Sacha Inchi (Plukenetia volubilis L.) Protein Hydrolysate as a New Ingredient of Functional Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Hydrolysis of Sacha Inchi Protein Isolate
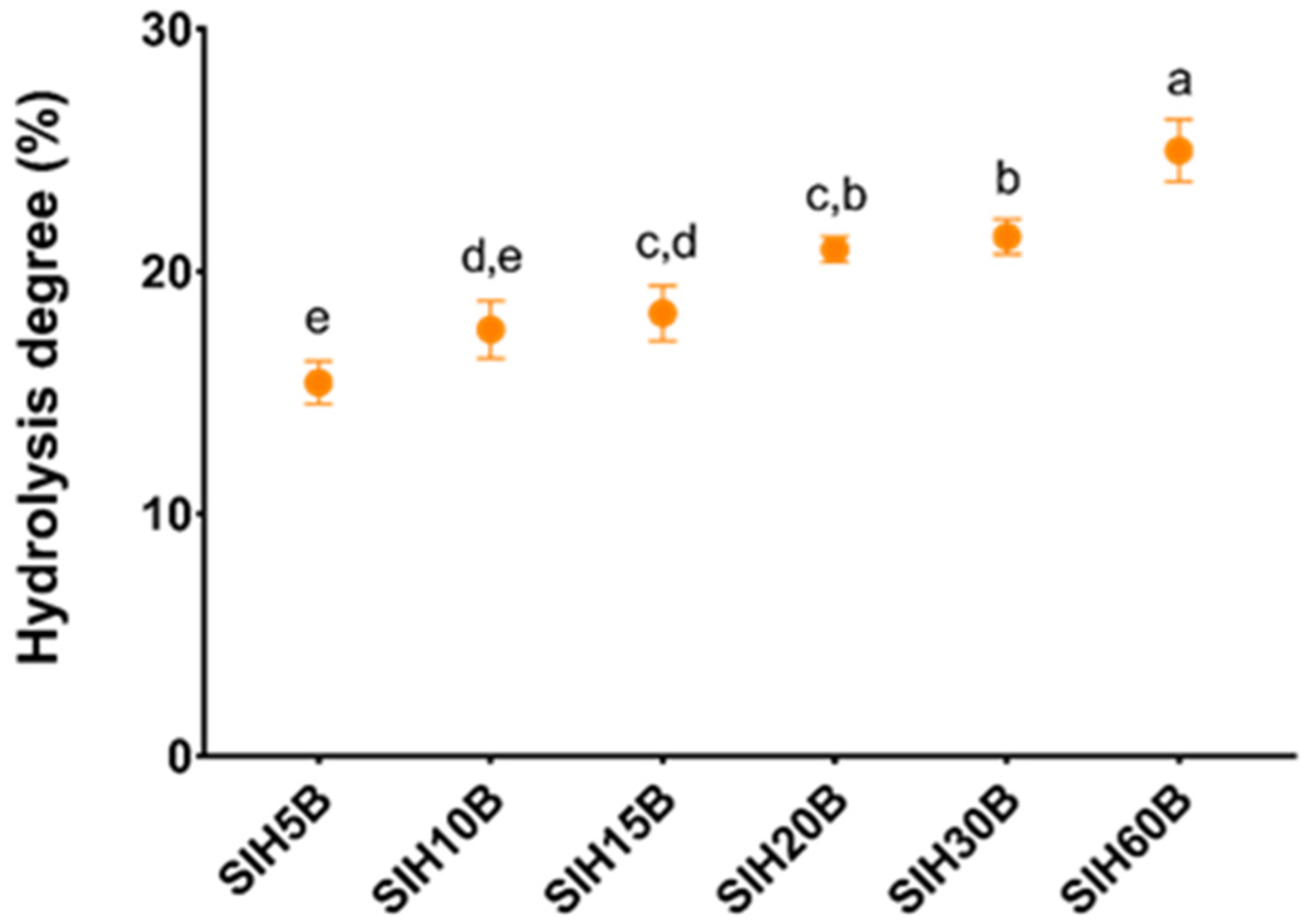
2.3. Evaluation of Hydrolysis Degree
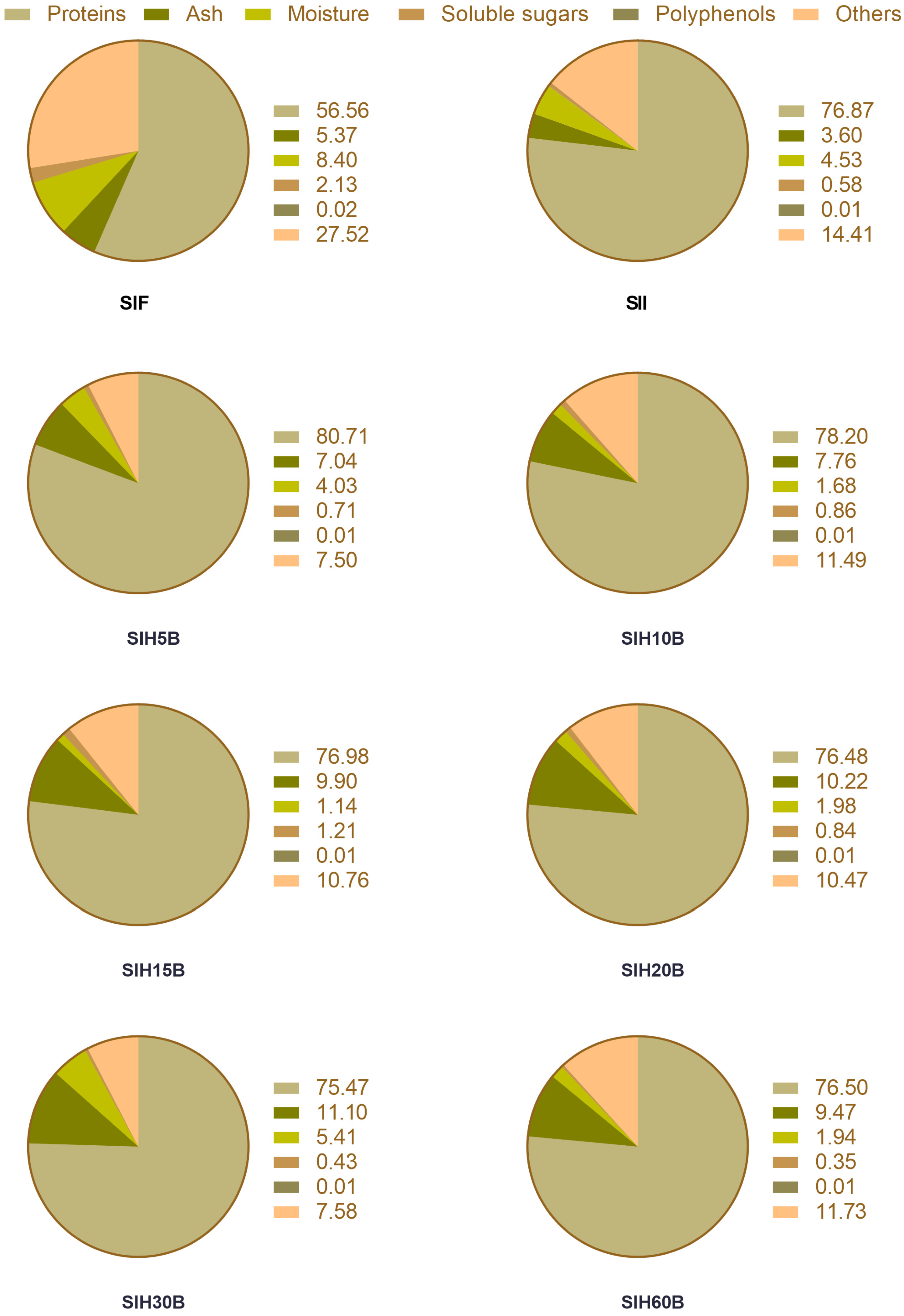
2.4. Proximate Composition Analysis of Sacha inchi Protein Products
2.5. Determination of Amino Acid Composition by Ultrahigh-Performance Liquid Chromatography (UHPLC)
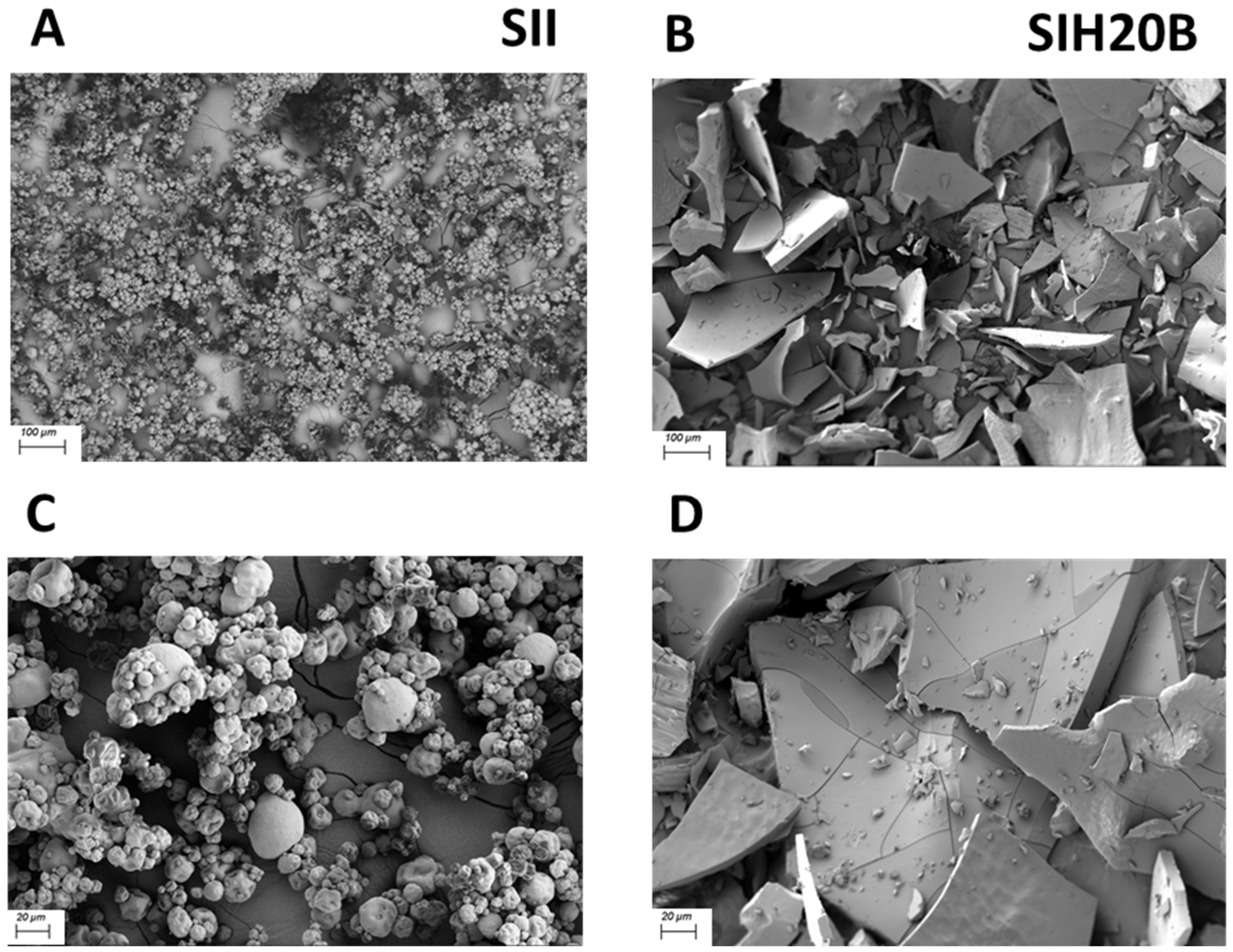
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. Pepsin-Pancreatic Digestibility
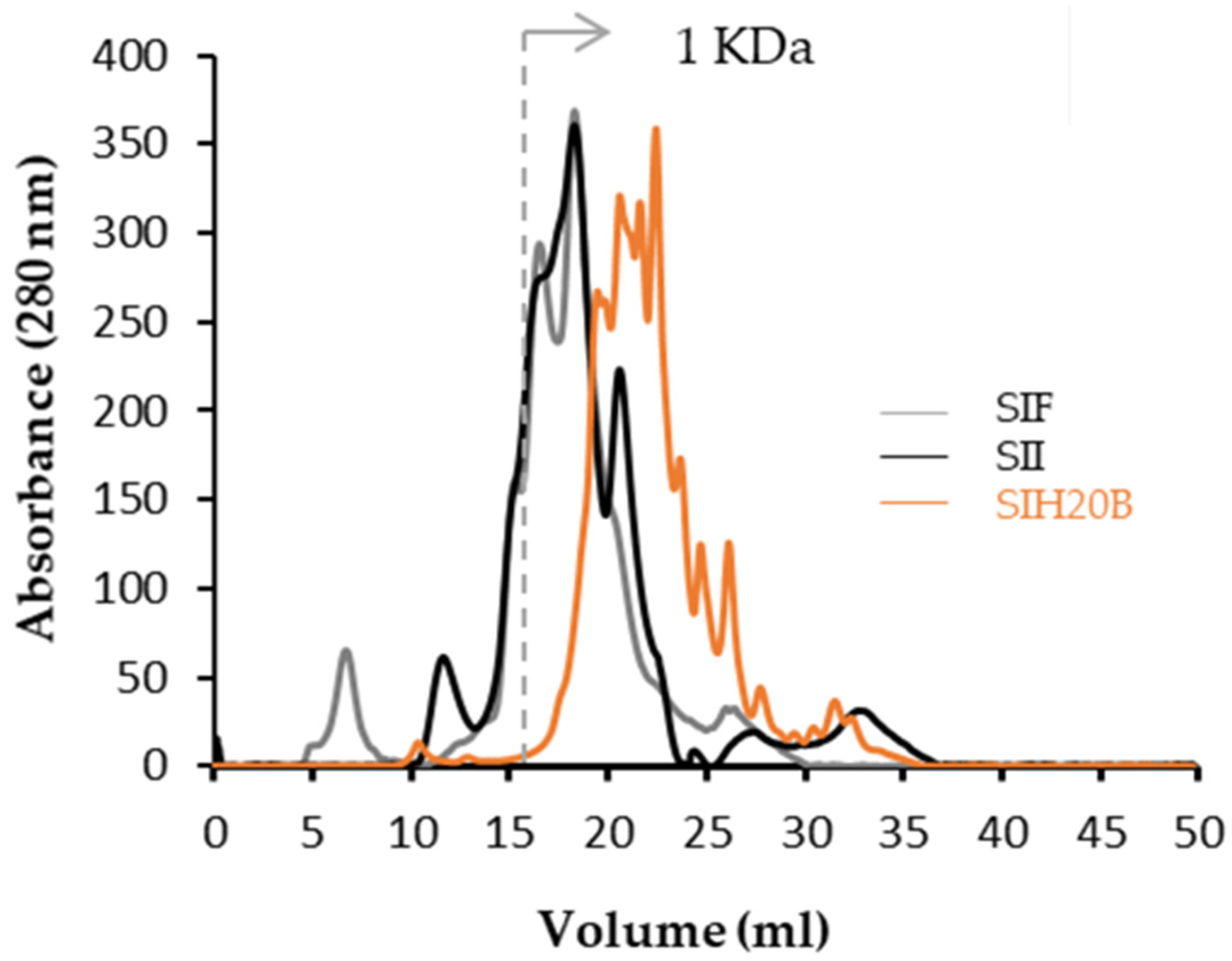
2.8. Molecular Weights (MWs) by Fast Protein Liquid Chromatography (FPLC)
2.9. Determination of Antioxidant Activity
2.9.1. DPPH Radical Scavenging Activity
2.9.2. Ferric Ion Reducing Antioxidant Power (FRAP)
2.9.3. Determination of the Antioxidant Activity by β-Carotene–Linoleic Acid Assay
2.10. THP-1 Culture
2.11. Cell Viability (MTT)
2.12. RNA Extraction and RT-qPCR
2.13. Extracellular Quantification of Cytokines
2.14. Peptide Extraction, Purification, and Sequence Identification by LC-TIMS-MS/MS
2.15. In Silico Analysis
2.16. Statistical Analysis
3. Results
3.1. Chemical Characterization of Sacha Inchi Protein Isolate and Different Sacha Inchi Hydrolysates
3.2. Amino Acid Composition of Sacha Inchi Protein Products
3.3. Antioxidant Effects of Sacha Inchi Protein Products
3.4. Sacha Inchi Products Remodel Their Surface Ultrastructure after Enzymatic Hydrolysis
3.5. Molecular Profile of Sacha Inchi Protein Products
3.6. Cell Viability
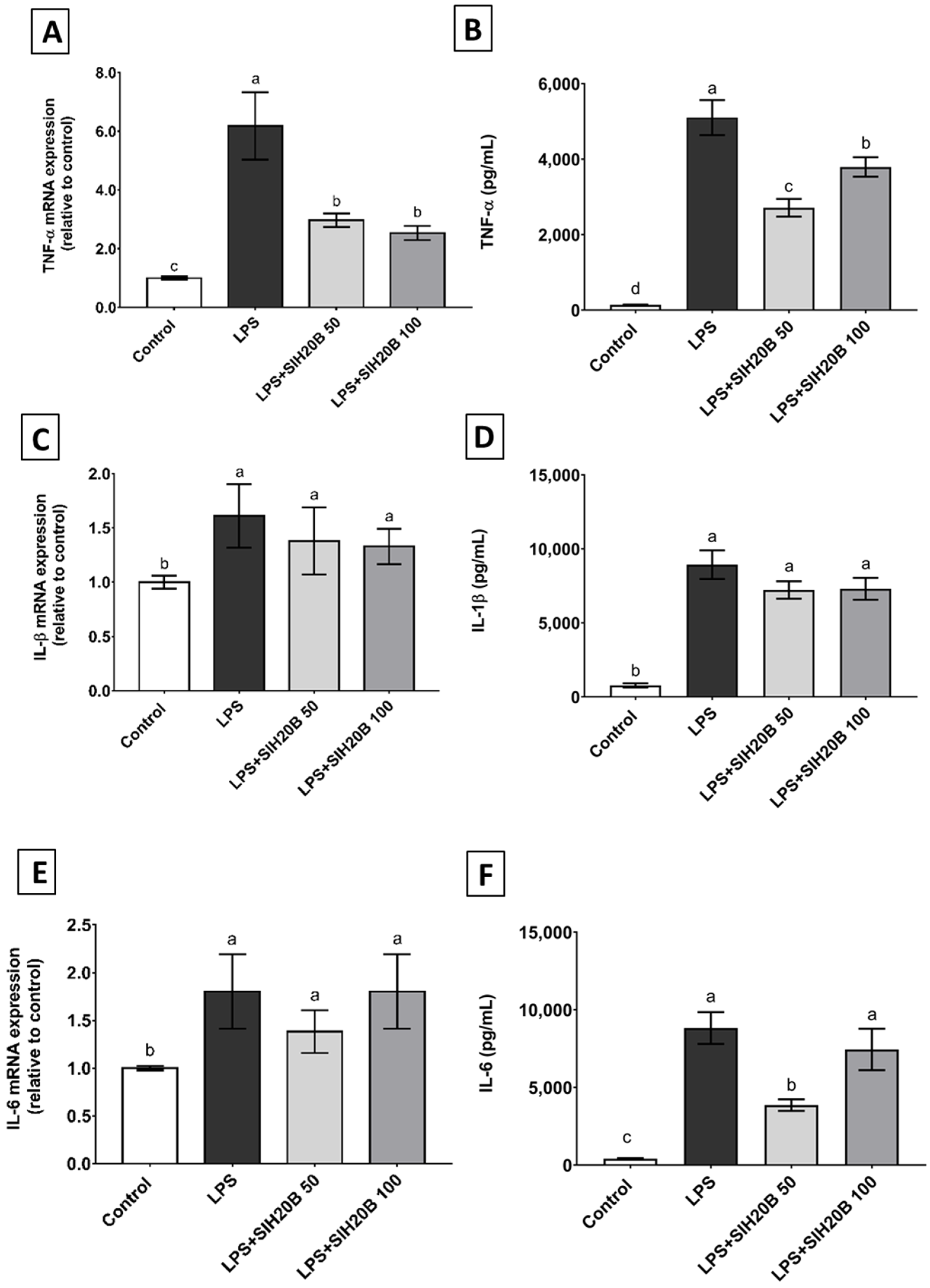
3.7. SIH20B on Cytokine Gene and Protein Expression in THP-1 Cell Culture
3.8. In Silico Analyses of Identified Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmieri, N.; Nervo, C.; Torri, L. Consumers’ attitudes towards sustainable alternative protein sources: Comparing seaweed, insects and jellyfish in Italy. Food Qual. Prefer. 2023, 104, 104735. [Google Scholar] [CrossRef]
- Paper, O. Solubilization, fractionation, and electrophoretic characterization of inca peanut (Plukenetia volubilis L.). Proteins 2012, 67, 247–255. [Google Scholar] [CrossRef]
- Wang, X.; Xu, R.; Wang, R.; Liu, A. Transcriptome analysis of Sacha inchi (Plukenetia volubilis L.) seeds at two developmental stages. BMC Genom. 2012, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Ramos-escudero, F.; Vin, A. Quality, stability, carotenoids and chromatic parameters of commercial Sacha inchi oil originating from Peruvian cultivars. J. Food Sci. Technol. 2019, 56, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wen, J.; Ma, X.; Lin, F.; Jiang, Z.; Du, B. Structural, functional properties and immunomodulatory activity of isolated Inca peanut (Plukenetia volubilis L.) seed albumin fraction. Int. J. Biol. Macromol. 2018, 118, 1931–1941. [Google Scholar] [CrossRef]
- Kodahl, N. Sacha inchi (Plukenetia volubilis L.)—From lost crop of the Incas to part of the solution to global challenges? Planta 2020, 251, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, Z.; Peng, Y.; Shang, X.; Xie, Y.; Arnold, R.J. Transcriptome analyses reveals the dynamic nature of oil accumulation during seed development of Plukenetia volubilis. Sci. Rep. 2020, 10, 20467. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, M.F.; Vilcacundo, R.; Carpio, C.; Carrillo, W. Digestibility and anti-inflammatory activity in vitro of Sacha inchi (Plukenetia volubilis L.) proteins. Asian J. Pharm. Clin. Res. 2016, 9, 303–306. Available online: https://journals.innovareacademics.in/index.php/ajpcr/article/view/11370 (accessed on 21 May 2024).
