Sequential Fermentation in Red Wine cv. Babić Production: The Influence of Torulaspora delbrueckii and Lachancea thermotolerans Yeasts on the Aromatic and Sensory Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Alcoholic Fermentation Trials
2.3. Identification and Quantification of Volatile Compounds
2.4. Determination of Organic Acids
2.5. Physicochemical Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters
3.2. Organic Acids in Wines
3.3. Volatile Aromatic Compounds in Wines
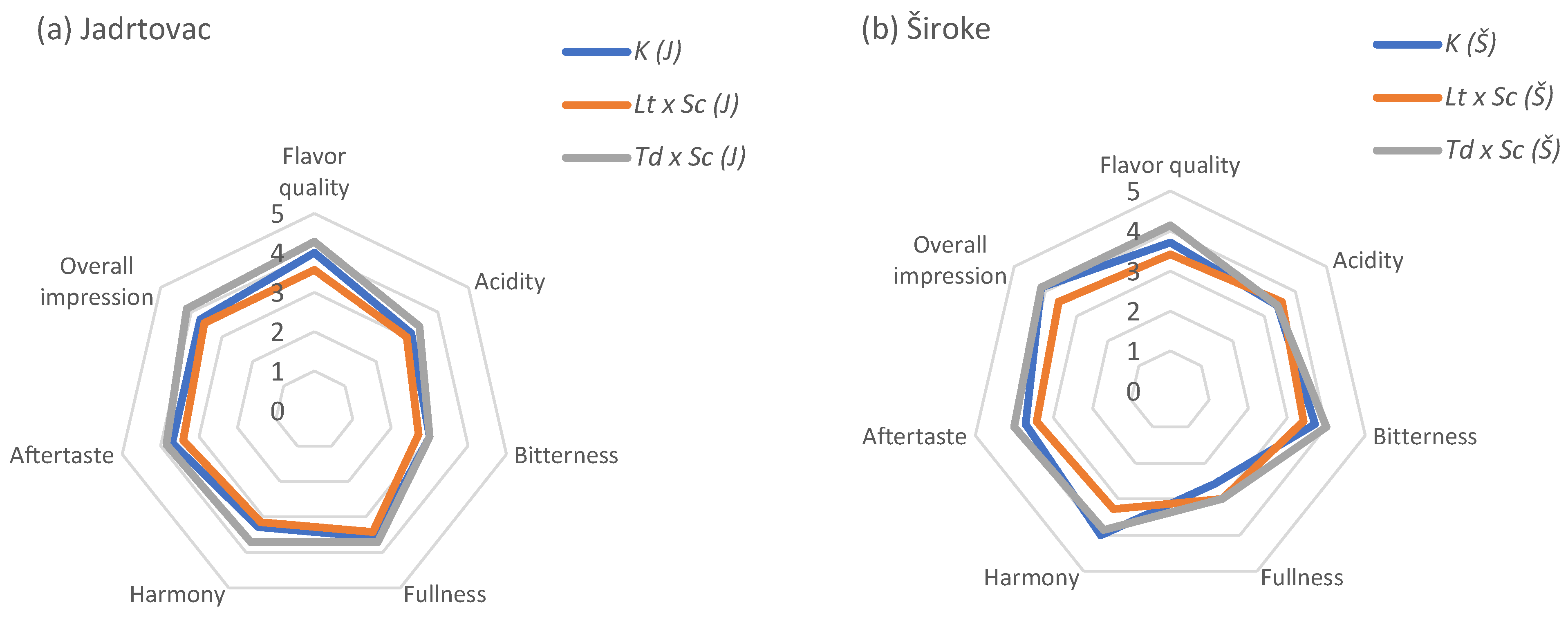
3.4. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 28, 1142. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Torres-Díaz, L.L.; Murillo-Peña, R.; Iribarren, M.; Sáenz de Urturi, I.; Marín-San Román, S.; González-Lázaro, M.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Exploring Metschnikowia pulcherrima as a Co-Fermenter with Saccharomyces cerevisiae: Influence on Wine Aroma during Fermentation and Ageing. Beverages 2024, 10, 26. [Google Scholar] [CrossRef]
- Albertin, W.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. New Insights into Wine Yeast Diversities, Yeasts in the Production of Wine, 1st ed.; Springer: New York, NY, USA, 2019; pp. 117–163. [Google Scholar]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Tzamourani, A.; Paramithiotis, S.; Favier, M.; Coulon, J.; Moine, V.; Paraskevopoulos, I.; Dimopoulou, M. New Insights into the Production of Assyrtiko Wines from the Volcanic Terroir of Santorini Island Using Lachancea thermotolerans. Microorganisms 2024, 12, 786. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Perez-Jiménez, M.; Esteban-Fernández, A.; Muñoz-González, C.; Pozo-Bayón, M.A. Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility. Molecules 2020, 25, 1701. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R. Wine Science—Principles and Applications, 4th ed.; Academic Press: London, UK, 2014; pp. 357–359. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Singleton, V.L.; Bisson, L.F. Principles and Practices of Winemaking, 1st ed.; Springer: New York, NY, USA, 1998; pp. 521–538. [Google Scholar]
- Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Skendrović Babojelić, M.; Jeromel, A. Aroma Profile of Monovarietal Pét-Nat Ciders: The Role of Croatian Traditional Apple Varieties. Horticulturae 2022, 8, 689. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organization of Vine and Wine: Paris, France, 2021; Volume 1, ISBN 978-2-85038-033-4. [Google Scholar]
- HRN EN ISO/IEC 17065:2013; Hrvatski Normativni Document. Croatian Accreditation Agency (HAA): Zagreb, Croatia, 2013.
- ISO 3591:1977; Sensory Analysis–Apparatus–Wine-Tasting Glass. International Organization for Standardization (ISO): Geneva, Switzerland, 1977.
