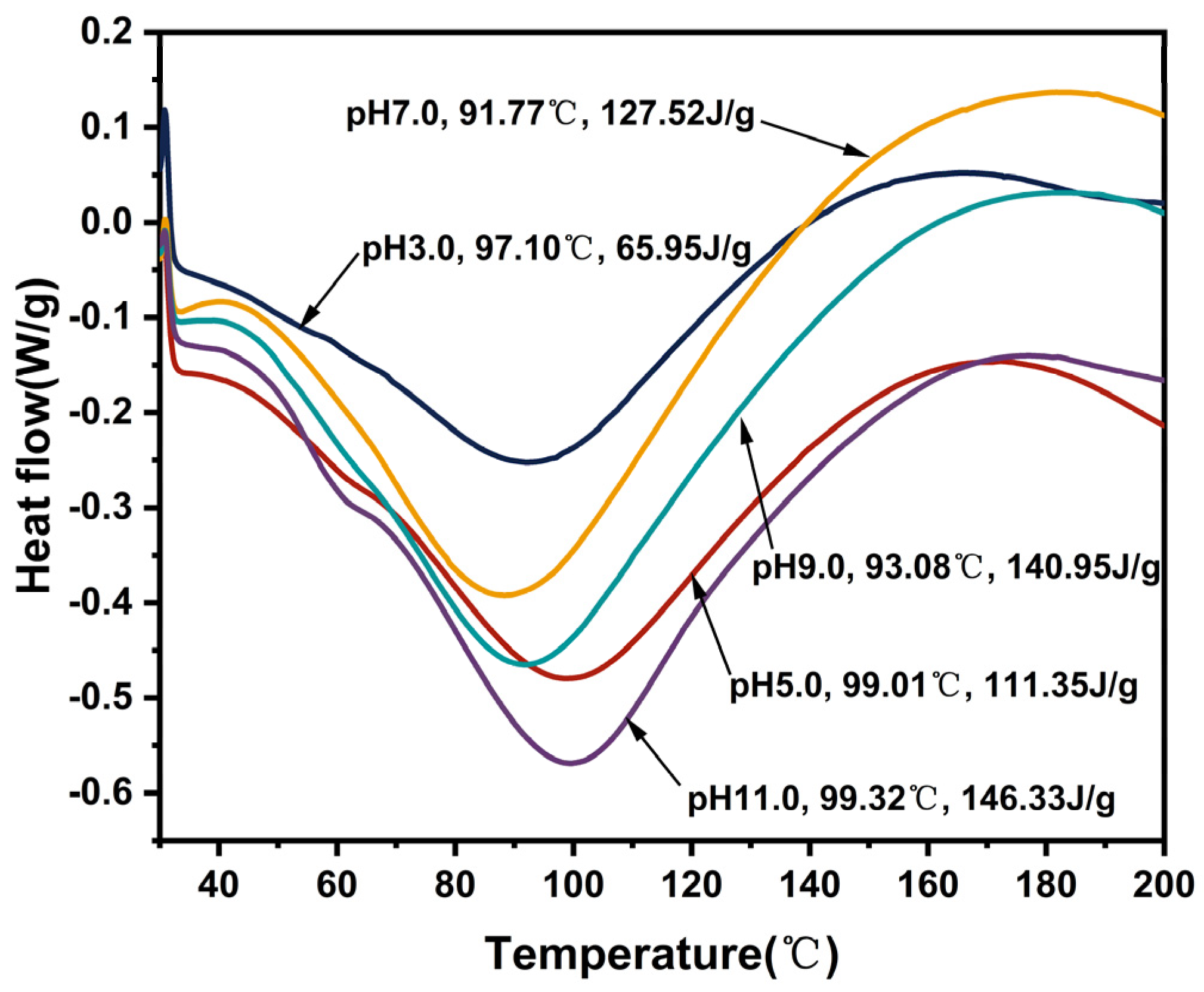
3.14. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kottuparambil, S.; Thankamony, R.L.; Agusti, S. Euglena as a potential natural source of value-added metabolites. A review. Algal Res. 2019, 37, 154–159. [Google Scholar] [CrossRef]
- Carcea, M.; Sorto, M.; Batello, C.; Narducci, V.; Aguzzi, A.; Azzini, E.; Fantauzzi, P.; Finotti, E.; Gabrielli, P.; Galli, V.; et al. Nutritional characterization of traditional and improved dihé, alimentary blue-green algae from the lake Chad region in Africa. LWT Food Sci. Technol. 2015, 62, 753–763. [Google Scholar] [CrossRef]
- Williamson, E.; Ross, I.L.; Wall, B.T.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2024, 29, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Lisboa, C.R.; Costa, J.A.V. High protein ingredients of microalgal origin: Obtainment and functional properties. Innov. Food Sci. Emerg. Technol. 2018, 47, 187–194. [Google Scholar] [CrossRef]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef]
- Piovan, A.; Brun, P.; Peron, G.; Filippini, R.; Dall’Acqua, S.; Caniato, R. Euglena gracilis as an alternative source of nutrients. Planta Med. 2016, 82, P829. [Google Scholar] [CrossRef]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts From Euglena gracilis: Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M.; Goktepe, I. Peanut protein concentrate: Production and functional properties as affected by processing. Food Chem. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, X.; Zhang, X.; Liu, H. Extraction, structural and functional properties of Haematococcus pluvialis protein after pigment removal. Int. J. Biol. Macromol. 2019, 140, 1073–1083. [Google Scholar] [CrossRef]
- Jeong, M.-S.; Cho, S.-J. Effect of pH-shifting on the water holding capacity and gelation properties of mung bean protein isolate. Food Res. Int. 2024, 177, 113912. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Zhong, Z.; Xiong, Y.L. Thermosonication-induced structural changes and solution properties of mung bean protein. Ultrason. Sonochem. 2020, 62, 104908. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, P.; Song, S.; Zhang, X.; Hayat, K.; Xia, S.; Jia, C.; Gu, F. Contribution of sulfur-containing compounds to the colour-inhibiting effect and improved antioxidant activity of Maillard reaction products of soybean protein hydrolysates. J. Sci. Food Agric. 2011, 91, 710–720. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhao, J.; Cao, X.; Ye, Y.; Wu, Z.; Yue, J.; Yang, L.; Jin, R.; Sun, H. Low pH-shifting treatment would improve functional properties of black turtle bean (Phaseolus vulgaris L.) protein isolate with immunoreactivity reduction. Food Chem. 2020, 330, 127217. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.A.; Aluko, R.E. Conversion of a low protein hemp seed meal into a functional protein concentrate through enzymatic digestion of fibre coupled with membrane ultrafiltration. Innov. Food Sci. Emerg. Technol. 2015, 31, 151–159. [Google Scholar] [CrossRef]
