Stability of Buriti Oil Microencapsulated in Mixtures of Azuki and Lima Bean Flours with Maltodextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pre-Treatment of Grains and Flour Production
2.2.2. Microencapsulation of Buriti Oil by Spray Drying
2.2.3. Characterization of Microcapsules
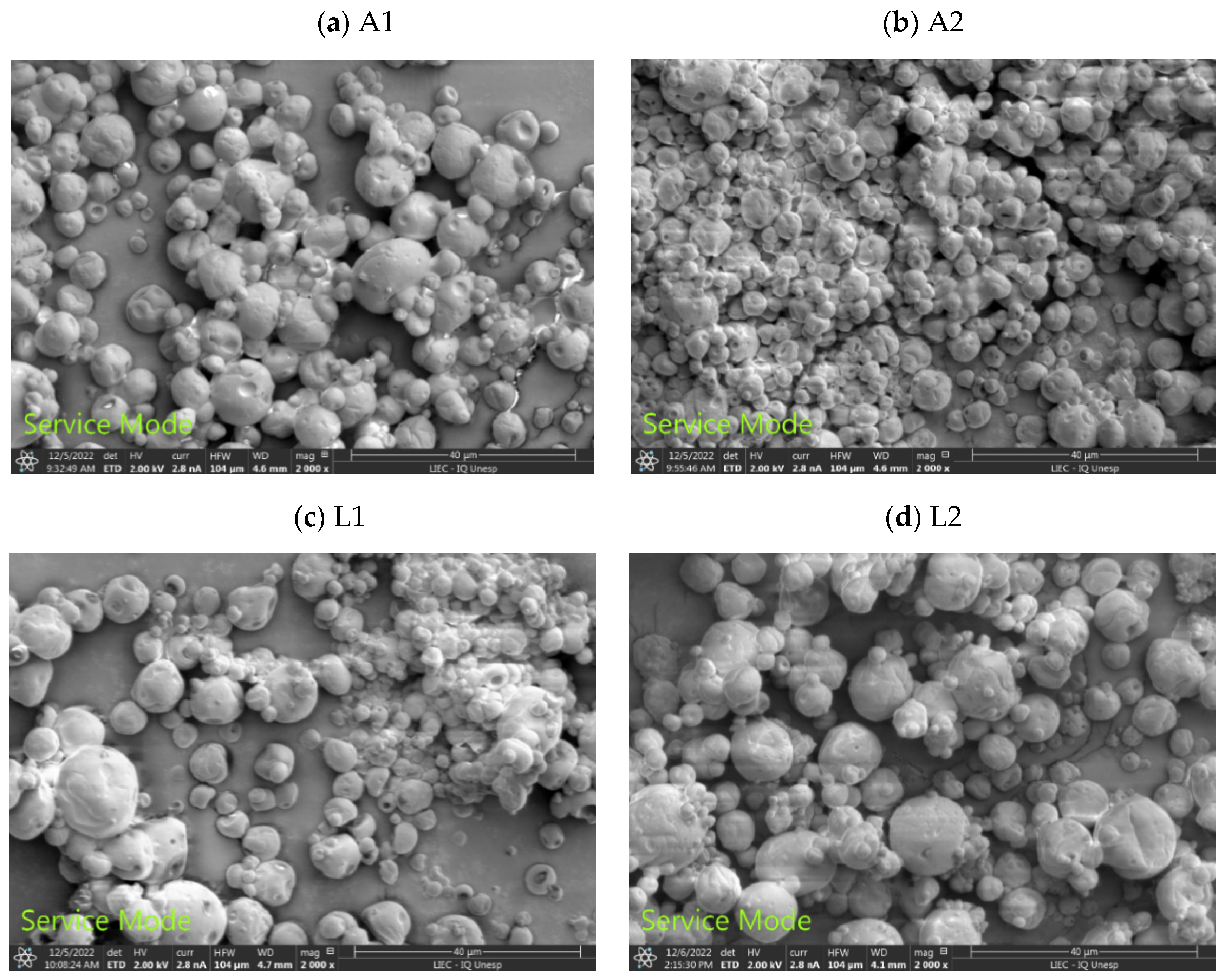
Scanning Electron Microscopy (SEM)
Moisture Content
Water Activity
Hygroscopicity
Solubility
Process Yield
Colorimetric Analysis
Encapsulation Efficiency (EEC) and Retention of Carotenoids (RTC)
Oil Encapsulation Efficiency (EEO) and Oil Retention (RTO)
Stability of Microcapsules
Statistical Analysis
3. Results and Discussion
3.1. Characterization of Microcapsules
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Physicochemical Properties
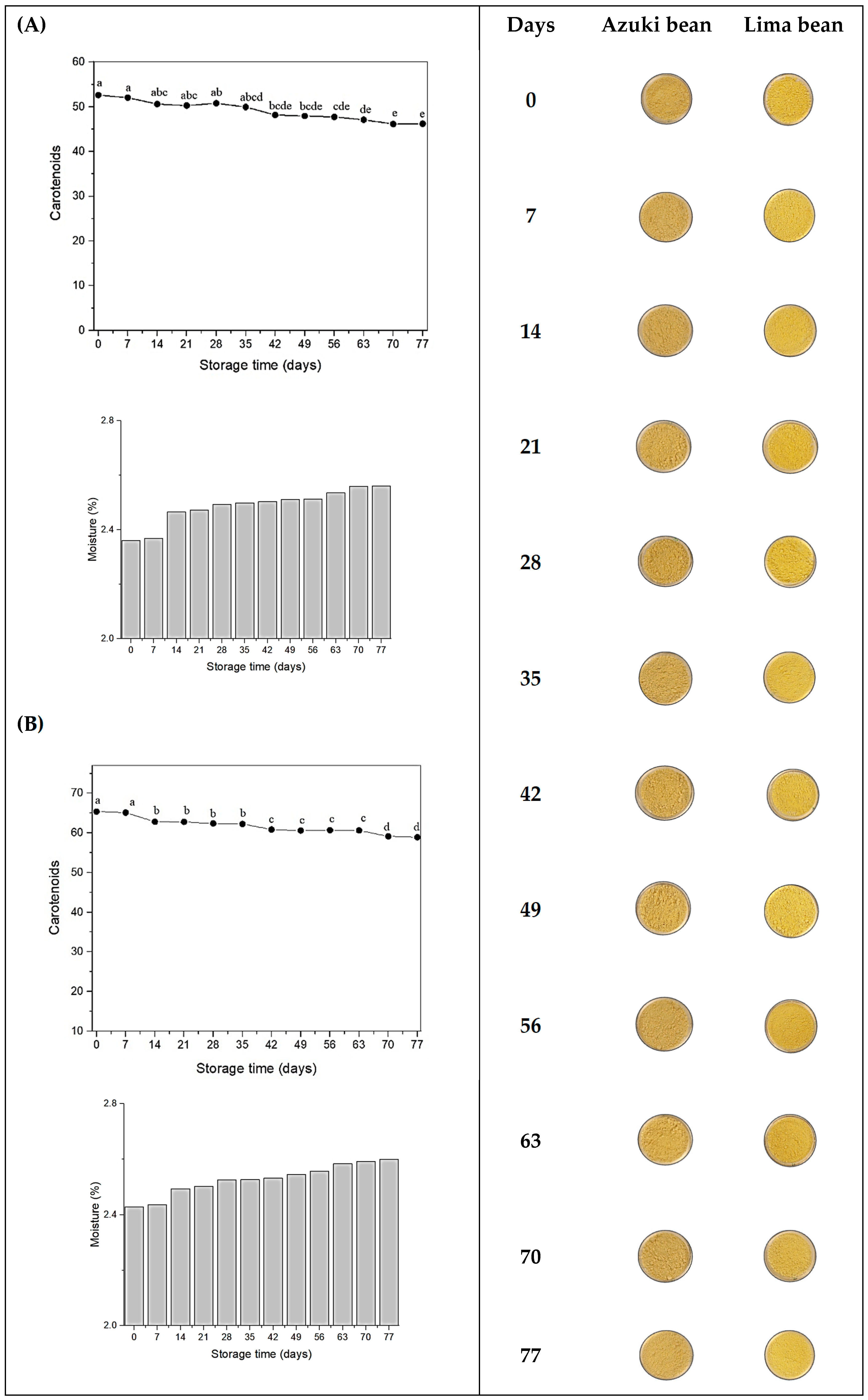
3.1.3. Stability of Microcapsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camelo-Silva, C.; Sanches, M.A.R.; Brito, R.M.; Devilla, I.A.; Tussolini, L.; Pertuzatti, P.B. Influence of buriti pulp (Mauritia flexuosa L.) concentration on thermophysical properties and antioxidant capacity. LWT 2021, 151, 112098. [Google Scholar] [CrossRef]
- Moser, P.; Nicoletti, V.R.; Drusch, S.; Brückner-Gührmann, M. Functional properties of chickpea protein-pectin interfacial complex in buriti oil emulsions and spray dried microcapsules. Food Hydrocoll. 2020, 107, 105929. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Almeida, O.P.; Campelo, P.H.; Carneiro, G.; Rocha, L.D.O.F.; Santos, J.H.; da Costa, J.M.G. Tailoring the physicochemical properties of freeze-dried buriti oil microparticles by combining inulin and gum Arabic as encapsulation agents. LWT 2022, 161, 113372. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Molina, C.V.; Netto, F.M.; Pinho, S.C. Encapsulation of Beta-carotene in lipid microparticles stabilized with hydrolyzed soy protein isolate: Production parameters, alpha-tocopherol coencapsulation and stability under stress conditions. J. Food Sci. 2017, 82, 659–669. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Dang, T.T.; Nguyen, T.V.L.; Nguyen, T.T.D.; Nguyen, N.N. Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of different carriers on selected physicochemical properties and antioxidant activities of spray-dried and freeze-dried powder. Int. J. Food Prop. 2022, 25, 359–374. [Google Scholar] [CrossRef]