- Romano, P.; Suzzi, G.; Domizio, P.; Fatichenti, F. Secondary products formation as a tool for discriminating non-Saccharomyces wine strains. Strain diversity in non-Saccharomyces wine yeasts. Antonie Van Leeuwenhoek 1997, 71, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Influence of Saccharomyces and non-Saccharomyces Yeasts in the Formation of Pyranoanthocyanins and Polymeric Pigments during Red Wine Making. Molecules 2019, 24, 4490. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The use of glucose oxidase and catalase for the enzymatic reduction of the potential ethanol content in wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; Portu, J.; Garijo, P.; López, R.; Santamaría, P.; López-Alfaro, I.; Gutiérrez, A.R.; González-Arenzana, L. Effect of the Sequential Inoculation of Non-Saccharomyces/Saccharomyces on the Anthocyans and Stilbenes Composition of Tempranillo Wines. Front. Microbiol. 2019, 10, 773. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation usingmixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Influence of Native Saccharomyces cerevisiae Strains from D.O.“Vinos de Madrid” in the Volatile Profile of White Wines. Fermentation 2019, 5, 94. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine Use of Selected Schizosaccharomyces pombe and Lachancea thermotolerans Yeast Strains as an Alternative to the Traditional Malolactic Fermentation in Red Wine Production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N.; Lavigne, V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. Food Agric. 1993, 62, 191–202. [Google Scholar] [CrossRef]
- Tomasino, E.; Bolman, S. The Potential Effect of β-Ionone and β-Damascenone on Sensory Perception of Pinot Noir Wine Aroma. Molecules 2021, 26, 1288. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic Acid Metabolism and the Impact of Fermentation Practices on Wine Acidity: A Review. S. Afr. J. Enol. Vitic. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Guise, R.; Filipe-Ribeiro, L.; Nascimento, D.; Bessa, O.; Nunes, F.M.; Cosme, F. Comparison between different types of carboxylmethylcellulose and other oenological additives used for white wine tartaric stabilization. Food Chem. 2014, 156, 250–257. [Google Scholar] [CrossRef]
- Caridi, A.; Cufari, A.; Lovino, R.; Palumbo, R.; Tedesco, I. Influence of yeast on polyphenol composition of wine. Food Technol. Biotechnol. 2004, 42, 37–40. [Google Scholar]
- Palomo, E.S.; González-Viñas, M.A.; Díaz-Maroto, M.C.; Soriano-Pérez, A.; Pérez-Coello, M.S. Aroma potential of Albillo wines and effect of skin-contact treatment. Food Chem. 2007, 103, 631–640. [Google Scholar] [CrossRef]
- Kapsopoulo, K.; Kapaklis, A.; Spyropoulos, H. Growth and Fermentation Characteristics of a Strain of the Wine Yeast Kluyveromyces thermotolerans Isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Masson, P.; Bertoni, G. Ground cover trials with soil-improving clover. Obstbau-Weinbau 1996, 33, 7–8. [Google Scholar]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 18, 183–191. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cersosimo, M.; Loscos, N.; Cacho, J.; Garcia-Moruno, E.; Ferreira, V. The development of varietal aroma from non-floral grapes by yeasts of different genera. Food Chem. 2008, 107, 1064–1077. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canalis, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Oliveira, P.; Baumes, R.L.; Maia, O. Changes in aromatic characteristics of Loureiro and Alvarinho wines during maturation. J. Food Compos. Anal. 2008, 21, 695–707. [Google Scholar] [CrossRef]