- Chee, K.L.; Ling, H.K.; Ayob, M.K. Optimization of trypsin-assisted extraction, physico-chemical characterization, nutritional qualities and functionalities of palm kernel cake protein. LWT Food Sci. Technol. 2012, 46, 419–427. [Google Scholar] [CrossRef]
- Lawal, O.S. Functionality of African locust bean (Parkia biglobossa) protein isolate: Effects of pH, ionic strength and various protein concentrations. Food Chem. 2004, 86, 345–355. [Google Scholar] [CrossRef]
- Plazzotta, S.; Moretton, M.; Calligaris, S.; Manzocco, L. Physical, chemical, and techno-functional properties of soy okara powders obtained by high pressure homogenization and alkaline-acid recovery. Food Bioprod. Process. 2021, 128, 95–101. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Dabbour, M.; Mintah, B.K.; Huang, L.; Dai, C.; He, R.; Ma, H. Changes in physicochemical, structural and functional properties, and lysinoalanine formation during the unfolding and refolding of pH-shifted black soldier fly larvae albumin. Int. J. Biol. Macromol. 2024, 272, 132801. [Google Scholar] [CrossRef]
- Xu, P.-W.; Yuan, X.-F.; Zhao, B. A study on pH-mediated variations and differences in the structure and function of hemp seed albumin, globulin, and protein isolate. Food Biosci. 2023, 56, 103452. [Google Scholar] [CrossRef]
- Abarghoei, M.; Goli, M.; Shahi, S. Investigation of cold atmospheric plasma effects on functional and physicochemical properties of wheat germ protein isolate. LWT 2023, 177, 114585. [Google Scholar] [CrossRef]
- He, Q.; Sun, X.; He, S.; Wang, T.; Zhao, J.; Yang, L.; Wu, Z.; Sun, H. PEGylation of black kidney bean (Phaseolus vulgaris L.) protein isolate with potential functironal properties. Colloids Surf. B Biointerfaces 2018, 164, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Y.; Ma, C.; Julian McClements, D.; Liu, F.; Liu, X. Pea protein isolate-inulin conjugates prepared by pH-shift treatment and ultrasonic-enhanced glycosylation: Structural and functional properties. Food Chem. 2022, 384, 132511. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, R.; Can Karaca, A. Physicochemical and functional properties of proteins isolated from local beans of Turkey. LWT 2020, 130, 109609. [Google Scholar] [CrossRef]
