Antioxidant Capacity, Phytochemicals, Minerals, and Chemical Pollutants in Worker Honey Bee (Apis mellifera L.) Broods from Northern Thailand: A Safe and Sustainable Food Source
Abstract
1. Introduction
2. Materials and Methods
2.1. HBB Samples
2.2. Bioactive Compounds Analysis
2.2.1. Extraction of HBB
2.2.2. DPPH Radical-Scavenging Activity
2.2.3. ABTS Radical-Scavenging Activity
2.2.4. Ferric Ion Reducing Antioxidant Power Assay (FRAP)
2.2.5. Total Phenolic Content (TCP)
2.2.6. Total Flavonoid Content (TFC)
2.3. LC-Q-TOF/MS Identification of Phytochemicals
2.4. Mineral Analysis
2.4.1. Sample Preparation
2.4.2. ICP-OES Determination of Minerals
2.5. Chemical Pollutants Analysis
2.5.1. Organophosphate and Synthetic Pyrethroid Insecticide Analysis
2.5.2. Organochlorine Pesticide Analysis (OCs)
2.5.3. Polycyclic Aromatic Hydrocarbons (PAHs)
2.6. Statistical Analysis
3. Results
3.1. Bioactive Compounds
3.1.1. Antioxidant Activity
3.1.2. Total Phenolic Content (TPC) and Total Flavonoids Content (TFC)
3.2. Phytochemical Analysis
3.3. Minerals
3.4. Chemical Pollutants Analysis
4. Discussion
4.1. Antioxidant Activities of the Bee Brood
4.2. Phytochemicals
4.3. Mineral Content
4.4. Food Safety
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Wang, F.; Lu, Y.; Wang, J.; Chen, J.; Yu, Y.; Tao, X.; Xiao, Y.; Peng, Y. A review on edible insects in China: Nutritional supply, environmental benefits, and potential applications. Curr. Res. Food Sci. 2023, 7, 100596. [Google Scholar] [CrossRef] [PubMed]
- Yucel, B.; Topal, E.; Kosoglu, M. Bee products as functional food. In Superfood and Functional Food—An Overview of Their Processing and Utilization; IntechOpen: London, UK, 2017; Volume 1, pp. 15–33. [Google Scholar] [CrossRef]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A.; et al. The Honey Bee Apis mellifera: An Insect at the Interface between Human and Ecosystem Health. Biology 2022, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.P.; Wongsiri, S.; Jamyanya, T.; Rinderer, T.E.; Vongsamanode, S.; Matsuka, M.; Sylvester, H.A.; Oldroyd, B.P. Honey Bees and other Edible Insects Used as Human Food in Thailand. Am. Entomol. 1998, 44, 24–29. [Google Scholar] [CrossRef]
- Schabel, H.G. Forest insects as food: A global review. In Forest Insects as Food: Humans Bite Back; FAO: Rome, Italy, 2010. [Google Scholar]
- Huis, A. Increasing academic interest in edible insects: The Journal of Insects as Food and Feed. J. Insects Food Feed. 2023, 9, 1–2. [Google Scholar] [CrossRef]
- Luo, Z.-Y. Insects as food in China. Ecol. Food Nutr. 1997, 36, 201–207. [Google Scholar] [CrossRef]
- Schiel, L.; Wind, C.; Ulmer, M.; Braun, P.G.; Koethe, M. Honey bee drone brood used as food. Ernahr. Umsch. 2022, 69, 96–104. [Google Scholar]