- Ribeiro, M.L.F.F.; Roos, Y.H.; Ribeiro, A.P.B.; Nicoletti, V.R. Effects of maltodextrin content in double-layer emulsion for production and storage of spray-dried carotenoid-rich microcapsules. Food Bioprod. Process. 2020, 124, 208–221. [Google Scholar] [CrossRef]
- Millinia, B.L.; Mashithah, D.; Nawatila, R.; Kartini, K. Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of maltodextrin and trehalose matrix on selected physicochemical properties and antioxidant activities of spray-dried powder. Future Foods 2024, 9, 100300. [Google Scholar] [CrossRef]
- Ré, M.I. Microencapsulation via spray drying. Drying Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-drying microencapsulation of anthocyanins by natural biopolymers: A review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- de Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Vegetable Proteins in Microencapsulation: A review of recent interventions and their effectiveness. Ind. Crops Prod. 2013, 42, 469–479. [Google Scholar] [CrossRef]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.L.; Cattelan, M.G.; Nicoletti, V.R. Microencapsulation of pink pepper essential oil: Properties of spray-dried pectin/SPI double-layer versus SPI single-layer stabilized emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123806. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, W.; Jin, W.; Shah, B.R.; Li, Y.; Li, B. Influence of anionic alginate and cationic chitosan on physicochemical stability and carotenoids bioaccessibility of soy protein isolate-stabilized emulsions. Food Res. Int. 2015, 77, 419–425. [Google Scholar] [CrossRef]
- MCClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid. Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Telis, V.R.N. O/W emulsions stabilized by interactions between proteins and polysaccharides. Encycl. Food Chem. 2019, 494–498. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Kubo, M.T.K.; Fuzetti, C.G.; Nicoletti, V.R. Functional properties of physically pretreated kidney bean and mung bean flours and their performance in microencapsulation of a carotenoid-rich oil. Front. Sustain. Food Syst. 2022, 6, 845566. [Google Scholar] [CrossRef]
- Aydin, Ö.K.; Baysan, U.; Altay, Ö.; Baysan, I.İ.; Ertekin, F.K.; Jafari, S.M. Vitamin delivery systems by spray-drying encapsulation within plant protein-based carriers: A review. Food Biosci. 2023, 103341. [Google Scholar] [CrossRef]
- Choe, U.; Osorno, J.M.; Ohm, J.B.; Chen, B.; Rao, J. Modification of physicochemical, functional properties, and digestibility of macronutrients in common bean (Phaseolus vulgaris L.) flours by different thermally treated whole seeds. Food Chem. 2022, 382, 132570. [Google Scholar] [CrossRef]
- Yadav, U.; Singh, N.; Kaur, A.; Thakur, S. Physico-chemical, hydration, cooking, textural and pasting properties of different adzuki bean (Vigna angularis) accessions. J. Food Sci. Technol. 2018, 55, 802–810. [Google Scholar] [CrossRef]
- Garcia, T.; Duitama, J.; Zullo, S.S.; Gil, J.; Ariani, A.; Dohle, S.; Chacón-Sánchez, M.I. Comprehensive genomic resources related to domestication and crop improvement traits in Lima bean. Nat. Commun. 2021, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) seed coats attenuate vascular oxidative stress and inflammation in spontaneously hypertensive rats. J. Nutr. Biochem. 2011, 22, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kitano-Okada, T.; Ito, A.; Koide, A.; Nakamura, Y.; Han, K.H.; Shimada, K.; Sasaki, K.; Ohba, K.; Sibayama, S.; Fukushima, M. Anti-Obesity Role of Adzuki Bean Extract Containing Polyphenols: In Vivo and in Vitro Effects. J. Sci. Food Agric. 2012, 92, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Seidu, K.T.; Osundahunsi, O.F.; Olaleye, M.T.; Oluwalana, I.B. Amino acid composition, mineral contents and protein solubility of some lima bean (Phaseolus lunatus L. Walp) seeds coat. Food Res. Int. 2015, 73, 130–134. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 1990. [Google Scholar]
