Anti-Inflammatory Effects of Barley Sprout Fermented by Lactic Acid Bacteria in RAW264.7 Macrophages and Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Barley and Culture of LABs
2.2. Sprouted Barley Sample Preparation and Fermentation
2.3. Sample Preparation for Cell Treatment and Antioxidative Activity, and HPLC Analyses
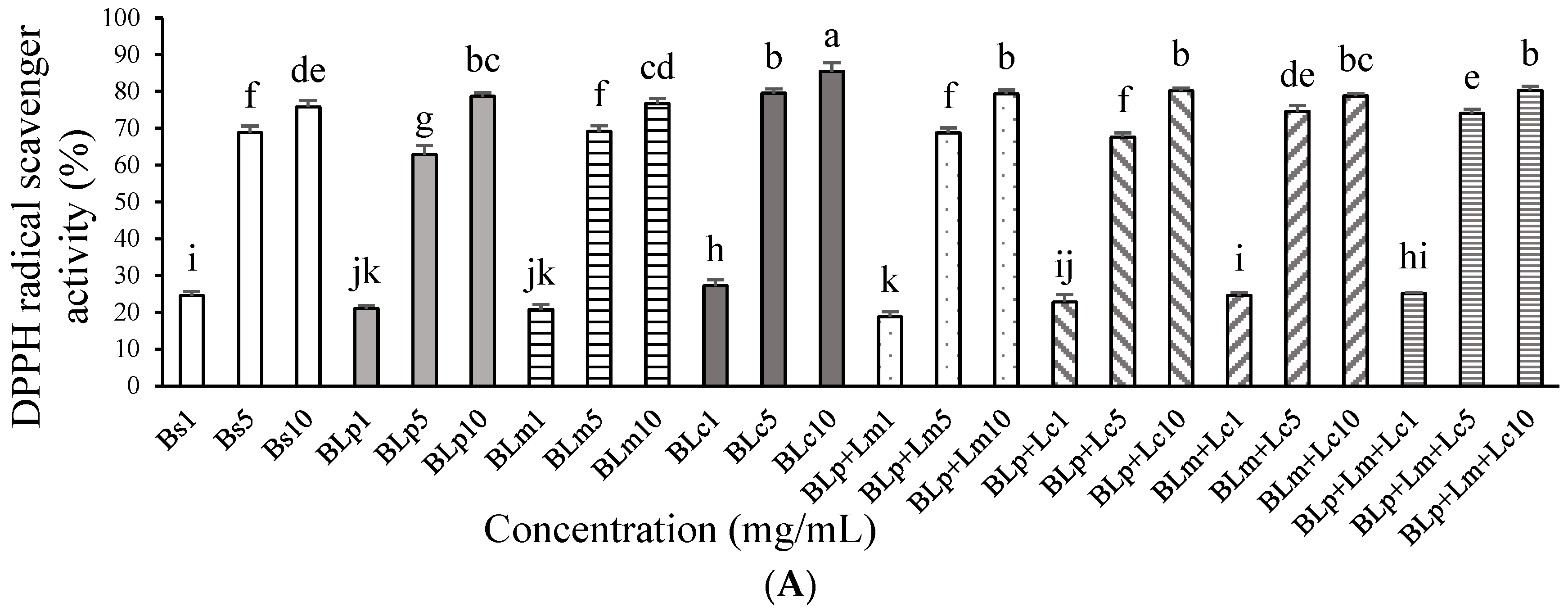
2.4. Determination of DPPH Radical Scavenging Activity