- Guiné, R.P.F.; Florença, S.G.; Correia, P.M.R.; Anjos, O.; Coelho, C.; Costa, C.A. Honey Bee (Apis mellifera L.) Broods: Composition, Technology and Gastronomic Applicability. Foods 2022, 11, 2750. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Meyer-Rochow, V.B.; Jung, C. Honey bees and their brood: A potentially valuable resource of food, worthy of greater appreciation and scientific attention. J. Ecol. Environ. 2021, 45, 31. [Google Scholar] [CrossRef]
- Finke, M.D. Nutrient composition of bee brood and its potential as human food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar] [CrossRef]
- Worthy, S.H.; Acorn, J.H.; Frost, C.M. Honey bees (Apis mellifera) modify plant-pollinator network structure, but do not alter wild species’ interactions. PLoS ONE 2023, 18, e0287332. [Google Scholar] [CrossRef]
- Travis, D.J.; Kohn, J.R. Honeybees (Apis mellifera) decrease the fitness of plants they pollinate. Proc. Biol. Sci. 2023, 290, 20230967. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Neov, B.; Shumkova, R.; Palova, N.; Hristov, P. The health crisis in managed honey bees (Apis mellifera). Which factors are involved in this phenomenon? Biologia 2021, 76, 2173–2180. [Google Scholar] [CrossRef]
- Quinlan, G.M.; Grozinger, C.M. Chapter Five—Honey bee nutritional ecology: From physiology to landscapes. In Advances in Insect Physiology; Harrison, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 64, pp. 289–345. [Google Scholar]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia-Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Balkanska, R.; Karadjova, I.; Ignatova, M. Comparative analyses of chemical composition of royal jelly and drone brood. Bulg. Chem. Commun. 2014, 46, 412–416. [Google Scholar]
- Sidor, E.; Miłek, M.; Tomczyk, M.; Dżugan, M. Antioxidant Activity of Frozen and Freeze-Dried Drone Brood Homogenate Regarding the Stage of Larval Development. Antioxidants 2021, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Farjan, M.; Dmitryjuk, M.; Lipiński, Z.; Biernat-Łopieńska, E.; Żółtowska, K. Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. J. Apic. Res. 2012, 51, 263–270. [Google Scholar] [CrossRef]
- Eshbah, H.; Mohamed, A.; Zedan, O.; Ghanem, A. Phytochemical components in different types of pollen and their effects on honey bees. Minia J. Agric. Res. Dev. 2021, 41, 1–13. [Google Scholar] [CrossRef]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.; H. S., A. Dietary Phytochemicals, Honey Bee Longevity and Pathogen Tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef]
- Borkovcová, M.; Mlček, J.; Adámková, A.; Adámek, M.; Bednářová, M.; Musilová, Z.; Ševčíková, V. Use of foods based on bee drone brood: Their sensory and microbiological evaluation and mineral composition. Sustainability 2022, 14, 2814. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci. World J. 2012, 2012, 930849. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.; Yusof, Y.; Tan, S.W.; Chua, L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2017, 20, S2723–S2738. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Jeeno, P.; Tongban, S.; Yana, P.; Wongta, A.; Sutan, K.; Yadoung, S.; Hongsibsong, S. Tentative identification of phytochemicals from smilax glabra and smilax corbularia extracts by LC-QTOF/MS and Their bioactive potential. Plants 2022, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Jeytawan, N.; Yadoung, S.; Jeeno, P.; Yana, P.; Sutan, K.; Naksen, W.; Wongkaew, M.; Sommano, S.R.; Hongsibsong, S. Antioxidant and phytochemical potential and phytochemicals in Gymnema inodorum (Lour.) Decne in Northern Thailand. Plants 2022, 11, 3498. [Google Scholar] [CrossRef] [PubMed]
- Kawichai, S.; Bootdee, S.; Sillapapiromsuk, S.; Janta, R. Epidemiological Study on Health Risk Assessment of Exposure to PM2.5-Bound Toxic Metals in the Industrial Metropolitan of Rayong, Thailand. Sustainability 2022, 14, 15368. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Hongsibsong, S. Organophosphorus pesticide residues in vegetables from farms, markets, and a supermarket around Kwan Phayao Lake of Northern Thailand. Arch. Environ. Contam. Toxicol. 2014, 67, 60–67. [Google Scholar] [CrossRef]
- Hongsibsong, S.; Wipasa, J.; Pattarawarapan, M.; Chantara, S.; Stuetz, W.; Nosten, F.; Prapamontol, T. Development and application of an indirect competitive enzyme-linked immunosorbent assay for the detection of p,p’-DDE in human milk and comparison of the results against GC-ECD. J. Agric. Food Chem. 2012, 60, 16–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tapiero, H.; Tew, K.D.; Ba, G.N.; Mathé, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Sawczuk, R.; Karpinska, J.; Filipowska, D.; Bajguz, A.; Hryniewicka, M. Evaluation of total phenols content, anti-DPPH activity and the content of selected antioxidants in the honeybee drone brood homogenate. Food Chem. 2022, 368, 130745. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Runikhina, S.A.; Afanasyev, O.I.; Biriukov, K.; Perekalin, D.S.; Klussmann, M.; Chusov, D. Aldehydes as Alkylating Agents for Ketones. Chemistry 2019, 25, 16225–16229. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Dżugan, M.; Sidor, E.; Miłek, M.; Tomczyk, M. The Possibility of Using Bee Drone Brood to Design Novel Dietary Supplements for Apitherapy. Appl. Sci. 2023, 13, 4687. [Google Scholar] [CrossRef]
- Devi, W.D.; Bonysana, R.; Kapesa, K.; Rai, A.K.; Mukherjee, P.K.; Rajashekar, Y. Potential of edible insects as source of functional foods: Biotechnological approaches for improving functionality. Syst. Microbiol. Biomanuf. 2022, 2, 461–472. [Google Scholar] [CrossRef]
- Hussain, A.; AlJabr, A.M. Plant Secondary Metabolites: Emerging Trends in Agricultural Pests Control. In New and Future Development in Biopesticide Research: Biotechnological Exploration; Springer: Berlin/Heidelberg, Germany, 2022; pp. 187–201. [Google Scholar]