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef]
- Wang, X.; Liu, A. Expression of Genes Controlling Unsaturated Fatty Acids Biosynthesis and Oil Deposition in Developing Seeds of Sacha inchi (Plukenetia volubilis L.). Lipids 2014, 49, 1019–1031. [Google Scholar] [CrossRef]
- Carren, C. Sacha inchi seeds from sub-tropical cultivation: Effects of roasting on antinutrients, antioxidant capacity and oxidative stability. J. Food Sci. Technol. 2018, 55, 4159–4166. [Google Scholar] [CrossRef]
- Goyal, A.; Tanwar, B.; Kumar, M.; Sharma, V. Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022, 373, 131459. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F.; Tello, J.; Zevallos-Concha, A.; Baquerizo, L.; Caballero, L. Nitrogen balance after a single oral consumption of Sacha inchi (Plukenetia volubilis L.) protein compared to soy protein. A randomized study in humans. Toxicol. Mech. Methods 2018, 28, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.F.; Rosada, L.M.; Jimenez, A. Chemical composition of Sacha inchi (Plukenetia volubilis L.) seeds and characteristics of their lipid fraction. Grasas Aceites 2011, 62, 76–83. [Google Scholar] [CrossRef]
- Vanegas-Azuero, A.M.; Gutierrez, L.F. Physicochemical and sensory properties of yogurts containing Sacha inchi (Plukenetia volubilis L.) seeds and β-glucans from Ganoderma lucidum. J. Dairy Sci. 2018, 101, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.; Diaz, C.; Anaya, J.; Rojas, R. Approximate analysis, antinutrients, fatty acids and amino acids profiles of seeds and cakes from 2 species of Sacha inchi: Plukenetia volubilis and Plukenetia huayllabambana. Rev. Soc. Química Peru 2013, 79, 29–36. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1810-634X2013000100005&lng=es&nrm=iso (accessed on 21 May 2021).
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanoeski, M.; Kmiecik, D.; Gramza-Michalowska, G. The Chemical Composition and Nutritional Value of Chia Seeds-Current State of Knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Zhao, M.; Zhang, J.; Wang, F.; Su, G. Two-stage selective enzymatic hydrolysis generates protein hydrolysates rich in Asn-Pro and Ala-His for enhancing taste attributes of soy sauce. Food Chem. 2021, 345, 128803. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Leo, E.E.; Martín-Ortega, A.M.; Acebedo-Fernández, J.J.; Moo-Puc, R.; Segura-Campos, M.R. Peptides from Mucuna pruriens L., with protection and antioxidant in vitro effect on HeLa cell line. J. Sci. Food Agric. 2019, 99, 4167–4173. [Google Scholar] [CrossRef]
- Wen, L.; Bi, H.; Zhou, X.; Jiang, Y.; Zhu, H.; Fu, X.; Yang, B. Structure characterization of soybean peptides and their protective activity against intestinal inflammation. Food Chem. 2022, 387, 132868. [Google Scholar] [CrossRef]
- Medeiros, A.F.; Queiroz, J.L.C.; Maciel, B.L.L.; Araújo Morais, A.H. Hydrolyzed proteins and vegetable peptides: Anti-inflammatory mechanisms in obesity and potential therapeutic targets. Nutrients 2022, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Protein digests and pure peptides from chia seed prevented adipogenesis and inflammation by inhibiting PPARγ and NF-κB pathways in 3T3L-1 adipocytes. Nutrients 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 176, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodriguez, A.; Gonzalez de Mejia, E.; Dia, V.P.; Reyes-Moreno, C.; Milan-Carrillo, J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-κB signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Yongsawatdigul, J. Immunomodulatory activity of protein hydrolysates derived from Virgibacillus halodenitrificans SK1-3-7 proteinase. Food Chem. 2017, 224, 320–328. [Google Scholar] [CrossRef]