- de Barros Fernandes, R.V.; Marques, G.R.; Borges, S.V.; Botrel, D.A. Effect of solids content and oil load on the microencapsulation process of rosemary essential oil. Ind. Crops Prod. 2014, 58, 173–181. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Fuzetti, C.G.; de Castilhos, M.B.M.; Nicoletti, V.R. Microencapsulation of natural blue dye from butterfly pea (Clitoria ternatea L.) flowers: The application of different carriers. J. Food Process. Preserv. 2022, 46, e16420. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Nizori, A.; Bui, L.T.; Jie, F.; Small, D.M. Impact of varying hydrocolloid proportions on encapsulation of ascorbic acid by spray drying. Int. J. Food Sci. Technol. 2018, 53, 1363–1370. [Google Scholar] [CrossRef]
- Alves, A.I.; Rodrigues, M.Z.; Ribeiro Pinto, M.R.M.; Lago Vanzela, E.S.; Stringheta, P.C.; Perrone, Í.T.; Ramos, A.M. Morphological characterization of pequi extract microencapsulated through spray drying. Int. J. Food Prop. 2017, 20, 1298–1305. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Rocha, J.d.C.G.; de Barros, F.A.R.; Perrone, T.; Viana, K.W.C.; Tavares, G.M.; Stephani, R.; Stringheta, P.C. Microencapsulation by atomization of the mixture of phenolic extracts. Powder Technol. 2019, 343, 317–325. [Google Scholar] [CrossRef]
- Schuck, P.; Ouest, A. Milk powder: Physical and functional properties of milk powders. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Jafari, S.M.; Ghalenoei, M.G.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Xiong, T.; Ye, X.; Su, Y.T.; Chen, X.; Sun, H.; Li, B.; Chen, Y. Identification and Quantification of Proteins at Adsorption Layer of Emulsion Stabilized by Pea Protein Isolates. Colloids Surf. B Biointerfaces 2018, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Campechano-Carrera, E.; Corona-Cruz, A.; Chel-Guerrero, L.; Betancur-Ancona, D. Effect of pyrodextrinization on available starch content of Lima bean (Phaseolus lunatus) and Cowpea (Vigna unguiculata) starches. Food Hydrocoll. 2007, 21, 472–479. [Google Scholar] [CrossRef]