- Torres-Castillo, J.A.; Olazarán-Santibáñez, F.E. Insects as source of phenolic and antioxidant entomochemicals in the food industry. Front. Nutr. 2023, 10, 1133342. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.N.; Dey, P.; Singhal, R.K.; Sahu, C.; Jena, D.; Nanda, S.; Chauhan, J. Plant phenolics: As antioxidants and potent compounds under multiple stresses. In Plant Phenolics in Abiotic Stress Management; Springer: Berlin/Heidelberg, Germany, 2023; pp. 215–234. [Google Scholar]
- Silva, C.S.; Moutinho, C.; Ferreira da Vinha, A.; Matos, C. Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. Int. J. Sci. Res. Methodol. 2019, 13, 57–80. [Google Scholar]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Baturin, A.K.; Sharafetdinov, K.K.; Kodentsova, V.M. Role of calcium in health and reducing the risk of non-communicable diseases. Vopr. Pitan. 2022, 91, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Pop, M.S.; Cheregi, D.C.; Onose, G.; Munteanu, C.; Popescu, C.; Rotariu, M.; Turnea, M.A.; Dogaru, G.; Ionescu, E.V.; Oprea, D.; et al. Exploring the Potential Benefits of Natural Calcium-Rich Mineral Waters for Health and Wellness: A Systematic Review. Nutrients 2023, 15, 3126. [Google Scholar] [CrossRef]
- Wu, F.Y.; Wu, C.-W. Zinc in DNA replication and transcription. Annu. Rev. Nutr. 1987, 7, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Płaczek, A.; Machaj, D.; Białas, F.; Cyboran, K.; Kuc, M. Consequences of zinc deficiency in selected diseases—Literature review. J. Educ. Health Sport 2022, 12, 775–779. [Google Scholar] [CrossRef]
- Di Fiore, C.; De Cristofaro, A.; Nuzzo, A.; Notardonato, I.; Ganassi, S.; Iafigliola, L.; Sardella, G.; Ciccone, M.; Nugnes, D.; Passarella, S. Biomonitoring of polycyclic aromatic hydrocarbons, heavy metals, and plasticizers residues: Role of bees and honey as bioindicators of environmental contamination. Environ. Sci. Pollut. Res. 2023, 30, 44234–44250. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Polo, R.O.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Guarna, M.M. Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Medina-Dzul, K.; Medina-Peralta, S.; Carrera-Figueiras, C.; Sánchez, M.; Muñoz-Rodríguez, D. Matrix solid-phase dispersion extraction of organophosphorous pesticides from beeswax. Int. J. Environ. Anal. Chem. 2017, 97, 831–840. [Google Scholar] [CrossRef]
- Ravoet, J.; Reybroeck, W.; de Graaf, D.C. Pesticides for apicultural and/or agricultural application found in Belgian honey bee wax combs. Bull. Environ. Contam. Toxicol. 2015, 94, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Karazafiris, E.; Tananaki, C.; Thrasyvoulou, A.; Menkissoglu-Spiroudi, U. Pesticide residues in bee products. In Pesticides in the Modern World-Risks and Benefits; IntechOpen: London, UK, 2011; pp. 89–126. [Google Scholar]