- Korenika, A.-M.J.; Tomaz, I.; Preiner, D.; Lavrić, M.; Šimić, B.; Jeromel, A. Influence of L. thermotolerans and S. cerevisiae Commercial Yeast Sequential Inoculation on Aroma Composition of Red Wines (Cv Trnjak, Babic, Blatina and Frankovka). Fermentation 2021, 7, 4. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Delač Salopek, D.; Horvat, I.; Hranilović, A.; Plavša, T.; Radeka, S.; Pasković, I.; Lukić, I. Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation. Foods 2022, 11, 3088. [Google Scholar] [CrossRef]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Shekhawat, K.; Porter, T.J.; Bauer, F.F.; Setati, M.E. Employing oxygen pulses to modulate Lachancea thermotolerans–Saccharomyces cerevisiae Chardonnay fermentations. Ann. Microbiol. 2018, 68, 93–102. [Google Scholar] [CrossRef]
- Mucalo, A.; Budić-Leto, I.; Zdunić, G. Effect of Sequential Fermentation with Lachancea thermotolerans/S. cerevisiae on Aromatic and Flavonoid Profiles of Plavac Mali Wine. Foods 2023, 12, 1912. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory Impact of Higher Alcohols on Red Wine Fruity Ester Aroma Expression in Model Solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Lilly, M.; Bauer, F.F.; Lambrechts, M.G.; Swiegers, J.H.; Cozzolino, D.; Pretorius, I.S. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 2006, 23, 641–659. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Gallardo-Chacón, J.J.; Vichi, S.; López-Tamames, E.; Buxaderas, S. Changes in the sorption of diverse volatiles by Saccharomyces cerevisiae lees during sparkling wine aging. J. Agric. Food Chem. 2010, 58, 12426–12430. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, 2nd ed.; Wiley: Chichester, UK, 2006; pp. 141–203. [Google Scholar]
- Jackson, R.S. Wine Science, 2nd ed.; Academic Press: Amsterdam, The Netherland, 2000; pp. 232–280. [Google Scholar]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015, 6, 1222. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C. Effects of Torulaspora delbrueckii and Saccharomyce cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food. Res. Technol. 2012, 2, 303–313. [Google Scholar] [CrossRef]
- Vaquero, C.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Marchante-Cuevas, L.; Heras, J.M.; Morata, A. Use of Lachancea thermotolerans for Biological vs. Chemical Acidification at Pilot-Scale in White Wines from Warm Areas. Fermentation 2021, 7, 193. [Google Scholar] [CrossRef]
- Bakker, J.; Clarke, R.J. Wine: Flavour Chemistry, 1st ed.; John Wiley and Sons: Chichester, UK, 2004; pp. 189–229. [Google Scholar]
- Morata, A.; Loira, I.; González, C.; Escott, C. Non-Saccharomyces as Biotools to Control the Production of Off-Flavors in Wines. Molecules 2021, 26, 4571. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Evaluation of the use of multiflora bee pollen on the volatile compounds and sensorial profile of Palomino fino and Riesling white young wines. Food Res. Int. 2018, 105, 197–209. [Google Scholar] [CrossRef]

| K | Lt x Sc | Td x Sc |
|---|---|---|
| Control treatment, alcoholic fermentation with the yeast strain Saccharomyces cerevisiae (Uvaferm BDX®, Lallemand Montreal, QC, Canada) | Sequential alcoholic fermentation with the yeast strains Lachancea thermotolerans (Laktia®, Lallemand, Montreal, QC, Canada) + Saccharomyces cerevisiae (Uvaferm BDX®, Lallemand, Montreal, QC, Canada) | Sequential alcoholic fermentation with the yeast strains Torulaspora delbrueckii (Biodiva®, Lallemand, Montreal, QC, Canada) + Saccharomyces cerevisiae (Lalvin ICV D254®, Lallemand, Montreal, QC, Canada) |