- Ha, S.M.; Kim, J.H.; Kim, J.W.; Kim, D.Y.; Ha, M.S. The Potential Role of Korean Mistletoe Extract as an Anti-Inflammatory Supplementation. J. Immunol. Res. 2021, 2021, 2183427. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. An inflammation classification system using cytokine parameters. Scand J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Huang, L.; Garcia, C.; Sakaki, J.R.; Blesso, C.N.; Chun, O.K.; Fernández, M.L. The Effects of Eggs in a Plant-Based Diet on Oxidative Stress and Inflammation in Metabolic Syndrome. Nutrients 2022, 14, 2548. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; Almaeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive compounds modulating Toll-like 4 receptor (TLR4)-mediated inflammation: Pathways involved and future perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Rivero-Pino, F.; Villanueva, A.; Toscano, R.; Grao-Cruces, E.; Marquez-Paradas, E.; Martin, M.E.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Kiwicha (Amaranthus caudatus L.) protein hydrolysates reduce intestinal inflammation by modulating NLRP3 inflammasome pathway. Food Funct. 2022, 13, 11604. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Villanueva, Á.; Montserrat-de-la-Paz, S.; Sanchez-Fidalgo, S.; Millán-Linares, M.C. Evidence of Immunomodulatory Food-Protein Derived Peptides in Human Nutritional Interventions: Review on the Outcomes and Potential Limitations. Nutrients 2023, 15, 2681. [Google Scholar] [CrossRef] [PubMed]
- Millan-Linares, M.C.; Yust, M.M.; Alcaide-Hidalgo, J.M.; Millan, F.; Pedroche, J. Lupine protein hydrolysates inhibit enzymes involved in the inflammatory pathway. Food Chem. 2014, 151, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Adler-Nissen, J. Determination of the Degree of Hydrolysis of Food Protein Hydrolysates by Trinitrobenzenesulfonic Acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, A.G.; Theander, O. Determination of Chlorogenic Acid in Potato Tubers. J. Agric. Food Chem. 1985, 33, 549–551. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smit, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Alaiz, M.; Navarro, J.L.; Giron, J.; Vioque, E. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J. Chromatogr. A. 1992, 591, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Yust, M.M.; Pedroche, J.; Giron-Calle, J.; Vioque, J.; Millan, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Sindayikengera, S.; Shui Xia, W. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J. Zhejiang Univ. Sci. B 2006, 7, 90–98. [Google Scholar] [CrossRef]
- Wu, H.C.; Chen, H.M.; Shiau, C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction, Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Marco, G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Villanueva-Lazo, A.; Montserrat-de la Paz, S.; Grao-Cruces, E.; Pedroche, J.; Toscano, R.; Millan, F.; Millan-Linares, M.C. Antioxidant and Immunomodulatory Properties of Chia Protein Hydrolysates in Primary Human Monocyte–Macrophage Plasticity. Foods 2022, 11, 623. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Luan, J.J.; Tang, X.H.; Zhu, W.H.; Xu, Y.X.; Bu, Y.; Li, J.; Cui, F.; Li, X. Identification of umami peptides based on virtual screening and molecular docking from Atlantic cod (Gadus morhua). Food Funct. 