- Ai, M.; Tang, T.; Zhou, L.; Ling, Z.; Guo, S.; Jiang, A. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2019, 87, 933–942. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D.J.; Wu, L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016, 61, 251–260. [Google Scholar] [CrossRef]
- Ma, X.; Huang, C.; Zheng, C.; Wang, W.; Ying, H.; Liu, C. Effect of oil extraction methods on walnut oil quality characteristics and the functional properties of walnut protein isolate. Food Chem. 2024, 438, 138052. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Ba, F.; Ursu, A.V.; Laroche, C.; Djelveh, G. Haematococcus pluvialis soluble proteins: Extraction, characterization, concentration/fractionation and emulsifying properties. Bioresour. Technol. 2016, 200, 147–152. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, C.-Y.; Wang, S.-T.; Li, Y.-Q.; Mo, H.-Z.; He, J.-X. Physicochemical properties and antioxidant activities of tree peony (Paeonia suffruticosa Andr.) seed protein hydrolysates obtained with different proteases. Food Chem. 2021, 345, 128765. [Google Scholar] [CrossRef]
- Mohammadi, M.; Soltanzadeh, M.; Ebrahimi, A.R.; Hamishehkar, H. Spirulina platensis protein hydrolysates: Techno-functional, nutritional and antioxidant properties. Algal Res. 2022, 65, 102739. [Google Scholar] [CrossRef]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose Tissue Branched Chain Amino Acid (BCAA) Metabolism Modulates Circulating BCAA Levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef] [PubMed]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Withana-Gamage, T.S.; Wanasundara, J.P.D.; Pietrasik, Z.; Shand, P.J. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): A comparative study with soy (Glycine max) and pea (Pisum sativum L.). J. Sci. Food Agric. 2011, 91, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, S.-M.; Xiong, S.-B.; Xie, B.-J.; Qin, L.-H. Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Sci. 2008, 80, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-F.; Li, Y.-Q.; Wang, C.-Y.; Zhao, X.-Z.; Liang, Y.; He, J.-X.; Mo, H.-Z. Impact of pH on the physicochemical and rheological properties of mung bean (Vigna radiata L.) protein. Process Biochem. 2021, 111, 274–284. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Li, J.; Pan, D.; Du, L. Enhancing the functionalities of chickpea protein isolate through a combined strategy with pH-shifting and cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2024, 93, 103607. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Li, S. Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Res. Int. 2012, 48, 299–306. [Google Scholar] [CrossRef]