| K | Lt x Sc | Td x Sc |
|---|---|---|
|
|
|
| Compound | Location | Treatment | ||
|---|---|---|---|---|
| K | Lt x Sc | Td x Sc | ||
| Alcohol (vol%) | J | 13.60 ± 0.01 c | 13.30 ± 0.06 b | 13.40 ± 0.02 b |
| Š | 13.00 ± 0.02 a | 13.01 ± 0.03 a | 12.93 ± 0.05 a | |
| Total dry extract (g/L) | J | 35.40 ± 0.20 c | 37.50 ± 0.40 d | 35.20 ± 0.20 bc |
| Š | 34.60 ± 0.20 ab | 36.20 ± 0.20 d | 34.40 ± 0.20 a | |
| Reducing sugars (g/L) | J | 3.80 ± 0.20 abc | 4.00 ± 0.10 bc | 3.40 ± 0.20 a |
| Š | 4.20 ± 0.10 c | 3.70 ± 0.10 ab | 3.40 ± 0.20 a | |
| Total acidity * (g/L) | J | 6.30 ± 0.10 a | 7.50 ± 0.10 b | 6.80 ± 0.20 ab |
| Š | 7.50 ± 0.10 b | 8.20 ± 0.10 c | 8.00 ± 0.20 c | |
| Volatile acidity ** (g/L) | J | 0.30 ± 0.02 ab | 0.40 ± 0.01 ab | 0.50 ± 0.01 a |
| Š | 0.42 ± 0.03 c | 0.44 ± 0.04 c | 0.40 ± 0.01 c | |
| pH | J | 3.62 ± 0.02 b | 3.51 ± 0.01 a | 3.53 ± 0.02 a |
| Š | 3.36 ± 0.01 a | 3.32 ± 0.02 a | 3.32 ± 0.02 a | |
| Ash (g/L) | J | 3.35 ± 0.01 a | 3.48 ± 0.01 b | 3.40 ± 0.10 ab |
| Š | 3.29 ± 0.01 a | 3.39 ± 0.01 ab | 3.35 ± 0.02 a | |
| Total phenols (mg/L) | J | 1775.00 ± 300.52 a | 1632.50 ± 38.89 a | 1610.00 ± 162.63 a |
| Š | 1567.50 ± 24.74 b | 1590.00 ± 134.35 b | 1520.00 ± 14.14 b | |
| Organic Acid (g/L) | Location | Treatment | ||
|---|---|---|---|---|
| K | Lt x Sc | Td x Sc | ||
| Tartaric | J | 2.76 ± 0.01 a | 2.83 ± 0.02 b | 2.96 ± 0.02 c |
| Š | 4.12 ± 0.04 d | 4.18 ± 0.02 d | 4.38 ± 0.02 e | |
| Malic | J | 0.29 ± 0.00 d | 0.26 ± 0.00 c | 0.27 ± 0.00 c |
| Š | 0.18 ± 0.01 b | 0.17 ± 0.01 b | 0.15 ± 0.01 a | |
| Lactic | J | 0.01 ± 0.01 a | 1.58 ± 0.01 d | 0.06 ± 0.01 b |
| Š | 0.01 ± 0.01 a | 0.62 ± 0.01 c | 0.06 ± 0.01 b | |
| Citric | J | 0.49 ± 0.01 c | 0.35 ± 0.01 b | 0.26 ± 0.00 a |
| Š | 0.33 ± 0.01 b | 0.34 ± 0.02 b | 0.27 ± 0.01 a | |
| Succinic | J | 0.43 ± 0.00 a | 0.71 ± 0.02 d | 0.58 ± 0.01 c |
| Š | 0.54 ± 0.01 b | 0.42 ± 0.02 a | 0.42 ± 0.01 a | |
| Compounds (μg/L) | Locality | Treatments | ||
|---|---|---|---|---|
| K | Lt x Sc | Td x Sc | ||
| Fatty acids | ||||
| Propanoic acid | J | 3.68 ± 0.28 a | 2.86 ± 0.26 a | 2.60 ± 0.00 a |
| Š | 2.70 ± 0.12 a | 2.70 ± 0.12 a | 3.20 ± 0.93 a | |
| 2-Methylpropanoic acid | J | 521.71 ± 17.41 a | 933.11 ± 558.58 a | 1052.60 ± 50.55 a |
| Š | 535.41 ± 0.79 a | 661.98 ± 19.50 a | 762.14 ± 86.67 a | |
| Butanoic acid | J | 159.07 ± 2.39 a | 191.02 ± 26.57 a | 264.78 ± 13.21 a |
| Š | 217.19 ± 4.58 a | 209.52 ± 14.63 a | 153.61 ± 154.60 a | |
| Isovaleric acid | J | 2.63 ± 0.02 a | 2.84 ± 0.13 a | 2.69 ± 0.09 a |
| Š | 2.74 ± 0.01 a | 2.79 ± 0.16 a | 3.29 ± 0.43 a | |
| Hexanoic acid | J | 338.12 ± 20.36 a | 309.98 ± 43.79 a | 431.63 ± 6.05 ab |
| Š | 599.29 ± 71.17 b | 467.86 ± 62.50 ab | 543.92 ± 83.35 ab | |
| Heptanoic acid | J | 5.35 ± 2.43 a | 8.03 ± 0.77 a | 5.65 ± 2.92 a |
| Š | 9.03 ± 0.36 a | 8.92 ± 0.68 a | 6.02 ± 3.46 a | |
| Nonanoic acid | J | 7.95 ± 0.09 a | 8.34 ± 0.21 a | 8.20 ± 0.15 a |
| Š | 8.26 ± 0.09 a | 8.11 ± 0.38 a | 8.43 ± 0.21 a | |
| Decanoic acid | J | 5.46 ± 0.47 a | 5.01 ± 1.56 a | 5.17 ± 0.01 a |
| Š | 5.04 ± 0.22 a | 4.76 ± 0.41 a | 5.00 ± 0.19 a | |
| Ʃ Fatty acids | J | 1044.00 ± 37.10 a | 1461.00 ± 489.00 a | 1773.00 ± 60.70 a |
| Š | 1380.00 ± 76.70 a | 1367.00 ± 97.90 a | 1486.00 ± 12.50 a | |
| Terpenes | ||||
| Farnesol | J | 10.38 ± 4.85 a | 8.90 ± 0.37 a | 10.57 ± 0.88 a |
| Š | 6.27 ± 3.66 a | 3.01 ± 0.22 a | 7.12 ± 5.42 a | |
| Tetrahydrolinalool | J | 6.43 ± 0.33 a | 11.07 ± 0.02 a | 30.10 ± 0.08 b |
| Š | 12.42 ± 0.67 a | 5.76 ± 8.15 a | 12.32 ± 1.66 a | |
| Linalyl format | J | 0.36 ± 0.14 a | 0.42 ± 0.00 a | 0.32 ± 0.12 |