2023, 14, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.; Cobb, S.L. Pep-Calc.com: A set of web utilities for the calculation of peptide and peptoid properties and automatic mass spectral peak assignment. J. Comput. Aided Mol. Des. 2016, 30, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mora, L.; Toldrá, F. Structure-function relationship of small peptides generated during the ripening of Spanish dry-cured ham: Peptidome, molecular stability and computational modelling. Food Chem. 2022, 375, 131673. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.S.; Hasan, M.M.; Kurata, H. PreAIP: Computational prediction of anti-inflammatory peptides by integrating multiple complementary features. Front Genet. 2019, 10, 129. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review. Trends Food Sci. Technol. 2021, 110, 778–797. [Google Scholar] [CrossRef]
- Villanueva-Lazo, A.; Montserrat-De la Paz, S.; Rodriguez-Martin, N.M.; Millan, F.; Carrera, C.; Pedroche, J.J.; Millan-Linares, M.D.C. Antihypertensive and antioxidant activity of chia protein techno-functional extensive hydrolysates. Foods 2021, 10, 2297. [Google Scholar] [CrossRef]
- Rodriguez-Martin, N.M.; Toscano, R.; Villanueva, A.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Neuroprotective protein hydrolysates from hemp (Cannabis sativa L.) seeds. Food Funct. 2019, 10, 6732–6739. [Google Scholar] [CrossRef] [PubMed]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy protein: Impacts, production and, applications. In Sustainable Protein Sources; Nadathur, S.R., Wanasundaea, J.P.D., Scanlin, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Villanueva, A.; Pedroche, J.; Millan, F.; Martin, M.E.; Millan-Linares, M.C. Antioxidant and anti-inflammatory properties of bioavailable protein hydrolysates from lupin-derived agri-waste. Biomolecules 2021, 11, 1458. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent advances in the recovery techniques of plant-based proteins from agro-industrial by- products recent advances in the recovery techniques of plant-based. Food Rev. Int. 2021, 37, 447–468. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of animal/plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Rodzi, N.; Pinijsuwan, S. Characterization of Sacha inchi protein hydrolysates produced by crude papain and Calotropis proteases. LWT-Food Sci. Technol. 2018, 98, 18–24. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Cronin, M.F.; Arendt, E.K. Impact of protease and amylase treatment on proteins and the product quality of a quinoa-based milk substitute. Food Funct. 2018, 9, 3500–3508. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the degree of hydrolysis on functional properties and antioxidant activity of enzymatic soybean protein hydrolysates. Molecules 2022, 27, 6110. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Ekezie, F.C.; Sarteshnizi, R.A.; Boachie, R.T.; Emenike, C.U.; Sun, X.; Nwachukwu, I.D.; Udenigwe, C.C. Legume Seed Protein Digestibility as Influenced by Traditional and Emerging Physical Processing Technologies. Foods 2022, 11, 2299. [Google Scholar] [CrossRef]
- Beaubier, S.; Pineda-vadillo, C.; Mesieres, O.; Framboisier, X.; Galet, O.; Kapel, R. Improving the in vitro digestibility of rapeseed albumins resistant to gastrointestinal proteolysis while preserving the functional properties using enzymatic hydrolysis. Food Chem. 2023, 407, 135132. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Mckenna, C.F.; Salvador, A.F.; Paulussen, K.J.M.; Moore, D.R. Dietary protein quantity, quality, and exercise are key to healthy living: A muscle-centric perspective across the lifespan. Front. Nutr. 2019, 6, 83. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Reynaud, Y.; Buffière, C.; Cohade, B.; Vauris, M.; Liebermann, K. True ileal amino acid digestibility and digestible indispensable amino acid scores (DIAASs) of plant-based protein foods. Food Chem. 2021, 338, 128020. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-bo, J.C.; Weiler, M.; Allgeier, C. Plant proteins: Assessing their nutritional quality and effects on health and physical function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Akbari-adergani, Z.K.B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhang, G.; Wang, W.; Sadiq, F.A.; Zhang, Y.; Li, X.; Chen, Q.; Xia, Q.; Wang, X.; Li, Y. Preparation and Antioxidant Properties of Germinated Soybean Protein Hydrolysates. Front. Nutr. 2022, 9, 866239. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pedrouso, M.; Lorenzo, J.M.; Borrajo, P.; Franco, D. In search of antioxidant peptides from porcine liver hydrolysates using analytical and peptidomic approach. Antioxidants 2022, 11, 27. [Google Scholar] [CrossRef]
- Suwanangul, S.; Aluko, R.E.; Sangsawad, P.; Kreungngernd, D.; Ruttarattanamongkol, K. Antioxidant and enzyme inhibitory properties of Sacha inchi (Plukenetia volubilis) protein hydrolysate and its peptide fractions. J. Food Biochem. 2022, 46, e14464. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of Sacha inchi (Plukenetia volubilis L.) by-products as valuable and sustainable sources of health benefits. Horticulturae 2022, 8, 344. [Google Scholar] [CrossRef]
- Islam, M.S.; Hongxin, W.; Admassu, H.; Noman, A.; Ma, C.; An wei, F. Degree of Hydrolysis, Functional and Antioxidant Properties of Protein Hydrolysates from Grass Turtle (Chinemys reevesii) as Influenced by Enzymatic Hydrolysis Conditions. Food Sci. Nutr. 2021, 9, 4031–4047. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, X.; Zhang, Y.; Zhu, T. Effect of soy protein hydrolysates incorporation on dough rheology, protein characteristic, noodle quality, and their correlations. J. Food Sci. 2022, 87, 3419–3432. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Rivero-Pino, F.; Villanueva, A.; Toscano-Sanchez, R.; Martin, M.E.; Millan, F.; Millan-Linares, M.C. Nutritional composition, ultrastructural characterization, and peptidome profile of antioxidant hemp protein hydrolysates. Food Biosci. 2023, 53, 102561. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, N.M.; Montserrat-de la Paz, S.; Toscano, R.; Grao-Cruces, E.; Villanueva, A.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. Hemp (Cannabis sativa L.) protein hydrolysates promote anti-inflammatory response in primary human monocytes. Biomolecules 2020, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Chi, Z.; Guo, Z.; Zhang, L. Mung bean protein hydrolysate modulates the immune response through NF-κB pathway in lipopolysaccharide-stimulated RAW 264.7 Macrophages. J. Food Sci. 2019, 84, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Tao, G.; Yang, L.; Wu, X.; Liu, J.W.; Dagher, F.; Ou, S.Y.; Song, Y.; Huang, J.Q. Dietary phytochemical and metabolic disease prevention: Focus on plant proteins. Front. Nutr. 2023, 10, 1089487. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Aruldhas, J.; Srinivasan, A.; Erusappan, T. Modulation of NF-κB and MAPK signalling pathways by hydrolysable tannin fraction from Terminalia chebula fruits contributes to its anti-inflammatory action in RAW 264.7 cells. J. Pharm. Pharmacol. 2022, 74, 718–729. [Google Scholar] [CrossRef]