- Niell, S.; Jesús, F.; Pérez, N.; Pérez, C.; Pareja, L.; Abbate, S.; Carrasco-Letelier, L.; Díaz, S.; Mendoza, Y.; Cesio, V. Neonicotinoids transference from the field to the hive by honey bees: Towards a pesticide residues biomonitor. Sci. Total Environ. 2017, 581, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Arjin, C.; Hongsibsong, S.; Pringproa, K.; Seel-Audom, M.; Ruksiriwanich, W.; Sutan, K.; Sommano, S.R.; Sringarm, K. Effect of ethanolic Caesalpinia sappan fraction on in vitro antiviral activity against porcine reproductive and respiratory syndrome virus. Vet. Sci. 2021, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Amekawa, Y.; Bumrungsri, S.; Wayo, K.; Gebre, G.G.; Hongsibsong, S. Pesticide Use under Public Good Agricultural Practices Standard: A Comparative Study in Thailand. Agriculture 2022, 12, 606. [Google Scholar] [CrossRef]
- Noël, A.; Dumas, C.; Rottier, E.; Beslay, D.; Costagliola, G.; Ginies, C.; Nicolè, F.; Rau, A.; Le Conte, Y.; Mondet, F. Detailed chemical analysis of honey bee (Apis mellifera) worker brood volatile profile from egg to emergence. PLoS ONE 2023, 18, e0282120. [Google Scholar] [CrossRef]
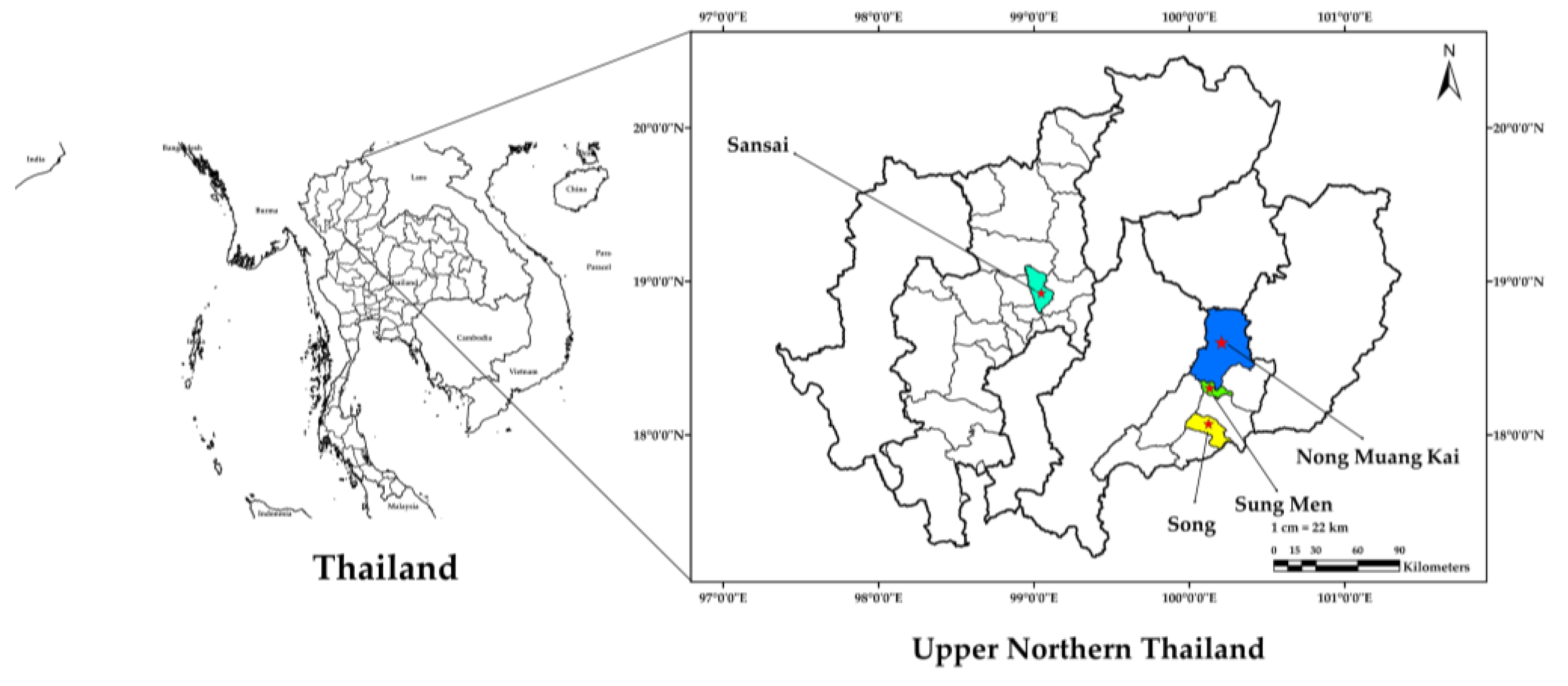
| Sample | Apiary Location | Water Content (%) |
|---|---|---|
| HB-NT-1 | Nong Muang Kai District, Phrae Province | 73.95 |
| HB-NT-2 | Song District, Phrae Province | 77.51 |
| HB-NT-3 | San Sai District, Chiang Mai Province | 76.61 |
| HB-NT-4 | Sung Men District, Phrae Province | 71.59 |
| HB-NT-5 | 68.75 |
| ID Farms | DPPH-Scavenging Activity | ABTS Radical-Scavenging Activity | Ferric Ion Reducing Antioxidant Power | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 (mg/mL) | IC50 (mg/mL) | (mg AAE/100 g Dry Weight) | |||||||
| Extracted | 70% EtOH | DI Water | p-Value | 70% EtOH | DI Water | p-Value | 70% EtOH | DI Water | p-Value |
| HB-NT-1 | 3.20 ± 0.34 a | 39.68 ± 6.72 b | 0.007 ** | 27.91 ± 6.47 ab | 13.37 ± 1.87 a | 0.063 | 94.90 ± 4.85 a | 146.92 ± 13.17 b | 0.038 ** |
| HB-NT-2 | 2.04 ± 0.10 b | 10.67 ± 2.64 c | 0.032 ** | 33.72 ± 1.69 a | 9.25 ± 1.21 b | 0.003 ** | 104.15 ± 25.43 a | 145.15 ± 6.67 b | 0.122 |
| HB-NT-3 | 2.43 ± 0.17 b | 14.39 ± 4.54 c | 0.004 ** | 33.90 ± 3.24 a | 11.33 ± 1.7 ab | 0.015 ** | 98.48 ± 40.16 a | 177.32 ± 14.53 a | 0.037 ** |
| HB-NT-4 | 3.37 ± 0.37 a | 84.97 ± 14.02 a | 0.010 ** | 33.91 ± 9.48 a | 13.54 ± 2.86 a | 0.077 | 79.21 ± 14.94 ab | 119.94 ± 12.43 c | 0.105 |
| HB-NT-5 | 3.28 ± 0.07 a | 77.52 ± 10.3 a | 0.000 ** | 21.22 ± 2.92 b | 9.40 ± 0.96 b | 0.025 ** | 50.07 ± 4.11 b | 57.66 ± 5.42 d | 0.011 ** |
| ID Farms | Total Phenolic Content | Total Flavonoid Content | ||||
|---|---|---|---|---|---|---|
| (mg GAE/100 g Dry Weight) | (mg QE/g Dry Weight) | |||||
| Extracted | 70% EtOH | DI Water | p-Value | 70% EtOH | DI Water | p-Value |
| HB-NT-1 | 488.95 ± 14.28 | 496.06 ± 10.93 b | 0.421 | 12.53 ±3.80 b | 4.32 ± 3.25 b | 0.074 |
| HB-NT-2 | 501.37 ± 18.01 | 508.80 ± 6.45 ab | 0.422 | 5.34 ± 1.71 c | 9.15 ± 4.48 ab | 0.325 |