| Š | 1.51 ± 0.61 a | 1.29 ± 0.24 a | 1.27 ± 0.17 a | |
| cis-Linalool oxide, fur. | J | 1.60 ± 0.19 a | 2.19 ± 0.03 ab | 1.59 ± 0.03 a |
| Š | 3.23 ± 0.24 bc | 3.59 ± 0.14 c | 3.98 ± 0.55 c | |
| Linalool | J | 4.48 ± 0.00 a | 4.38 ± 0.74 a | 4.43 ± 0.16 a |
| Š | 4.82 ± 0.85 a | 4.39 ± 0.09 a | 4.34 ± 0.62 a | |
| Terpinen-4-ol | J | 4.14 ± 1.30 a | 7.81 ± 0.92 b | 5.89 ± 0.22 ab |
| Š | 4.57 ± 0.09 a | 5.58 ± 0.22 ab | 4.44 ± 0.17 a | |
| Hotrienol | J | 0.59 ± 0.24 a | 1.56 ± 1.01 ab | 1.55 ± 1.32 ab |
| Š | 4.54 ± 2.44 a | 3.34 ± 0.67 ab | 3.12 ± 0.53 a | |
| β-Ionone-5,6-epoxide | J | 0.13 ± 0.02 a | 0.10 ± 0.05 b | 0.16 ± 0.04 ab |
| Š | 0.14 ± 0.04 a | 0.09 ± 0.10 ab | 0.15 ± 0.08 a | |
| cis-β-Farnesene | J | 0.56 ± 0.02 a | 0.59 ± 0.00 a | 0.56 ± 0.01 a |
| Š | 0.83 ± 0.22 a | 0.71 ± 0.24 a | 0.55 ± 0.02 a | |
| trans-β-Farnesene | J | 0.87 ± 0.04 a | 1.05 ± 0.14 a | 1.24 ± 0.02 a |
| Š | 0.90 ± 0.17 a | 1.06 ± 0.07 a | 0.90 ± 0.49 a | |
| Menthol | J | 0.29 ± 0.16 a | 0.19 ± 0.24 a | 0.12 ± 0.04 a |
| Š | 0.25 ± 0.17 a | 0.25 ± 0.21 a | 0.33 ± 0.04 a | |
| Ocimenol | J | 0.18 ± 0.20 a | 0.20 ± 0.20 a | 0.05 ± 0.01 a |
| Š | 0.40 ± 0.25 a | 0.18 ± 0.06 a | 0.36 ± 0.18 a | |
| Nerolic acid | J | 11.68 ± 1.50 b | 2.48 ± 0.14 a | 2.39 ± 0.38 a |
| Š | 2.53 ± 0.02 a | 12.59 ± 0.90 b | 12.95 ± 0.45 b | |
| 2,6-Dimethyl-3,7-octadien-2,6-diol | J | 0.19 ± 0.02 a | 0.33 ± 0.07 a | 0.37 ± 0.00 a |
| Š | 0.47 ± 0.51 a | 0.16 ± 0.02 a | 0.32 ± 0.06 a | |
| α-Terpineol | J | 1.73 ± 0.51 a | 2.23 ± 0.65 a | 1.98 ± 0.19 a |
| Š | 1.75 ± 0.14 a | 1.67 ± 0.00 a | 2.14 ± 0.48 a | |
| Terpendiol I | J | 4.2 ± 0.64 b | 4.15 ± 0.72 b | 3.32 ± 0.07 ab |
| Š | 2.48 ± 0.04 ab | 1.60 ± 0.12 a | 1.86 ± 0.24 a | |
| Citronellol | J | 31.77 ± 3.91 a | 28.02 ± 4.12 a | 31.98 ± 0.60 a |
| Š | 27.83 ± 0.10 a | 21.01 ± 0.51 a | 32.52 ± 3.51 a | |
| Nerol | J | 1.02 ± 0.05 a | 2.55 ± 0.14 b | 3.26 ± 0.00 c |
| Š | 2.52 ± 0.09 b | 2.66 ± 0.14 bc | 2.35 ± 0.25 b | |
| Geraniol | J | 6.85 ± 0.74 a | 10.58 ± 1.41 b | 10.33 ± 0.09 b |
| Š | 5.57 ± 0.05 a | 5.08 ± 0.09 a | 4.50 ± 0.31 a | |
| Terpendiol II | J | 0.63 ± 0.77 a | 0.16 ± 0.09 a | 0.35 ± 0.31 a |
| Š | 1.14 ± 0.24 a | 0.49 ± 0.23 a | 0.93 ± 0.84 a | |
| 6,7-Dihydro-7-hydroxylinalool | J | 10.11 ± 0.53 a | 14.01± 0.50 a | 13.71 ± 2.72 a |
| Š | 23.01 ± 0.50 a | 20.56 ± 1.84 a | 23.18 ± 6.07 a | |
| 2,6-Dimethyl-7-octen-2,6-diol | J | 9.33 ± 1.65 a | 12.59 ± 1.37 ab | 12.11 ± 1.38 ab |
| Š | 21.38 ± 1.35 bc | 19.29 ± 1.83 abc | 22.86 ± 3.93 c | |
| Nerolidol | J | 0.59 ± 0.09 a | 0.63 ± 0.03 a | 0.89 ± 0.35 a |
| Š | 0.76 ± 0.02 a | 0.76 ± 0.26 a | 0.82 ± 0.07 a | |
| 1,8-Terpin | J | 1.10 ± 0.96 a | 1.66 ± 0.41 a | 1.17 ± 1.15 a |
| Š | 1.45 ± 1.68 a | 1.10 ± 1.15 a | 1.32 ± 1.56 a | |
| Geranyl acetate | J | 9.84 ± 0.33 a | 11.20 ± 0.06 a | 11.79 ± 1.90 a |
| Š | 14.39 ± 1.34 a | 11.69 ± 0.48 a | 12.07 ± 1.65 a | |
| 8-Hydroksylinalool | J | 1.86 ± 1.83 a | 1.28 ± 1.18 a | 2.15 ± 2.02 a |
| Š | 8.86 ± 11.59 a | 0.89 ± 0.28 a | 1.89 ± 1.40 a | |
| Ethyl linalyl acetate | J | 1.91 ± 1.21 a | 0.33 ± 0.24 a | 0.95 ± 0.45 a |
| Š | 0.87 ± 0.12 a | 0.72 ± 0.04 a | 1.06 ± 0.16 a | |
| Ʃ Terpenes | J | 122.49 ± 14.10 a | 130.98 ± 9.56 a | 153.68 ± 3.55 a |
| Š | 154.55 ± 0.84 a | 129.82 ± 11.70 a | 159.85 ± 27.60 a | |
| C13-norisoprenoids | ||||
| β-Damascenone | J | 2.23 ± 0.19 b | 3.75 ± 0.28 c | 3.25 ± 0.05 c |
| Š | 1.18 ± 0.12 a | 1.65 ± 0.26 ab | 1.53 ± 0.18 ab | |
| TDN | J | n.d. | n.d. | n.d. |
| Š | n.d. | n.d. | n.d. | |
| β-Ionone | J | 0.12 ± 0.04 a | 0.13 ± 0.02 ab | 0.12 ± 0.04 a |