- Wang, K.; Wu, S.; Li, P.; Xiao, N.; Wen, J.; Lin, J.; Lu, S.; Cai, X.; Xu, Y.; Du, B. Sacha inchi oil press-cake protein hydrolysates exhibit anti-hyperuricemic activity via attenuating renal damage and regulating gut microbiota. Foods 2022, 11, 2534. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chamorro, I.; Alvarez-Sanchez, N.; Millan-Linares, M.C.; Yust, M.M.; Pedroche, J.; Millan, F.; Lardone, P.J.; Carrera-Sanchez, C.; Guerrero, J.M.; Carrillo-Vico, A. Lupine protein hydrolysates decrease the inflammatory response and improve the oxidative status in human peripheral lymphocytes. Food Res. Int. 2019, 126, 108585. [Google Scholar] [CrossRef] [PubMed]
- Lemus-Conejo, A.; Millan-Linares, M.C.; Toscano, R.; Millan, F.; Pedroche, J.; Muriana, F.J.G.; Montserrat-de la Paz, S. GPETAFLR, a peptide from Lupinus angustifolius L. prevents inflammation in microglial cells and confers neuroprotection in brain. Nutr. Neurosci. 2022, 25, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Millan-Linares, M.C.; Millan, F.; Pedroche, J.; Yust, M.M. GPETAFLR: A new anti-inflammatory peptide from Lupinus angustifolius L. protein hydrolysate. J. Funct. Foods 2015, 18, 358–367. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Lima, S.L.S.; Alves, N.E.G.; Assis, A.; Moreira, M.E.C.; Toledo, R.C.L.; Rosa, C.O.B.; Teixeira, O.R.; Bassinello, P.Z.; de Mejia, E.G.; et al. Common bean protein hydrolysate modulates lipid metabolism and prevents endothelial dysfunction in BALB/c mice fed an atherogenic diet. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 141–150. [Google Scholar] [CrossRef]
- Aguchem, R.N.; Okagu, I.U.; Okagu, O.D.; Ndefo, J.C.; Udenigwe, C.C. A review on the techno-functional, biological, and health-promoting properties of hempseed-derived proteins and peptides. J. Food Biochem. 2022, 46, e14127. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, G.; Zhang, X.; Song, G.; Zhang, L.; Zheng, L.; Zhao, M. Characterization and exploration of potential neuroprotective peptides in walnut (Juglans regia) protein hydrolysate against cholinergic system damage and oxidative stress in scopolamine-induced cognitive and memory impairment mice and zebrafish. J. Agric. Food Chem. 2021, 69, 2773–2783. [Google Scholar] [CrossRef]
- Yuan, H.; Luo, Z.; Ban, Z.; Reiter, R.J.; Ma, Q.; Liang, Z.; Yang, M.; Li, X.; Li, L. Bioactive peptides of plant origin: Distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef]
- Peng, L.; Kong, X.; Wang, Z.; Ai-lati, A.; Ji, Z.; Mao, J. Baijiu vinasse as a new source of bioactive peptides with antioxidant and anti-inflammatory activity. Food Chem. 2021, 339, 128159. [Google Scholar] [CrossRef]
- Kalafatovic, D.; Giralt, E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22, 1929. [Google Scholar] [CrossRef]
- Li, Y.; Lammi, C.; Boschin, G.; Arnoldi, A.; Aiello, G. Recent Advances in Microalgae Peptides: Cardiovascular Health Benefits and Analysis. J. Agric. Food Chem. 2019, 67, 11825–11838. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Recio, I.; Heimo, D.; Dubois, S.; Moughan, P.J.; Hodgkinson, S.M.; Portmann, R.; Egger, L. In vitro digestibility of dietary proteins and in vitro DIAAS analytical workflow based on the INFOGEST static protocol and its validation with in vivo data. Food Chem 2023, 404, 134720. [Google Scholar] [CrossRef] [PubMed]


| Gen | No. GenBank | Forward Reverse | Sequence (5′→3′) |
|---|---|---|---|
| TNF-α | NM_000594 | Forward Reverse | TCCTTCAGACACCCTCAACC AGGCCCCAGTTTGAATTCTT |
| IL-1 β | NM_000576 | Forward Reverse | GGGCCTCAAGGAAAAGAATC TTCTGCTTGAGAGGTGCTGA |
| IL-6 | NM_000600 | Forward Reverse | TACCCCCAGGAGAAGATTCC TTTTCTGCCAGTGCCTCTTT |
| IL-10 | NM_000572 | Forward Reverse | GCCTAACATGCTTCGAGATC TGATGTCTGGGTCTTGGTTC |
| IL-4 | NM_000589 | Forward Reverse | TCAACCCCCAGCTAGTTGTC TGTTCTTCGTTGCTGTGAGG |