- Moser, P.; Ferreira, S.; Nicoletti, V.R. Buriti oil microencapsulation in chickpea protein-pectin matrix as affected by spray drying parameters. Food Bioprod. Process. 2019, 117, 183–193. [Google Scholar] [CrossRef]
- Vieira, T.R.R.; Lima, A.B.; Ribeiro, C.M.C.M.; de Medeiros, P.V.Q.; Converti, A.; Lima, M.d.S.; Maciel, M.I.S. Red pitaya (Hylocereus polyrhizus) as a source of betalains and phenolic compounds: Ultrasound extraction, microencapsulation, and evaluation of stability. LWT 2024, 115755. [Google Scholar] [CrossRef]
- Guadarrama-Lezama, A.Y.; Jaramillo-Flores, E.; Gutiérrez-López, G.F.; Pérez-Alonso, C.; Dorantes-Álvarez, L.; Alamilla-Beltrán, L. Effects of storage temperature and water activity on the degradation of carotenoids contained in microencapsulated chili extract. Dry. Technol. 2014, 32, 1435–1447. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of pineapple peel extract by spray drying using maltodextrin, inulin, and Arabic gum as wall matrices. Foods 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-López, J.A. Production of a red–purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]

| Flour | Water | Buriti Oil | Maltodextrin | |
|---|---|---|---|---|
| (g/100 g of Emulsion) | ||||
| (A) Azuki bean | 5 | 55 | 10 | 30 |
| 10 | 55 | 10 | 25 | |
| (B) Lima bean | 5 | 55 | 10 | 30 |
| 10 | 55 | 10 | 25 | |
| Properties | A1 | A2 | L1 | L2 |
|---|---|---|---|---|
| Moisture (%) | 2.61 ± 0.21 A | 2.44 ± 0.04 A | 2.70 ± 0.06 A | 2.38 ± 0.24 A |
| aW | 0.25 ± 0.09 AB | 0.23 ± 0.05 B | 0.26 ± 0.07 A | 0.24 ± 0.01 AB |
| Hygroscopicity (%) | 7.47 ± 0.13 B | 7.94 ± 0.17 A | 7.74 ± 0.01 AB | 7.78 ± 0.20 AB |
| Solubility (%) | 81.58 ± 0.26 B | 83.97 ± 0.14 A | 81.28 ± 0.18 B | 83.45 ± 1.09 A |
| Process Yield (%) | 60.97 ± 5.21 A | 57.9 ± 7.5 A | 58.28 ± 4.04 A | 56.50 ± 3.54 A |
| L* | 84.36 ± 0.32 B | 80.77 ± 0.58 C | 86.88 ± 0.44 A | 86.45 ± 0.72 A |
| a* | 3.58 ± 0.16 A | 3.56 ± 0.25 A | 3.46 ± 0.26 A | 3.10 ± 0.10 B |