2.5. Cell Line Culture
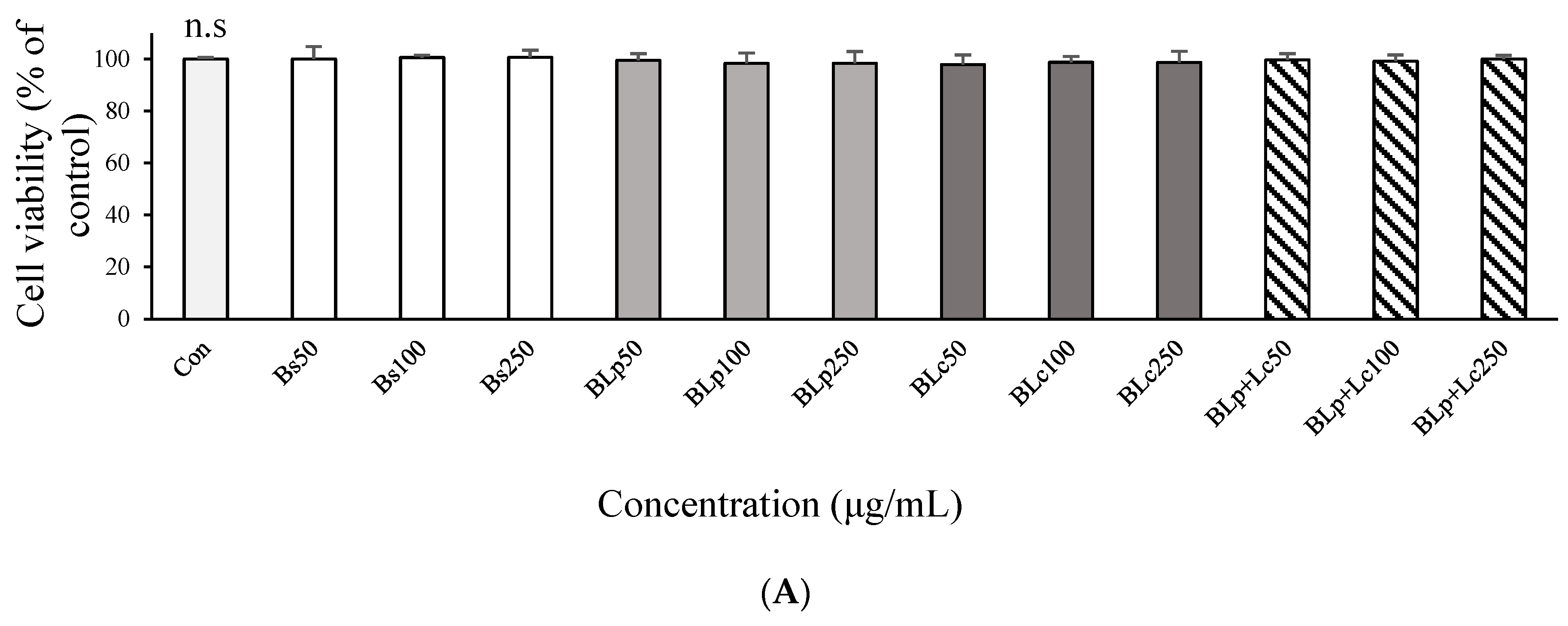
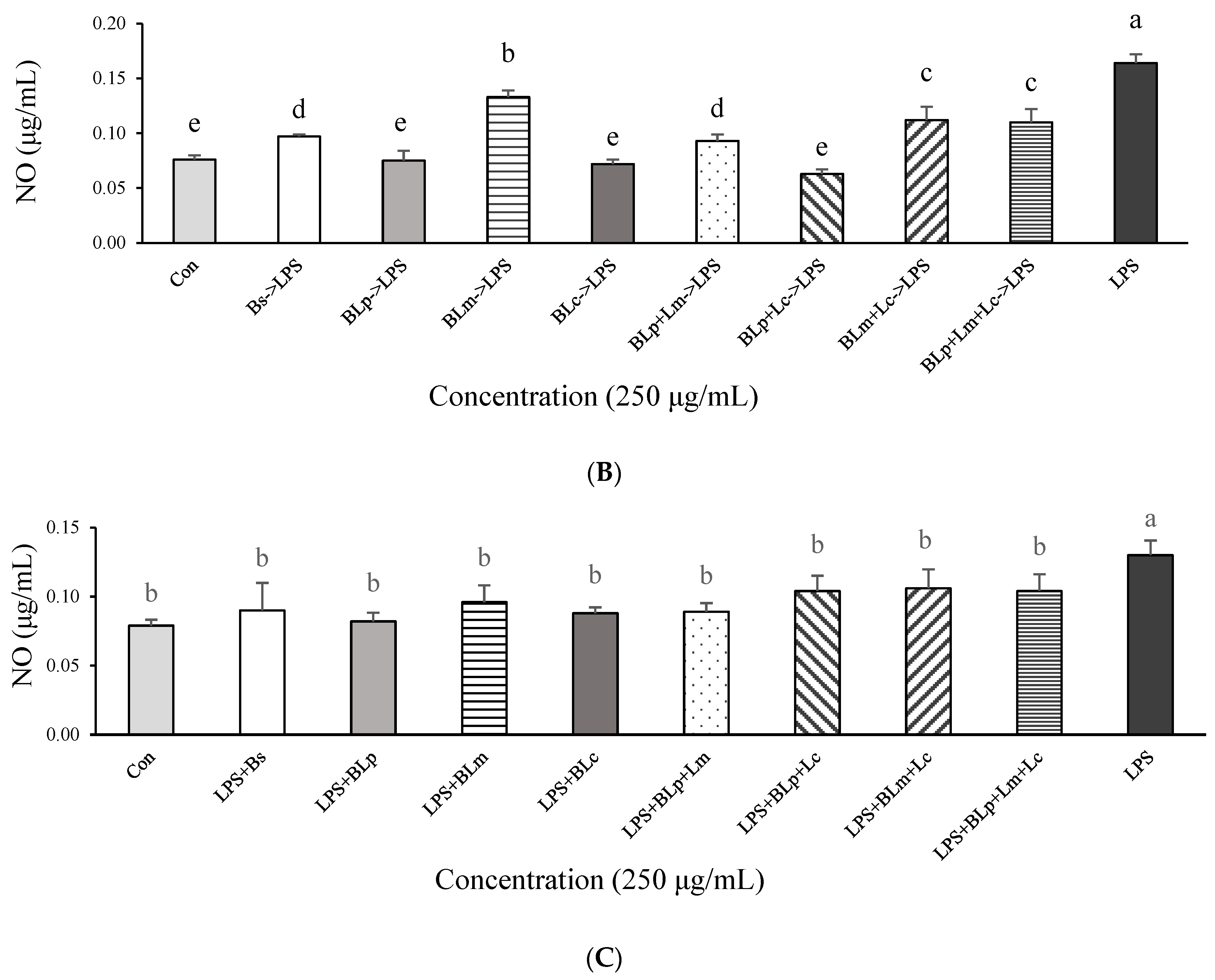
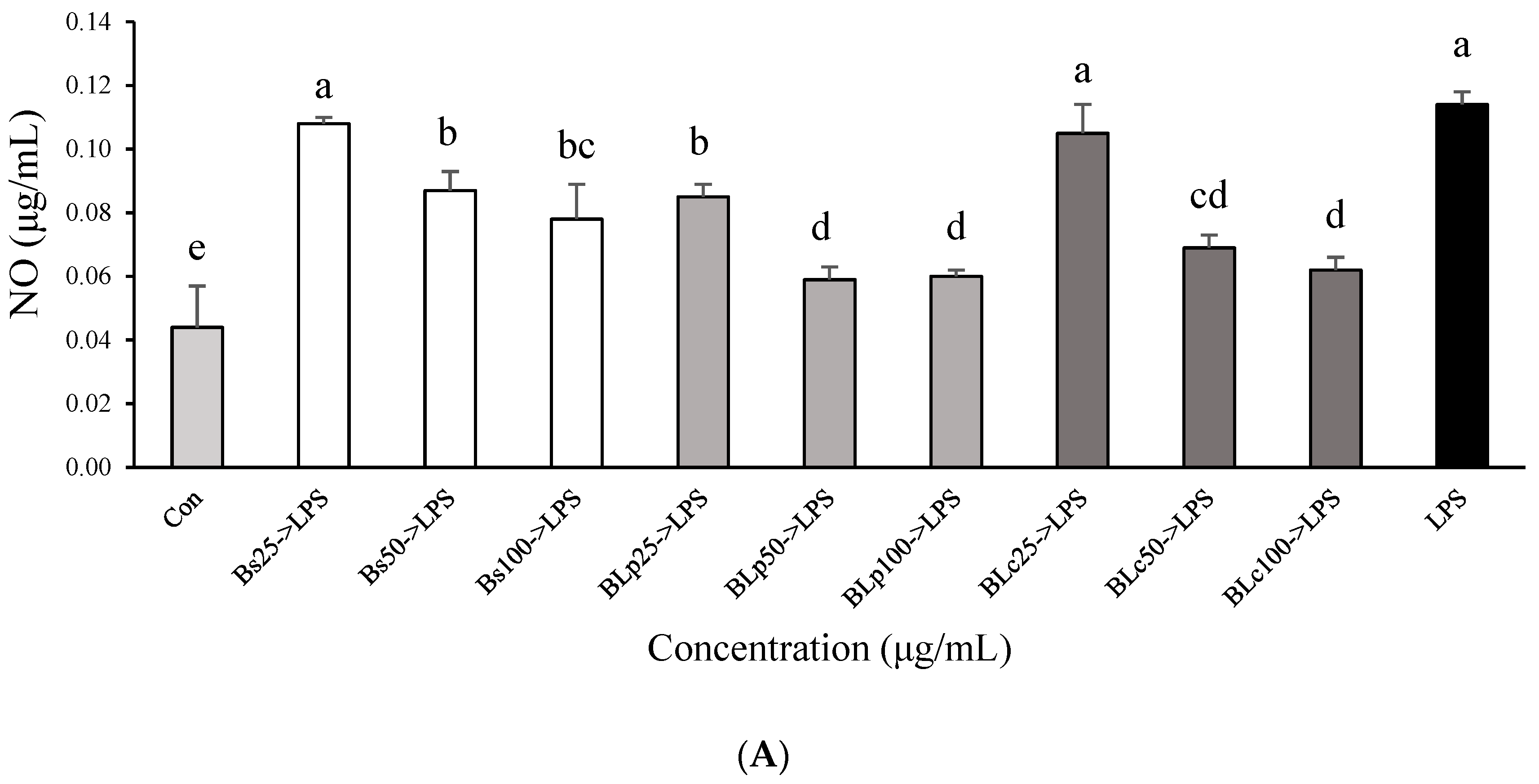
2.6. Measurement of Cell Viability and Determination of Nitric Oxide (NO) and Cytokines