| HPRT | NM_002046 | Forward Reverse | GAGTCAACGGATTTGGTCGT GACAAGCTTCCCGTTCTCAG |
| GADPH | NM_001289745 | Forward Reverse | ACAGTCAGCCGCATCTTCTT ACGACCAAATCCGTTGACTC |
| Essential Aminoacid | SIF | SII | SIH5B | SIH10B | SIH15B | SIH20B | SI3H0B | SIH60B | FAO 1,2 |
|---|---|---|---|---|---|---|---|---|---|
| His | 14.03 ± 0.49 | 22.22 ± 0.15 | 48.33 ± 1.60 | 16.95 ± 1.02 | 15.11 ± 0.60 | 17.64 ± 2.14 | 15.99 ± 1.11 | 16.06 ± 0.06 | 15 |
| Ile | 25.45 ± 1.33 | 37.76 ± 0.29 | 35.37 ± 3.20 | 37.15 ± 2.06 | 31.65 ± 1.59 | 37.24 ± 4.31 | 37.55 ± 2.26 | 37.61 ± 0.45 | 30 |
| Leu | 41.69 ± 1.17 | 64.77 ± 0.35 | 53.41 ± 1.17 | 58.66 ± 3.52 | 48.22 ± 2.59 | 57.79 ± 7.70 | 50.61 ± 2.78 | 51.43 ± 0.83 | 59 |
| Lys | 29.12 ± 0.92 | 39.35 ± 0.14 | 35.42 ± 1.01 | 39.01 ± 2.54 | 32.02 ± 1.67 | 37.55 ± 4.76 | 34.23 ± 1.95 | 34.54 ± 0.60 | 45 |
| Met + Cys | 19.86 ± 3.56 | 25.16 ± 0.71 | 24.34 ± 1.68 | 24.01 ± 3.38 | 17.90 ± 1.69 | 25.62 ± 4.06 | 22.35 ± 2.77 | 18.45 ± 0.39 | 22 |
| Met | 5.40 ± 2.30 | 4.44 ± 0.07 | 8.83 ± 0.28 | 5.57 ± 1.69 | 3.50 ± 0.57 | 8.15 ± 1.37 | 8.20 ± 1.90 | 6.42 ± 0.31 | 16 |
| Cys | 14.46 ± 1.26 | 20.72 ± 0.64 | 15.51 ± 1.40 | 18.43 ± 1.69 | 14.36 ± 1.12 | 17.47 ± 2.69 | 14.15 ± 0.87 | 11.93 ± 0.08 | 6 |
| Phe + Tyr | 46.92 ± 1.27 | 72.29 ± 0.66 | 55.91 ± 1.62 | 61.85 ± 4.04 | 49.89 ± 2.47 | 64.79 ± 7.92 | 51.76 ± 2.76 | 52.06 ± 0.79 | 38 |
| Thr | 29.13 ± 0.47 | 44.29 ± 0.20 | 44.69 ± 0.58 | 42.13 ± 2.57 | 34.88 ± 2.00 | 40.80 ± 4.93 | 35.16 ± 1.80 | 35.40 ± 0.49 | 23 |
| Trp | 17.53 ± 0.00 | 24.29 ± 0.00 | 20.44 ± 0.01 | 19.25 ± 0.00 | 19.87 ± 0.00 | 19.36 ± 0.00 | 19.01 ± 0.00 | 19.49 ± 0.00 | 6 |
| Val | 30.74 ± 1.54 | 44.47 ± 0.22 | 32.01 ± 1.96 | 33.10 ± 1.42 | 29.39 ± 1.12 | 33.17 ± 2.74 | 32.48 ± 1.50 | 32.47 ± 0.30 | 39 |
| Total essential aminoacids | 274.33 | 399.76 | 374.26 | 356.11 | 296.79 | 359.58 | 321.49 | 315.86 | 277.00 |
| Non-Essential Aminoacid | SIF | SII | SIH5B | SIH10B | SIH15B | SIH20B | SIH30B | SIH60B |
|---|---|---|---|---|---|---|---|---|
| Asp + Asn | 69.15 ± 1.98 | 114.83 ± 1.02 | 138.36 ± 2.99 | 150.17 ± 8.65 | 124.28 ± 5.39 | 148.22 ± 19.63 | 126.39 ± 11.48 | 128.34 ± 1.92 |
| Glu + Gln | 78.37 ± 1.72 | 132.43 ± 1.05 | 132.23 ± 2.79 | 145.30 ± 8.41 | 118.86 ± 6.38 | 139.15 ± 17.32 | 122.12 ± 7.32 | 122.59 ± 1.53 |
| Ser | 39.43 ± 0.70 | 57.81 ± 0.22 | 48.33 ± 1.60 | 54.68 ± 3.41 | 44.09 ± 2.32 | 51.85 ± 6.38 | 43.01 ± 2.08 | 43.46 ± 0.72 |
| Gly | 63.38 ± 0.76 | 45.53 ± 1.37 | 33.38 ± 0.44 | 37.33 ± 2.31 | 30.35 ± 1.51 | 35.70 ± 4.34 | 31.52 ± 1.96 | 31.89 ± 0.36 |
| Arg | 61.84 ± 1.30 | 98.52 ± 0.46 | 83.39 ± 1.59 | 91.66 ± 5.59 | 75.32 ± 3.82 | 87.65 ± 3.20 | 79.03 ± 4.10 | 79.55 ± 1.30 |
| Ala | 21.79 ± 0.57 | 34.15 ± 0.23 | 31.33 ± 0.61 | 34.49 ± 2.15 | 27.75 ± 1.43 | 33.03 ± 2.25 | 28.89 ± 1.80 | 29.50 ± 0.47 |
| Pro | 1.00 ± 0.35 | 2.47 ± 0.20 | 29.39 ± 8.89 | 17.60 ± 2.33 | 29.10 ± 6.89 | 37.81 ± 6.05 | 28.46 ± 7.10 | 21.18 ± 1.16 |
| Total non-essential aminoacids | 334.96 | 485.74 | 496.41 | 531.23 | 449.75 | 533.41 | 459.42 | 456.51 |
| Sample | DPPH (mg/mL) | Reducing Power (mg/mL) | Β-Carotene (mg/mL) |
|---|---|---|---|
| BHT | 0.014 ± 0.004 a | 0.016 ± 0.002 a | 2.130 × 10−9 ± 0.030 × 10−9a |
| SII | 146.120 ± 7.573 f | 2.417 ± 0.101 b | 1.224 ± 0.070 e |
| SIH5B | 66.143 ± 10.412 e | 2.178 ± 0.410 b | 0.855 ± 0.147 d |
| SIH10B | 28.865 ± 1.151 c | 2.200 ± 0.294 b | 0.260 ± 0.123 c |
| SIH15B | 28.351 ± 1.204 c | 2.280 ± 0.074 b | 0.242 ± 1.204 c |
| SIH20B | 16.670 ± 0.589 b | 2.486 ± 0.130 b | 4.180 × 10−4 ± 4.330 × 10−4 b |