- Bing, S.-J.; Li, Y.-Q.; Sun, G.-J.; Wang, C.-Y.; Liang, Y.; Hua, D.-L.; Chen, L.; Mo, H.-Z. Effect of different acidic or alkaline environments on structural characteristics, functional and physicochemical properties of lentinus edodes protein. Process Biochem. 2023, 130, 15–25. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Huang, S.; Ruan, X.; Zhou, Q.; Sun, W. Effects of Spray Drying and Freeze Drying on the Properties of Protein Isolate from Rice Dreg Protein. Food Bioprocess. Technol. 2013, 6, 1759–1769. [Google Scholar] [CrossRef]
- Gao, L.-L.; Li, Y.-Q.; Wang, Z.-S.; Sun, G.-J.; Qi, X.-M.; Mo, H.-Z. Physicochemical characteristics and functionality of tree peony (Paeonia suffruticosa Andr.) seed protein. Food Chem. 2018, 240, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Sun, C.-X.; Mata, A.; Corke, H.; Gan, R.-Y.; Fang, Y. Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Das, D.; Panesar, P.S.; Saini, C.S. pH shifting treatment of ultrasonically extracted soybean meal protein isolate: Effect on functional, structural, morphological and thermal properties. Process Biochem. 2022, 120, 227–238. [Google Scholar] [CrossRef]
- Xi, C.; Kang, N.; Zhao, C.; Liu, Y.; Sun, Z.; Zhang, T. Effects of pH and different sugars on the structures and emulsification properties of whey protein isolate-sugar conjugates. Food Biosci. 2020, 33, 100507. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Zhao, X.; Xu, X. Comparison of the interfacial properties of native and refolded myofibrillar proteins subjected to pH-shifting. Food Chem. 2022, 380, 131734. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-X.; Sun, X.-H.; Chen, Z.-C.; Peng, W.; Qian, H.-F.; Zhou, H.-M. Comparison of functional properties and secondary structures of defatted wheat germ proteins separated by reverse micelles and alkaline extraction and isoelectric precipitation. Food Chem. 2010, 123, 1163–1169. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Ganesan, K.; Selvaraj, K.; Subba Rao, P.V. Studies on the functional properties of protein concentrate of Kappaphycus alvarezii (Doty) Doty—An edible seaweed. Food Chem. 2014, 153, 353–360. [Google Scholar] [CrossRef]
- Aletor, O.; Oshodi, A.A.; Ipinmoroti, K. Chemical composition of common leafy vegetables and functional properties of their leaf protein concentrates. Food Chem. 2002, 78, 63–68. [Google Scholar] [CrossRef]
- Julakanti, S.; Charles, A.P.R.; Zhao, J.; Bullock, F.; Syed, R.; Myles, Y.; Wu, Y. Hempseed protein (Cannabis sativa L.): Influence of extraction pH and ball milling on physicochemical and functional properties. Food Hydrocoll. 2023, 143, 108835. [Google Scholar] [CrossRef]
- Silva, A.M.M.d.; Almeida, F.S.; Silva, M.F.d.; Goldbeck, R.; Sato, A.C.K. How do pH and temperature influence extraction yield, physicochemical, functional, and rheological characteristics of brewer spent grain protein concentrates? Food Bioprod. Process. 2023, 139, 34–45. [Google Scholar] [CrossRef]