| b* | 30.78 ± 0.72 A | 27.09 ± 1.14 B | 31.67 ± 1.36 A | 30.70 ± 1.03 A |
| C* | 30.99 ± 0.73 A | 27.32 ± 1.16 B | 31.86 ± 1.38 A | 30.85 ± 1.02 A |
| h° | 83.36 ± 0.16 C | 82.50 ± 0.23 D | 83.75 ± 0.23 B | 84.21 ± 0.30 A |
| EEO (%) | 61.91 ± 0.20 B | 60.73 ± 0.62 B | 62.21 ± 3.12 AB | 68.67 ± 0.62 A |
| RTO (%) | 68.91 ± 1.92 A | 64.04 ± 0.87 A | 64.82 ± 0.96 A | 73.57 ± 4.73 A |
| EEC (%) | 51.31 ± 3.87 AB | 43.52 ± 3.33 C | 44.09 ± 1.64 BC | 51.94 ± 1.55 A |
| RTC (%) | 76.76 ± 6.97 A | 64.13 ± 4.80 B | 65.64 ± 2.19 AB | 77.49 ± 3.22 A |
| (A) | ||||||
| Days | L* | a* | b* | C | h° | ΔE |
| 0 | 83.54 ± 0.35 e | 3.30 ± 0.15 a | 25.97 ± 0.54 a | 26.18 ± 0.55 a | 82.74 ± 0.18 e | |
| 7 | 83.53 ± 0.01 e | 3.34 ± 0.015 a | 26.18 ± 0.02 a | 26.39 ± 0.02 a | 82.72 ± 0.03 e | 0.20 ± 0.02 f |
| 14 | 84.20 ± 0.09 cd | 2.89 ± 0.032 bc | 24.78 ± 0.13 b | 24.96 ± 0.13 b | 83.33 ± 0.05 d | 1.42 ± 0.16 e |
| 21 | 84.36 ± 0.04 abc | 2.76 ± 0.02 cd | 24.26 ± 0.03 c | 24.42 ± 0.03 c | 83.50 ± 0.04 cd | 1.97 ± 0.05 d |
| 28 | 84.23 ± 0.1 bcd | 2.89 ± 0.41 bc | 24.92 ± 0.08 b | 25.09 ± 0.09 b | 83.37 ± 0.07 cd | 1.32 ± 0.14 e |
| 35 | 83.98 ± 0.01 d | 2.92 ± 0.011 b | 24.95 ± 0.01 b | 25.12 ± 0.01 b | 83.31 ± 0.02 d | 1.17 ± 0.01 e |
| 42 | 84.68 ± 0.05 a | 2.72 ± 0.02 de | 24.15 ± 0.13 cd | 24.30 ± 0.13 cd | 83.55 ± 0.03 c | 2.23 ± 0.14 cd |
| 49 | 84.51 ± 0.02 abc | 2.55 ± 0.02 fg | 23.91 ± 0.07 cd | 24.05 ± 0.07 cd | 83.91 ± 0.03 ab | 2.40 ± 0.07 bc |
| 56 | 84.54 ± 0.01 ab | 2.60 ± 0.011 ef | 24.16 ± 0.02 cd | 24.30 ± 0.02 cd | 83.83 ± 0.02 ab | 2.18 ± 0.02 cd |
| 63 | 84.52 ± 0.03 abc | 2.70 ± 0.03 de | 23.67 ± 0.10 de | 23.83 ± 0.09 de | 83.47 ± 0.04 cd | 2.57 ± 0.10 b |
| 70 | 84.43 ± 0.02 abc | 2.51 ± 0.03 fg | 23.25 ± 0.08 e | 23.38 ± 0.08 e | 83.81 ± 0.05 b | 2.97 ± 0.08 a |
| 77 | 84.70 ± 0.05 a | 2.44 ± 0.03 g | 23.33 ± 0.09 e | 23.45 ± 0.10 e | 84.03 ± 0.06 a | 3.01 ± 0.11 a |
| (B) | ||||||
| Days | L* | a* | b* | C | h° | ΔE |
| 0 | 88.19 ± 0.03 e | 2.39 ± 0.03 b | 27.79 ± 0.02 b | 27.90 ± 0.03 b | 85.08 ± 0.07 f | |