| SIH30B | 33.708 ± 2.643 c | 2.507 ± 0.308 b | 3.980 × 10−4 ± 1.470 × 10−4b |
| SIH60B | 40.650 ± 3.713 d | 2.393 ± 0.185 b | 3.170 × 10−3 ± 1. 270 × 10−4 b |
| Peptide | Area | Length | Molec. Weight | Charge 1 | PI 1 | Hydrophobicity 1 | Steric Hindrance 1 | Amphipathicity 1 | Solubility 2 | PeptideRanker 3 | PreAIP 4 | AnOxPePred 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LATMMDDE | 6.47 × 10+7 | 8 | 956.35 | −3.0 | 0.59 | −0.12 | 0.67 | 0.16 | Good | 0.1166 | 0.373 | 0.335 |
| LGDECYGSG | 6.47 × 10+7 | 9 | 956.35 | −2.1 | 0.62 | −0.06 | 0.65 | 0.14 | Good | 0.3430 | 0.383 | 0.457 |
| LGDDSCYQ | 6.47 × 10+7 | 8 | 956.35 | −2.1 | 0.61 | −0.20 | 0.66 | 0.16 | Good | 0.2995 | 0.386 | 0.398 |
| GNGSLRML | 3.39 × 10+7 | 8 | 863.42 | 1.0 | 10.84 | −0.13 | 0.65 | 0.31 | Poor | 0.7512 | 0.471 | 0.403 |
| GDGSLRLM | 3.39 × 10+7 | 8 | 863.42 | 0.0 | 6.64 | −0.14 | 0.65 | 0.31 | Good | 0.8027 | 0.446 | 0.365 |
| TAGSLRME | 3.39 × 10+7 | 8 | 863.42 | 0.0 | 6.55 | 0.29 | 0.62 | 0.47 | Good | 0.2015 | 0.438 | 0.377 |
| GDGSLRML | 3.39 × 10+7 | 8 | 863.42 | 0.0 | 6.64 | −0.14 | 0.65 | 0.31 | Good | 0.8074 | 0.443 | 0.389 |
| PGVLKAPPP | 8.73 × 10+6 | 9 | 874.53 | 1.0 | 10.57 | 0.01 | 0.51 | 0.41 | Poor | 0.5328 | 0.360 | 0.435 |
| PGVLKKHP | 8.73 × 10+6 | 8 | 874.53 | 2.1 | 10.91 | −0.19 | 0.50 | 1.10 | Good | 0.2851 | 0.399 | 0.410 |
| LLSGAGVSFQ | 4.11 × 10+6 | 10 | 977.52 | 0.0 | 3.70 | 0.16 | 0.61 | 0.12 | Poor | 0.2366 | 0.458 | 0.371 |
| YLMLATPGL | 4.11 × 10+6 | 9 | 977.53 | 0.0 | 3.70 | 0.23 | 0.57 | 0.00 | Poor | 0.4295 | 0.530 | 0.404 |
| PQREVQSQ | 1.92 × 10+6 | 8 | 970.48 | 0.0 | 7.31 | −0.53 | 0.62 | 0.93 | Good | 0.0553 | 0.405 | 0.300 |
| SAVSLGTHVL | 1.77 × 10+6 | 10 | 982.54 | 0.1 | 7.54 | 0.15 | 0.53 | 0.14 | Poor | 0.2637 | 0.401 | 0.444 |
| MVPLAQLVN | 1.58 × 10+6 | 9 | 983.55 | 0.1 | 7.54 | 0.14 | 0.62 | 0.14 | Poor | 0.1605 | 0.396 | 0.379 |
| MNACESC | 1.07 × 10+6 | 7 | 870.27 | −1.1 | 1.12 | −0.13 | 0.64 | 0.18 | Good | 0.2798 | 0.422 | 0.353 |
| AAGALKKFL | 0.98 × 10+6 | 9 | 917.57 | 2.0 | 10.74 | 0.04 | 0.60 | 0.82 | Good | 0.7997 | 0.478 | 0.372 |
| LGVKFKGGL | 0.98 × 10+6 | 9 | 917.57 | 2.0 | 10.73 | 0.05 | 0.65 | 0.82 | Good | 0.6273 | 0.370 | 0.345 |
| NGVLKKFL | 0.98 × 10+6 | 8 | 917.57 | 2.0 | 10.68 | −0.06 | 0.66 | 0.92 | Good | 0.4740 | 0.465 | 0.334 |
| LFPPPGKA | 0.84 × 10+6 | 8 | 825.47 | 1.0 | 10.12 | 0.03 | 0.52 | 0.46 | Poor | 0.7289 | 0.365 | 0.521 |
| LLFGHATLE | 0.75 × 10+6 | 9 | 999.54 | −0.9 | 5.10 | 0.16 | 0.52 | 0.30 | Poor | 0.2124 | 0.473 | 0.498 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemus-Conejo, A.; Villanueva-Lazo, A.; Martin, M.E.; Millan, F.; Millan-Linares, M.C. Sacha Inchi (Plukenetia volubilis L.) Protein Hydrolysate as a New Ingredient of Functional Foods. Foods 2024, 13, 2045. https://doi.org/10.3390/foods13132045
Lemus-Conejo A, Villanueva-Lazo A, Martin ME, Millan F, Millan-Linares MC. Sacha Inchi (Plukenetia volubilis L.) Protein Hydrolysate as a New Ingredient of Functional Foods. Foods. 2024; 13(13):2045. https://doi.org/10.3390/foods13132045
Chicago/Turabian StyleLemus-Conejo, Ana, Alvaro Villanueva-Lazo, Maria E. Martin, Francisco Millan, and Maria C. Millan-Linares. 2024. "Sacha Inchi (Plukenetia volubilis L.) Protein Hydrolysate as a New Ingredient of Functional Foods" Foods 13, no. 13: 2045. https://doi.org/10.3390/foods13132045
APA StyleLemus-Conejo, A., Villanueva-Lazo, A., Martin, M. E., Millan, F., & Millan-Linares, M. C. (2024). Sacha Inchi (Plukenetia volubilis L.) Protein Hydrolysate as a New Ingredient of Functional Foods. Foods, 13(13), 2045. https://doi.org/10.3390/foods13132045