| Š | 0.30 ± 0.02 b | 0.20 ± 0.00 ab | 0.27 ± 0.04 ab | |
| α-Ionone | J | 0.10 ± 0.14 a | 0.08 ± 0.12 a | 0.22 ± 0.00 a |
| Š | 0.18 ± 0.01 a | 0.20 ± 0.04 a | 0.17 ± 0.00 a | |
| Ʃ C13-norisoprenoids | J | 2.45 ± 0.38 a | 3.97 ± 0.18 b | 3.60 ± 0.00 b |
| Š | 1.66 ± 0.16 a | 2.06 ± 0.21 a | 1.98 ± 0.14 a | |
| Higher alcohols | ||||
| Isobutanol | J | 5130.80 ± 278.80 bc | 5415.53 ± 71.36 ab | 3941.29 ± 237.07 a |
| Š | 429.55 ± 467.41 abc | 4206.45 ± 69.14 c | 4322.40 ± 137.48 abc | |
| 1-Butanol | J | 139.92 ± 16.40 a | 167.33 ± 5.61 a | 136.29 ± 2.85 a |
| Š | 140.27 ± 31.10 a | 196.66 ± 4.29 a | 170.33 ± 8.69 a | |
| 2-Methyl-1-butanol | J | 11,114.39 ± 1069.30 a | 21,785.33 ± 250.97 a | 20,076.06 ± 473.04 a |
| Š | 20,415.29 ± 628.22 a | 20,973.72 ± 51.15 a | 21,340.38 ± 131.95 a | |
| Isoamyl alcohol | J | 12,793.67 ± 8.23 a | 8048.26 ± 9.82 a | 8419.33 ± 8.10 a |
| Š | 10,245.44 ± 3.73 a | 8827.16 ± 1.13 a | 8914.66 ± 5.97 a | |
| 4-Methyl-1-pentanol | J | 37.455 ± 1.47 a | 32.15 ± 6.20 a | 26.20 ± 5.18 a |
| Š | 27.72 ± 0.75 a | 26.13 ± 1.20 a | 30.34 ± 5.21 a | |
| 1-Octanol | J | 0.75 ± 0.94 a | 1.00 ± 0.52 a | 0.03 ± 0.00 a |
| Š | 0.13 ± 0.03 a | 0.28 ± 0.31 a | 0.33 ± 0.33 a | |
| 1-Nonanol | J | 7.23 ± 1.12 ab | 5.53 ± 1.29 ab | 4.85 ± 0.07 a |
| Š | 7.49 ± 0.04 ab | 6.06 ± 0.57 ab | 8.70 ± 0.53 b | |
| 2-Penten-1-ol | J | 8.66 ± 0.39 a | 8.61 ± 1.34 a | 6.46 ± 1.4 a |
| Š | 5.91 ± 0.48 a | 6.01 ± 0.09 a | 6.80 ± 1.35 a | |
| 1-Hexanol | J | 1179.26 ± 47.80 a | 1239.97 ± 203.88 a | 1412.61 ± 27.33 a |
| Š | 1649.18 ± 57.89 a | 1409.09 ± 30.79 a | 1510.73 ± 195.77 a | |
| trans-3-Hexen-1-ol | J | 22.65 ± 2.11 a | 24.76 ± 4.24 a | 22.96 ± 0.19 a |
| Š | 42.88 ± 0.10 b | 41.63 ± 0.94 b | 43.26 ± 6.54 b | |
| 3-Etoxy-1-propanol | J | 7.08 ± 1.35 a | 121.48 ± 27.28 b | 48.35 ± 2.80 a |
| Š | 15.13 ± 0.30 a | 42.08 ± 2.51 a | 7.67 ± 0.72 a | |
| cis-3-Hexen-1-ol | J | 12.49 ± 0.16 ab | 15.15 ± 2.48 ab | 10.46 ± 0.26 a |
| Š | 18.96 ± 0.16 ab | 20.61 ± 0.28 b | 20.50 ± 3.76 b | |
| trans-3-Hexen-1-ol | J | 4.83 ± 0.26 a | 5.90 ± 0.14 a | 3.77 ± 0.16 a |
| Š | 12.01 ± 0.26 b | 11.39 ± 0.41 b | 12.40 ± 1.38 b | |
| 2-Ethyl-1-hexanol | J | 0.11 ± 0.08 a | 0.14 ± 0.14 a | 0.04 ± 0.00 a |
| Š | 0.41 ± 0.48 a | 0.65 ± 0.07 a | 0.25 ± 0.19 a | |
| 1-Decanol | J | 3.83 ± 0.40 bc | 2.10 ± 0.14 a | 2.48 ± 0.06 ab |
| Š | 4.22 ± 0.23 c | 2.31 ± 0.33 a | 3.87 ± 0.48 bc | |
| Phenylethyl alcohol | J | 5847.45 ± 171.74 a | 5207.13 ± 28.62 a | 5302.40 ± 29.76 a |
| Š | 4634.85 ± 72.85 a | 2768.87 ± 2452.29 a | 2721.91 ± 1921.65 a | |
| Ʃ Higher alcohols | J | 36,311.00 ± 236.00 a | 42,080.00 ± 592.00 a | 39,414.00 ± 565.00 a |
| Š | 41,516.00 ± 623.00 a | 38,539.00 ± 3504.00 a | 39,163.00 ± 189.00 a | |
| Esters | ||||
| Isobutyl acetate | J | 80.59 ± 30.38 a | 107.63 ± 0.47 a | 64.89 ± 0.49 a |
| Š | 81.74 ± 1.02 a | 81.30 ± 0.17 a | 98.57 ± 3.09 a | |
| Ethyl butanoate | J | 113.9 ± 6.06 a | 113.25 ± 4.53 a | 159.915 ± 2.58 ab |
| Š | 172.53 ± 0.26 ab | 176.33 ± 7.00 ab | 202.57 ± 34.18 b | |
| Isoamyl acetate | J | 333.97 ± 38.13 a | 514.63 ± 89.22 a | 576.81 ± 38.76 a |
| Š | 607.65 ± 9.61 a | 658.69 ± 40.51 a | 688.53 ± 196.17 a | |
| Ethyl hexanoate | J | 68.59 ± 15.28 ab | 59.06 ± 9.04 a | 93.98 ± 4.96 ab |
| Š | 163.6 ± 0.85 c | 128.17 ± 1.45 bc | 173.26 ± 25.58 c | |
| Ethyl lactate | J | 185.44 ± 1.35 a | 1826.19 ± 347.42 b | 385.48 ± 27.91 a |
| Š | 282.66 ± 15.52 a | 608.89 ± 8.36 b | 402.41 ± 46.77 a | |
| Ethyl 2-hydroxy-3-methylbutanoate | J | 1.71 ± 0.04 a | 3.45 ± 0.07 b | 10.14 ± 0.03 c |