| HB-NT-3 | 497.93 ± 15.87 | 518.74 ± 14.92 ab | 0.001 ** | 4.46 ± 3.07 c | 6.14 ± 5.04 b | 0.321 |
| HB-NT-4 | 508.87 ± 20.16 | 508.19 ± 13.14 ab | 0.961 | 18.88 ± 3.59 a | 21.60 ± 9.81 a | 0.758 |
| HB-NT-5 | 491.62 ± 4.29 | 512.60 ± 16.10 a | 0.040 ** | 4.98 ± 3.25 c | 21.48 ± 7.98 a | 0.097 |
| No. | Phytochemicals | Mass | RT | Score | Formula | m/z | Location of Samples | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HB-NT-1 | HB-NT-2 | HB-NT-3 | HB-NT-4 | HB-NT-5 | |||||||
| 1 | 5Z-Caffeoylquinic acid | 354.097 | 1.813 | 90.34 | C16 H18 O9 | 353.089 | + | − | + | − | + |
| 2 | Gallic acid | 170.019 | 2.166 | 90.55 | C7 H6 O5 | 188.054 | + | + | − | + | − |
| 3 | Protocatechuic acid-3-glucoside | 316.078 | 3.45 | 89.35 | C13 H16 O9 | 334.111 | + | + | − | − | − |
| 4 | Protocatechuic acid | 154.026 | 4.381 | 85.99 | C7 H6 O4 | 153.019 | + | + | + | + | + |
| 5 | Ellagic acid | 302.007 | 9.095 | 88.52 | C14 H6 O8 | 303.014 | + | − | + | − | − |
| 6 | Rutin | 610.152 | 9.786 | 97.83 | C27 H30 O16 | 633.141 | + | + | + | + | - |
| 7 | Orientin | 448.1 | 10.988 | 98.76 | C21 H20 O11 | 447.092 | + | + | + | + | + |
| 8 | Epicatechin | 290.078 | 11.372 | 99.57 | C15 H14 O6 | 289.071 | + | + | − | − | − |
| 9 | Vitexin | 432.104 | 13.168 | 93.96 | C21 H20 O10 | 477.103 | − | − | + | + | + |
| 10 | Quercetin | 302.042 | 15.235 | 98.91 | C15 H10 O7 | 301.035 | + | + | + | + | − |
| 11 | Pinobanksin | 272.068 | 15.586 | 99.28 | C15 H12 O5 | 273.075 | + | + | + | + | + |
| 12 | Kaempferol | 286.047 | 17.77 | 99.29 | C15 H10 O6 | 287.054 | + | + | + | + | + |
| 13 | Caffeic acid | 180.042 | 29.383 | 98.72 | C9 H8 O4 | 203.031 | + | + | + | + | + |
| Minerals | HB-NT-1 | HB-NT-2 | HB-NT-3 | HB-NT-4 | HB-NT-5 |
|---|---|---|---|---|---|
| Ca | 69.60 ± 1.22 a | 43.68 ± 6.70 b | 67.87 ± 2.44 a | 66.69 ± 10.54 a | 36.36 ± 2.10 b |
| Co | ND | ND | ND | ND | ND |
| K | 579.81 ± 21.28 b | 533.36 ± 3.17 c | 680.15 ± 3.36 a | 530.59 ± 10.31 c | 522.19 ± 9.02 c |
| Mg | 62.28 ± 1.07 a | 62.97 ± 0.84 a | 64.29 ± 0.41 a | 58.44 ± 2.28 b | 58.23 ± 0.47 b |
| Na | 36.53 ± 0.07 c | 40.97 ± 0.96 b | 27.11 ± 0.04 e | 30.41 ± 1.30 d | 43.35 ± 0.85 a |
| Zn | 6.24 ± 0.19 a | 6.11 ± 0.17 a | 5.78 ± 0.06 b | 5.59 ± 0.26 bc | 5.32 ± 0.13 c |
| Fe | 5.08 ± 0.63 a | 5.25 ± 0.30 a | 4.05 ± 0.03 b | 3.99 ± 0.07 b | 4.02 ± 0.25 b |
| Cu | 1.26 ± 0.04 a | 1.06 ± 0.02 c | 1.15 ± 0.01 b | 1.16 ± 0.01 b | 1.11 ± 0.03 b |
| Mn | 0.51 ± 0.02 a | 0.44 ± 0.01 b | 0.28 ± 0.00 d | 0.52 ± 0.01 a | 0.35 ± 0.01 c |
| Heavy Metal | HB-NT-1 | HB-NT-2 | HB-NT-3 | HB-NT-4 | HB-NT-5 |
|---|---|---|---|---|---|
| Al | 2.01 ± 0.20 a | 1.43 ± 0.23 ab | 1.55 ± 0.45 ab | 1.89 ± 0.42 a | 1.14 ± 0.12 b |
| Ag | ND | ND | ND | ND | ND |
| As | 0.04 ± 0.02 | 0.05 ± 0.30 | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.00 |
| B | 0.62 ± 0.02 a | 0.34 ± 0.04 b | 0.23 ± 0.04 b | 0.25 ± 0.04 b | 0.23 ± 0.02 b |