- Das, D.; Mir, N.A.; Chandla, N.K.; Singh, S. Combined effect of pH treatment and the extraction pH on the physicochemical, functional and rheological characteristics of amaranth (Amaranthus hypochondriacus) seed protein isolates. Food Chem. 2021, 353, 129466. [Google Scholar] [CrossRef]
- Mondor, M.; Ippersiel, D.; Lamarche, F.; Boye, J.I. Effect of electro-acidification treatment and ionic environment on soy protein extract particle size distribution and ultrafiltration permeate flux. J. Membr. Sci. 2004, 231, 169–179. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, J.; Xie, Q.; Pi, X.; Peng, Z.; Sun, Y.; Cheng, J. pH-induced physiochemical and structural changes of milk proteins mixtures and its effect on foaming behavior. Int. J. Biol. Macromol. 2024, 254, 127838. [Google Scholar] [CrossRef]
- Ai, M.-M.; Guo, S.-G.; Zhou, Q.; Wu, W.-L.; Jiang, A.-M. The investigation of the changes in physicochemical, texture and rheological characteristics of salted duck egg yolk during salting. LWT 2018, 88, 119–125. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhang, Z.; Wang, Y.; Mintah, B.K.; Dabbour, M.; Jiang, H.; He, R.; Ma, H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochem. 2020, 69, 105240. [Google Scholar] [CrossRef]
- Rahmati, N.F.; Koocheki, A.; Varidi, M.; Kadkhodaee, R. Introducing Speckled sugar bean (Phaseolus vulgaris) protein isolates as a new source of emulsifying agent. Food Hydrocoll. 2018, 79, 498–508. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Z.; Cao, Y.; Ming, Y.; Wu, M. Improving the solubility and interfacial absorption of hempseed protein via a novel high pressure homogenization-assisted pH-shift strategy. Food Chem. 2024, 442, 138447. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, R.; Xue, J.; Yang, X.; Lin, D. Characterization of bacterial cellulose nanofibers/soy protein isolate complex particles for Pickering emulsion gels: The effect of protein structure changes induced by pH. Int. J. Biol. Macromol. 2023, 226, 254–266. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S.; Surasani, V.K.R. Influence of processing and pH on amino acid profile, morphology, electrophoretic pattern, bioactive potential and functional characteristics of alfalfa protein isolates. Food Chem. 2020, 333, 127503. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the domain structure of the 7S and 11S globulins from soy proteins by XRD and FTIR. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef]
- Malik, M.A.; Saini, C.S. Heat treatment of sunflower protein isolates near isoelectric point: Effect on rheological and structural properties. Food Chem. 2019, 276, 554–561. [Google Scholar] [CrossRef]