| Š | 3.88 ± 0.23 b | 3.46 ± 0.14 b | 3.92 ± 0.70 b | |
| Ethyl octanoate | J | 31.08 ± 1.67 ab | 19.89 ± 1.35 a | 27.32 ± 3.30 ab |
| Š | 72.50 ± 3.13 ab | 47.96 ± 3.36 ab | 80.78 ± 29.86 b | |
| Ethyl 3-hydroxybutanoate | J | 11.70 ± 0.63 a | 11.57 ± 2.31 a | 14.03 ± 0.82 a |
| Š | 18.42 ± 1.01 a | 16.81 ± 0.14 a | 31.02 ± 3.73 b | |
| Ethyl furoate | J | 2.24 ± 0.17 b | 2.62 ± 0.31 bc | 3.51 ± 0.04 c |
| Š | 0.28 ± 0.04 a | 3.06 ± 0.02 bc | 3.82 ± 0.54 c | |
| Diethyl succinate | J | 155.10 ± 7.17 b | 73.13 ± 7.87 a | 184.26 ± 3.63 b |
| Š | 167.58 ± 1.04 b | 149.10 ± 2.34 b | 191.31 ± 27.18 b | |
| 2-Phenylethyl acetate | J | 1.10 ± 0.20 ab | 1.86 ± 0.29 b | 1.66 ± 0.19 ab |
| Š | 1.05 ± 0.02 ab | 1.04 ± 0.01 a | 0.94 ± 0.11 a | |
| Diethyl malate | J | 5.62 ± 0.05 a | 5.62 ± 0.31 a | 10.21 ± 0.82 ab |
| Š | 8.25 ± 0.50 ab | 8.69 ± 0.02 ab | 12.90 ± 3.22 b | |
| Ethyl hydrogen succinate | J | 0.22 ± 0.19 a | 0.50 ± 0.07 a | 0.83 ± 0.15 a |
| Š | 0.77 ± 0.20 a | 0.71 ± 0.20 a | 0.37 ± 0.07 a | |
| Ethyl linoleate | J | 0.16 ± 0.02 a | 0.37 ± 0.05 a | 0.21 ± 0.01 a |
| Š | 0.27 ± 0.00 a | 0.00 ± 0.25 a | 0.32 ± 0.20 a | |
| Ethyl vanillate | J | 5.30 ± 0.73 b | 6.09 ± 0.26 bc | 6.72 ± 0.24 bc |
| Š | 7.62 ± 0.08 c | 7.16 ± 0.08 c | 0.00 ± 0.00 a | |
| Ʃ Esters | J | 995.83 ± 25.90 a | 2745.56 ± 273.00 c | 1540.36 ± 20.60 ab |
| Š | 1588.58 ± 25.20 ab | 1891.05 ± 31.40 bc | 1890.31 ± 366.00 bc | |
| Aldehydes | ||||
| 2,4-Hexadienal | J | 1.22 ± 0.00 a | 1.29 ± 0.02 ab | 1.27 ± 0.14 ab |
| Š | 1.56 ± 0.05 b | 1.50 ± 0.02 ab | 1.52 ± 0.06 ab | |
| Benzaldehyde | J | 12.62 ± 1.13 ab | 10.18 ± 0.89 a | 10.27 ± 0.09 a |
| Š | 16.15 ± 0.81 b | 15.34 ± 1.17 b | 12.11 ± 1.30 ab | |
| 2,4-Heptadienal (E) | J | 8.14 ± 0.65 a | 10.26 ± 1.18 a | 9.62 ± 0.29 a |
| Š | 10.27 ± 0.39 a | 9.10 ± 0.38 a | 11.21 ± 1.61 a | |
| Decanal | J | 1.59 ± 0.18 a | 2.53 ± 0.44 a | 1.81 ± 0.12 a |
| Š | 3.22 ± 0.79 a | 2.38 ± 0.17 a | 2.71 ± 0.16 a | |
| Acetylfuran | J | 1.13 ± 0.09 a | 1.25 ± 0.27 a | 1.10 ± 0.07 a |
| Š | 1.18 ± 0.19 a | 1.02 ± 0.12 a | 1.43 ± 0.28 a | |
| 2,4-Nonadienal | J | 1.33 ± 0.19 b | 1.32 ± 0.20 b | 1.04 ± 0.03 ab |
| Š | 0.79 ± 0.05 ab | 0.52 ± 0.06 a | 0.66 ± 0.06 a | |
| 2,4-Decadienal | J | 0.17 ± 0.22 a | 0.07 ± 0.09 a | 0.21 ± 0.25 a |
| Š | 0.12 ± 0.03 a | 9.02 ± 12.68 a | 2.74 ± 1.24 a | |
| 2,4-Heptadienal (Z) | J | 0.40 ± 0.00 a | 0.11 ± 0.15 a | n.d. |
| Š | 0.28 ± 0.19 a | 0.25 ± 0.00 a | 0.35 ± 0.07 a | |
| Ʃ Aldehydes | J | 26.59 ± 0.45 a | 27.06 ± 3.59 a | 25.33 ± 1.30 a |
| Š | 33.60 ± 0.60 a | 39.15 ± 14.6 a | 32.72 ± 2.88 a | |
| Lactones | ||||
| γ-Decalactone | J | 1.27 ± 0.12 a | 3.29 ± 0.03 bc | 1.65 ± 0.24 ab |
| Š | 1.44 ± 0.33 a | 3.96 ± 0.76 c | 1.72 ± 0.28 ab | |
| γ-Nonalactone | J | 33.74 ± 2.25 a | 31.44 ± 0.45 a | 39.84 ± 5.61 a |
| Š | 35.64 ± 0.96 a | 34.71 ± 0.89 a | 49.18 ± 12.77 a | |
| γ-Hexalactone | J | 6.30 ± 0.28 a | 6.82 ± 0.79 a | 6.70 ± 0.37 a |
| Š | 8.38 ± 0.45 a | 9.34 ± 0.04 ab | 12.31 ± 1.62 b | |
| γ-Octalactone | J | 0.84 ± 0.07 b | 1.93 ± 0.02 c | 0.53 ± 0.03 ab |
| Š | 0.53 ± 0.07 ab | 0.45 ± 0.01 ab | 0.31 ± 0.20 a | |
| δ-Decalactone | J | 2.79 ± 0.15 a | 2.71 ± 0.07 a | 3.71 ± 0.16 b |
| Š | 2.71 ± 0.14 a | 2.50 ± 0.11 a | 2.89 ± 0.13 a | |
| γ-Undecalactone | J | 0.46 ± 0.04 ab | 0.45 ± 0.07 ab | 0.43 ± 0.02 a |
| Š | 0.41 ± 0.06 a | 0.43 ± 0.01 a | 0.60 ± 0.02 b | |
| γ-Butyrolactone | J | 325.83 ± 11.60 ab | 543.00 ± 90.58 b | 299.10 ± 35.63 a |
| Š | 201.61 ± 9.75 a | 243.26 ± 5.72 a | 327.91 ± 77.36 ab | |
| Ʃ Lactones | J | 371.00 ± 14.40 ab | 590.00 ± 91.80 b | 352.00 ± 30.00 ab |