| Ba | 0.15 ± 0.01 a | 0.06 ± 0.03 b | 0.12 ± 0.00 a | 0.12 ± 0.04 a | 0.05 ± 0.01 b |
| Be | 00 ± 0.01 | 00 ± 0.00 | 00 ± 0.00 | 00 ± 0.00 | 00 ± 0.00 |
| Cd | ND | ND | ND | ND | ND |
| Ti | 0.05 ± 0.00 | 0.11 ± 0.05 | 0.06 ± 0.00 | 0.09 ± 0.06 | 0.07 ± 0.02 |
| Tl | ND | ND | ND | ND | ND |
| Cr | 0.08 ± 0.02 b | 0.13 ± 0.00 a | 0.09 ± 0.01 b | 0.02 ± 0.00 c | 0.04 ± 0.02 c |
| Mo | 0.03 ± 0.02 a | 0.02 ± 0.00 ab | 0.02 ± 0.00 ab | 0.01 ± 0.00 b | 0.01 ± 0.00 ab |
| Ni | 0.02 ± 0.03 | ND | 0.01 ± 0.018 | ND | ND |
| Pb | 0.02 ± 0.02 | ND | ND | ND | 0.02 ± 0.041 |
| Sb | 0.04 ± 0.03 | 0.03 ± 0.00 | 0.03 ±0.02 | 0.03 ± 0.02 | 0.03 ± 0.017 |
| Se | 0.03 ± 0.02 b | 0.02 ± 0.00 b | 0.07 ± 0.02 a | 0.04 ± 0.02 b | ND |
| Si | 6.12 ± 1.51 a | 4.49 ± 0.38 b | 4.26 ± 0.12 b | 4.66 ± 0.11 b | 3.37 ± 0.15 b |
| V | 0.00 ± 0.00 b | 0.00 ± 0.00 bc | 0.02 ± 0.00 a | 0.00 ± 0.00 bc | 0.00 ± 0.00 c |
| Parameter (Unit) | Chemical Pollutants | |||
|---|---|---|---|---|
| OPs | PYs | OC | PAHs | |
| LOD (mg/kg−1) | 0.001–0.008 | 0.001–0.007 | 0.002–0.007 | 1.00–51.78 |
| LOQ (mg/kg−1) | 0.020–0.060 | 0.010–0.020 | 0.010–0.030 | 11.00–71.00 |
| Working standard range (μg/L) | 50–600 | 50–600 | 50–600 | 1–50 |
| Correlation of the calibration curve (R2) | 0.9919–0.9993 | 0.99192–0.9992 | 0.9907–0.9988 | 0.9919–0.9998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tongchai, P.; Yadoung, S.; Sutan, K.; Kawichai, S.; Danmek, K.; Maitip, J.; Ghosh, S.; Jung, C.; Chuttong, B.; Hongsibsong, S. Antioxidant Capacity, Phytochemicals, Minerals, and Chemical Pollutants in Worker Honey Bee (Apis mellifera L.) Broods from Northern Thailand: A Safe and Sustainable Food Source. Foods 2024, 13, 1998. https://doi.org/10.3390/foods13131998
Tongchai P, Yadoung S, Sutan K, Kawichai S, Danmek K, Maitip J, Ghosh S, Jung C, Chuttong B, Hongsibsong S. Antioxidant Capacity, Phytochemicals, Minerals, and Chemical Pollutants in Worker Honey Bee (Apis mellifera L.) Broods from Northern Thailand: A Safe and Sustainable Food Source. Foods. 2024; 13(13):1998. https://doi.org/10.3390/foods13131998
Chicago/Turabian StyleTongchai, Phannika, Sumed Yadoung, Kunrunya Sutan, Saweang Kawichai, Khanchai Danmek, Jakkrawut Maitip, Sampat Ghosh, Chuleui Jung, Bajaree Chuttong, and Surat Hongsibsong. 2024. "Antioxidant Capacity, Phytochemicals, Minerals, and Chemical Pollutants in Worker Honey Bee (Apis mellifera L.) Broods from Northern Thailand: A Safe and Sustainable Food Source" Foods 13, no. 13: 1998. https://doi.org/10.3390/foods13131998
APA StyleTongchai, P., Yadoung, S., Sutan, K., Kawichai, S., Danmek, K., Maitip, J., Ghosh, S., Jung, C., Chuttong, B., & Hongsibsong, S. (2024). Antioxidant Capacity, Phytochemicals, Minerals, and Chemical Pollutants in Worker Honey Bee (Apis mellifera L.) Broods from Northern Thailand: A Safe and Sustainable Food Source. Foods, 13(13), 1998. https://doi.org/10.3390/foods13131998