- Xu, H.; Pan, J.; Dabbour, M.; Kumah Mintah, B.; Chen, W.; Yang, F.; Zhang, Z.; Cheng, Y.; Dai, C.; He, R.; et al. Synergistic effects of pH shift and heat treatment on solubility, physicochemical and structural properties, and lysinoalanine formation in silkworm pupa protein isolates. Food Res. Int. 2023, 165, 112554. [Google Scholar] [CrossRef]
- Mo, X.; Zhong, Z.; Wang, D.; Sun, X. Soybean Glycinin Subunits: Characterization of Physicochemical and Adhesion Properties. J. Agric. Food Chem. 2006, 54, 7589–7593. [Google Scholar] [CrossRef]
- Tang, C.-H.; Ten, Z.; Wang, X.-S.; Yang, X.-Q. Physicochemical and Functional Properties of Hemp (Cannabis sativa L.) Protein Isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Rawiwan, P.; Quek, S.Y. Physicochemical and functional attributes of RuBisCo-enriched Brassicaceae leaf protein concentrates. Food Hydrocoll. 2024, 151, 109887. [Google Scholar] [CrossRef]
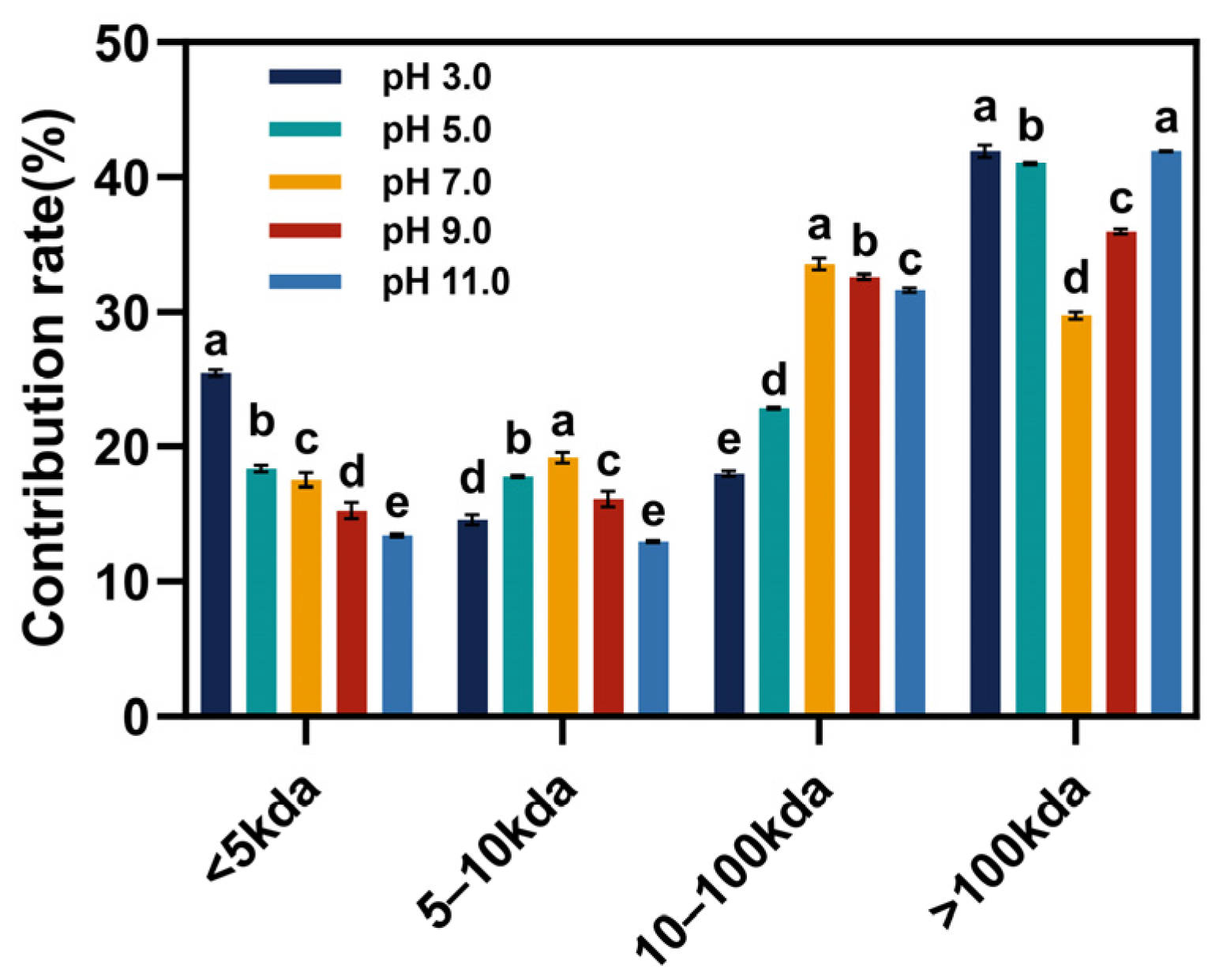
| Sample | Molecular Weight Distribution (%) | |||
|---|---|---|---|---|
| >100 kDa | 10–100 kDa | 5–10 kDa | <5 kDa | |
| pH 3.0 | 41.95 ± 0.46 a | 17.97 ± 0.20 e | 14.61 ± 0.38 d | 25.47 ± 0.26 a |
| pH 5.0 | 41.03 ± 0.08 b | 22.85 ± 0.09 d | 17.77 ± 0.09 b | 18.35 ± 0.24 b |
| pH 7.0 | 29.75 ± 0.28 d | 33.53 ± 0.44 a | 19.18 ± 0.40 a | 17.54 ± 0.52 c |
| pH 9.0 | 35.95 ± 0.20 c | 32.61 ± 0.19 b | 16.15 ± 0.57 c | 15.29 ± 0.59 d |
| pH 11.0 | 41.94 ± 0.50 a | 31.65 ± 0.17 c | 12.97 ± 0.08 e | 13.44 ± 0.11 e |
| Amino Acid | EGP (mg/g) | FAO/WHO Reference for Children (mg/g) | FAO/WHO Reference for Adults (mg/g) |
|---|---|---|---|
| Asparagine (Asp) | 76.81 ± 0.07 | - | - |
| Threonine (Thr) | 45.70 ± 0.03 | 14 | 9 |
| Serine (Ser) | 44.24 ± 0.18 | - | - |
| Glutarnine (Glu) | 87.82 ± 0.19 | - | - |
| Glycine (Gly) | 40.12 ± 0.03 | - | - |
| Alaine (Ala) | 50.82 ± 0.11 | - | - |
| Cystine (Cys) | 27.93 ± 0.04 | 25 | 17 |
| Valine (Val) | 52.45 ± 0.05 | 35 | 13 |
| Methionine (Met) | 28.14 ± 0.12 | - | - |
| Isoleucine (Ile) | 34.40 ± 0.27 | 28 | 13 |
| Leucine (Leu) | 63.66 ± 0.14 | 66 | 19 |
| Tyrosine (Tyr) | 47.42 ± 0.08 | 63 | 19 |
| Phenylalanine (Phe) | 45.04 ± 0.19 | - | - |
| Histidine (His) | 33.21 ± 0.17 | 19 | - |
| Lysine (Lys) | 56.09 ± 0.05 | 58 | 16 |
| Arginine (Arg) | 58.69 ± 0.16 | - | - |
| Proline (Pro) | 50.14 ± 0.11 | - | - |
| Essential amino acids | 434.06 ± 0.27 | - | - |
| Non-essential amino acids | 408.65 ± 0.76 | - | - |
| Hydrophobic amino acids | 364.78 ± 0.30 | - | - |
| Hydrophilic amino acids | 165.30 ± 0.29 | - | - |
| Acidic amino acids | 164.63 ± 0.23 | - | - |
| Basic amino acids | 148.00 ± 0.29 | - | - |