| Š | 251.00 ± 9.45 a | 295.00 ± 7.42 a | 395.00 ± 91.80 ab | |
| Volatile phenols | ||||
| Guaiacol | J | 3.51 ± 0.19 b | 3.10 ± 0.38 a | 3.18 ± 0.00 b |
| Š | 1.32 ± 0.02 a | 1.01 ± 0.01 a | 1.48 ± 0.33 a | |
| Homovanillyl alcohol | J | 70.75 ± 1.93 a | 75.03 ± 2.63 a | 79.80 ± 0.96 a |
| Š | 115.85 ± 1.43 b | 112.26 ± 2.77 b | 132.01 ± 14.79 b | |
| Eugenol | J | 0.60 ± 0.12 ab | 0.55 ± 0.04 ab | 0.81 ± 0.015 b |
| Š | 0.30 ± 0.01 a | 0.27 ± 0.03 a | 0.36 ± 0.11 a | |
| 4-Ethylphenol | J | 3.25 ± 0.04 b | 1.90 ± 0.00 a | 3.08 ± 0.14 b |
| Š | 2.53 ± 0.33 ab | 2.52 ± 0.11 ab | 3.40 ± 0.36 b | |
| 4-Vinylphenol | J | 12.09 ± 1.32 a | 9.76 ± 0.05 a | 9.30 ± 0.73 a |
| Š | 7.30 ± 0.98 a | 9.46 ± 1.35 a | 7.60 ± 1.68 a | |
| Vanillin | J | 9.29 ± 2.74 a | 12.13 ± 1.56 a | 15.22 ± 1.48 a |
| Š | 15.28 ± 0.73 a | 15.34 ± 0.48 a | 17.58 ± 2.67 a | |
| Ʃ Volatile phenols | J | 99.50 ± 0.77 a | 102.00 ± 1.47 ab | 111.00 ± 1.70 b |
| Š | 143.00 ± 0.00 c | 141.00 ± 2.06 c | 162.00 ± 20.00 d | |
| Other compounds | ||||
| 2-Pentylfuran | J | 245.51 ± 9.17 a | 255.59 ± 4.92 a | 264.88 ± 14.15 a |
| Š | 233.42 ± 2.08 a | 248.72 ± 23.94 a | 246.61 ± 23.49 a | |
| Acetoin | J | 12.84 ± 1.32 a | 14.23 ± 6.73 a | 8.69 ± 3.98 a |
| Š | 12.96 ± 0.79 a | 15.82 ± 6.83 a | 18.61 ± 4.90 a | |
| 6-Methyl-5-hepten-2-one | J | 34.90 ±1.22 a | 362.02 ± 67.38 b | 73.19 ± 5.53 a |
| Š | 53.61 ± 4.17 a | 117.57 ± 1.58 a | 75.73 ± 8.88 a | |
| Furfuryl alcohol | J | 0.26 ± 0.24 a | 0.47 ± 0.08 a | 0.45 ± 0.25 a |
| Š | 3.78 ± 0.07 bc | 3.02 ± 0.16 b | 4.95 ± 0.53 c | |
| 4-Ethyl-cyclohexanol | J | 8.75 ± 0.77 a | 11.28 ± 1.40 a | 10.51 ± 0.35 a |
| Š | 11.28 ± 0.46 a | 9.89 ± 0.45 a | 12.40 ± 1.90 a | |
| Furfural | J | 1.69 ± 0.37 a | 2.08 ± 0.08 a | 1.87 ± 0.67 a |
| Š | 1.96 ± 0.01 a | 1.66 ± 0.31 a | 1.33 ± 0.55 a | |
| Benzyl alcohol | J | 9.65 ± 0.38 a | 8.36 ± 0.26 a | 9.84 ± 0.74 a |
| Š | 7.82 ± 0.03 a | 7.03 ± 0.00 a | 8.79 ± 1.18 a | |
| Ʃ Other compounds | J | 314.00 ± 4.61 a | 653.69 ±72.80 b | 369.41 ± 8.25 a |
| Š | 327.57 ± 7.15 a | 404.11 ± 19.00 a | 368.83 ±38.70 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivić, S.; Jeromel, A.; Kozina, B.; Prusina, T.; Budić-Leto, I.; Boban, A.; Vasilj, V.; Jagatić Korenika, A.-M. Sequential Fermentation in Red Wine cv. Babić Production: The Influence of Torulaspora delbrueckii and Lachancea thermotolerans Yeasts on the Aromatic and Sensory Profile. Foods 2024, 13, 2000. https://doi.org/10.3390/foods13132000
Ivić S, Jeromel A, Kozina B, Prusina T, Budić-Leto I, Boban A, Vasilj V, Jagatić Korenika A-M. Sequential Fermentation in Red Wine cv. Babić Production: The Influence of Torulaspora delbrueckii and Lachancea thermotolerans Yeasts on the Aromatic and Sensory Profile. Foods. 2024; 13(13):2000. https://doi.org/10.3390/foods13132000
Chicago/Turabian StyleIvić, Stipe, Ana Jeromel, Bernard Kozina, Tihomir Prusina, Irena Budić-Leto, Ana Boban, Višnja Vasilj, and Ana-Marija Jagatić Korenika. 2024. "Sequential Fermentation in Red Wine cv. Babić Production: The Influence of Torulaspora delbrueckii and Lachancea thermotolerans Yeasts on the Aromatic and Sensory Profile" Foods 13, no. 13: 2000. https://doi.org/10.3390/foods13132000
APA StyleIvić, S., Jeromel, A., Kozina, B., Prusina, T., Budić-Leto, I., Boban, A., Vasilj, V., & Jagatić Korenika, A.-M. (2024). Sequential Fermentation in Red Wine cv. Babić Production: The Influence of Torulaspora delbrueckii and Lachancea thermotolerans Yeasts on the Aromatic and Sensory Profile. Foods, 13(13), 2000. https://doi.org/10.3390/foods13132000