| 7 | 87.61 ± 0.05 f | 2.56 ± 0.08 a | 28.25 ± 0.28 a | 28.37 ± 0.29 a | 84.81 ± 0.10 g | 0.77 ± 0.23 e |
| 14 | 88.63 ± 0.06 a | 2.17 ± 0.02 c | 26.63 ± 0.07 c | 26.72 ± 0.07 c | 85.32 ± 0.04 e | 1.26 ± 0.08 d |
| 21 | 88.38 ± 0.03 d | 2.04 ± 0.02 d | 26.51 ± 0.08 c | 26.59 ± 0.08 c | 85.59 ± 0.04 d | 1.34 ± 0.09 d |
| 28 | 88.36 ± 0.03 d | 2.03 ± 0.05 d | 26.33 ± 0.02 c | 26.41 ± 0.02 c | 85.57 ± 0.01 d | 1.51 ± 0.02 d |
| 35 | 88.33 ± 0.02 d | 1.82 ± 0.02 e | 25.72 ± 0.12 d | 25.78 ± 0.12 d | 85.94 ± 0.05 c | 2.15 ± 0.11 c |
| 42 | 88.40 ± 0.02 cd | 1.97 ± 0.07 de | 25.61 ± 0.13 de | 25.69 ± 0.14 de | 85.58 ± 0.14 d | 2.22 ± 0.14 c |
| 49 | 88.34 ± 0.04 d | 1.67 ± 0.02 fg | 25.34 ± 0.04 ef | 25.40 ± 0.04 ef | 86.22 ± 0.05 ab | 2.55 ± 0.04 b |
| 56 | 88.36 ± 0.01 d | 1.76 ± 0.01 ef | 25.30 ± 0.06 ef | 25.37 ± 0.06 ef | 86.01 ± 0.02 bc | 2.57 ± 0.05 b |
| 63 | 88.49 ± 0.02 bc | 1.63 ± 0.02 g | 25.24 ± 0.04 f | 25.30 ± 0.04 f | 86.30 ± 0.05 a | 2.67 ± 0.03 b |
| 70 | 88.40 ± 0.01 cd | 1.58 ± 0.04 g | 24.71 ± 0.08 g | 24.76 ± 0.08 g | 86.33 ± 0.08 a | 3.19 ± 0.08 a |
| 77 | 88.51 ± 0.01 b | 1.57 ± 0.04 g | 24.55 ± 0.07 g | 24.60 ± 0.07 g | 86.33 ± 0.09 a | 3.36 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuzetti, C.G.; Nicoletti, V.R. Stability of Buriti Oil Microencapsulated in Mixtures of Azuki and Lima Bean Flours with Maltodextrin. Foods 2024, 13, 1968. https://doi.org/10.3390/foods13131968
Fuzetti CG, Nicoletti VR. Stability of Buriti Oil Microencapsulated in Mixtures of Azuki and Lima Bean Flours with Maltodextrin. Foods. 2024; 13(13):1968. https://doi.org/10.3390/foods13131968
Chicago/Turabian StyleFuzetti, Caroline Gregoli, and Vânia Regina Nicoletti. 2024. "Stability of Buriti Oil Microencapsulated in Mixtures of Azuki and Lima Bean Flours with Maltodextrin" Foods 13, no. 13: 1968. https://doi.org/10.3390/foods13131968
APA StyleFuzetti, C. G., & Nicoletti, V. R. (2024). Stability of Buriti Oil Microencapsulated in Mixtures of Azuki and Lima Bean Flours with Maltodextrin. Foods, 13(13), 1968. https://doi.org/10.3390/foods13131968