| Aromatic amino acids | 92.47 ± 0.12 | - | - |
| Branched-chain amino acids | 150.51 ± 0.32 | - | - |
| Negatively charged amino acids | 254.57 ± 0.36 | - | - |
| Positively charged amino acids | 148.00 ± 0.29 | - | - |
| Sample | β-Sheets (%) | α-Helices (%) | β-Turns (%) | Random Coils (%) |
|---|---|---|---|---|
| pH 3.0 | 36.05 ± 0.25 a | 11.91 ± 0.17 d | 33.85 ± 0.28 c | 18.19 ± 0.15 ab |
| pH 5.0 | 35.34 ± 0.37 b | 11.93 ± 0.11 d | 35.06 ± 0.30 b | 17.67 ± 0.53 b |
| pH 7.0 | 30.37 ± 0.07 c | 12.66 ± 0.06 c | 38.35 ± 0.19 a | 18.62 ± 0.20 a |
| pH 9.0 | 28.72 ± 0.16 d | 18.04 ± 0.13 b | 35.50 ± 0.42 b | 17.74 ± 0.21 b |
| pH 11.0 | 24.56 ± 0.31 e | 19.22 ± 0.46 a | 38.03 ± 0.93 a | 18.19 ± 0.23 ab |
| Samples | Average Particle Size (µm) | Zeta Potential (mV) | Hydrophobicity |
|---|---|---|---|
| pH = 3.0 | 23.04 ± 0.41 b | 3.58 ± 0.78 a | 3244.67 ± 16.01 a |
| pH = 5.0 | 25.13 ± 0.42 a | −7.36 ± 0.18 b | 598.77 ± 7.95 e |
| pH = 7.0 | 16.52 ± 0.10 c | −16.14 ± 0.06 c | 2039.67 ± 37.45 b |
| pH = 9.0 | 16.34 ± 0.45 c | −25.98 ± 0.60 d | 1326.67 ± 10.50 c |
| pH = 11.0 | 3.81 ± 0.08 d | −28.06 ± 0.22 e | 977.70 ± 8.45 d |
| Sample | To (°C) | Td (°C) | Te (°C) | ΔH (J/g) |
|---|---|---|---|---|
| pH 3.0 | 55.45 ± 0.42 a | 97.10 ± 0.28 b | 136.82 ± 0.34 d | 65.95 ± 0.16 e |
| pH 5.0 | 53.31 ± 0.36 a | 99.01 ± 0.14 a | 149.55 ± 0.21 a | 111.35 ± 0.25 d |
| pH 7.0 | 51.36 ± 0.38 b | 91.77 ± 0.23 d | 145.91 ± 0.12 b | 127.52 ± 0.47 c |
| pH 9.0 | 49.82 ± 0.12 c | 93.08 ± 0.47 c | 143.89 ± 0.65 c | 140.95 ± 0.34 b |
| pH 11.0 | 46.94 ± 0.43 d | 99.32 ± 0.31 a | 145.33 ± 0.23 b | 146.33 ± 0.37 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Liu, M.; Wang, Y.; Zhu, Z.; Zhao, X. Euglena gracilis Protein: Effects of Different Acidic and Alkaline Environments on Structural Characteristics and Functional Properties. Foods 2024, 13, 2050. https://doi.org/10.3390/foods13132050
Zhu L, Liu M, Wang Y, Zhu Z, Zhao X. Euglena gracilis Protein: Effects of Different Acidic and Alkaline Environments on Structural Characteristics and Functional Properties. Foods. 2024; 13(13):2050. https://doi.org/10.3390/foods13132050
Chicago/Turabian StyleZhu, Laijing, Meng Liu, Yanli Wang, Zhunyao Zhu, and Xiangzhong Zhao. 2024. "Euglena gracilis Protein: Effects of Different Acidic and Alkaline Environments on Structural Characteristics and Functional Properties" Foods 13, no. 13: 2050. https://doi.org/10.3390/foods13132050
APA StyleZhu, L., Liu, M., Wang, Y., Zhu, Z., & Zhao, X. (2024). Euglena gracilis Protein: Effects of Different Acidic and Alkaline Environments on Structural Characteristics and Functional Properties. Foods, 13(13), 2050. https://doi.org/10.3390/foods13132050