2.7. HPLC Analysis of Lutonarin and Saponarin
2.8. Statistical Analysis
3. Results and Discussion
3.1. DPPH Radical Scavenger Activity and Anti-Inflammatory Effects of Fermented Barley Sprout with LAB in LPS-Stimulated RAW264.7 Cells
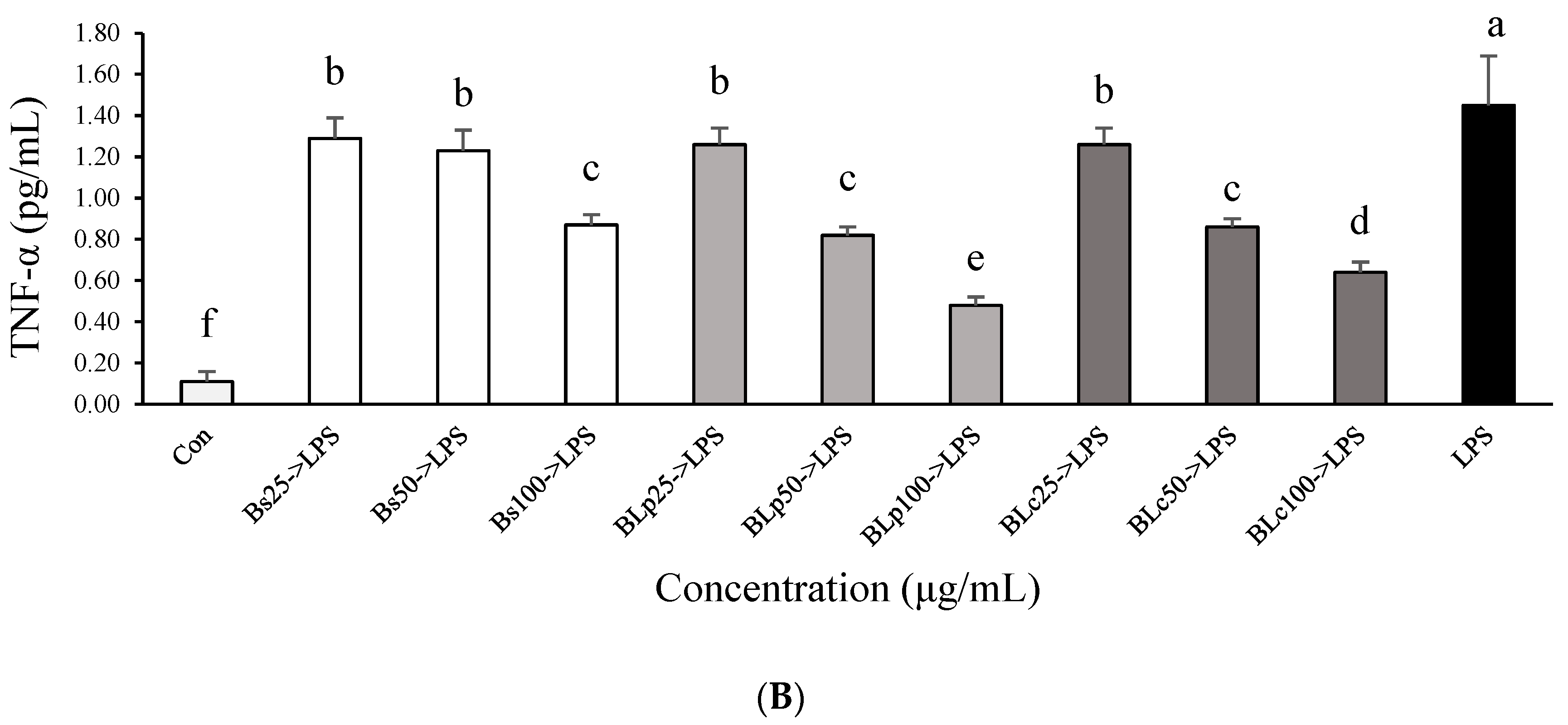
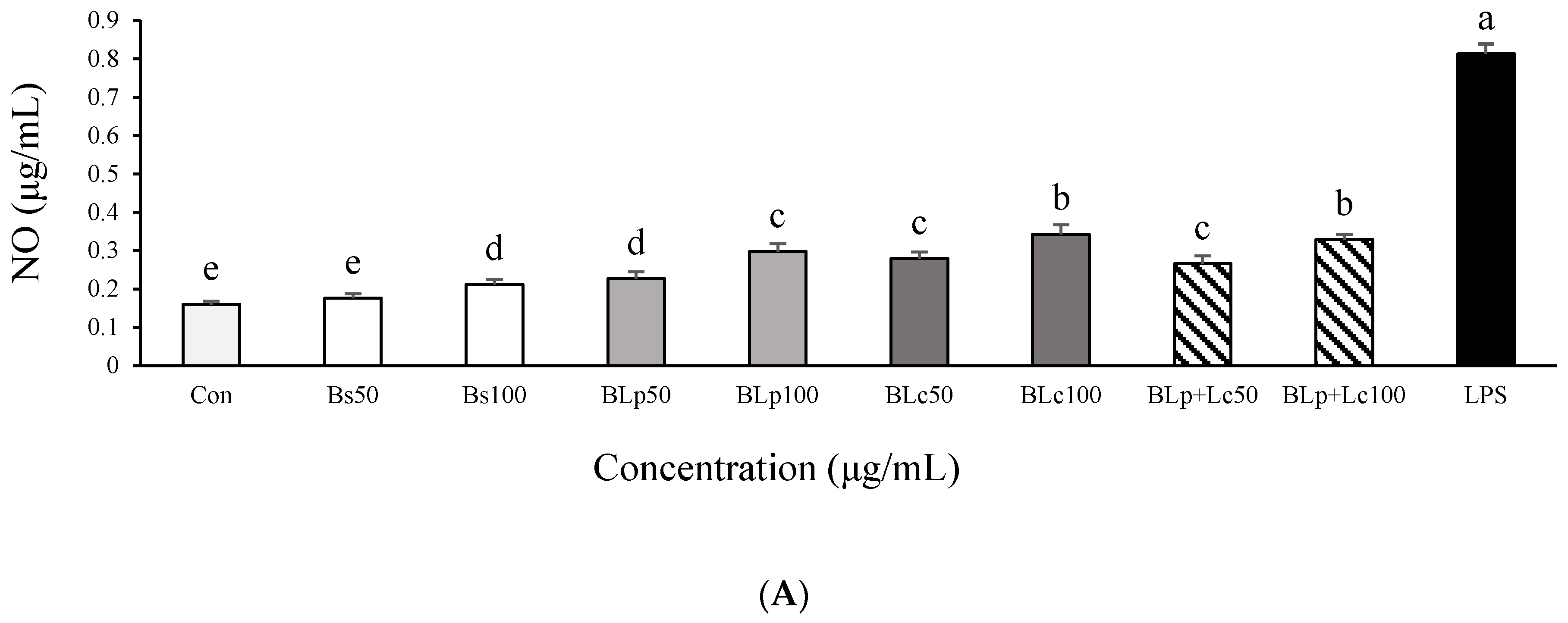
3.2. Effects of Fermented Barley Sprout with LAB on NO and TNF-α Production in LPS-Stimulated Caco-2 Cells
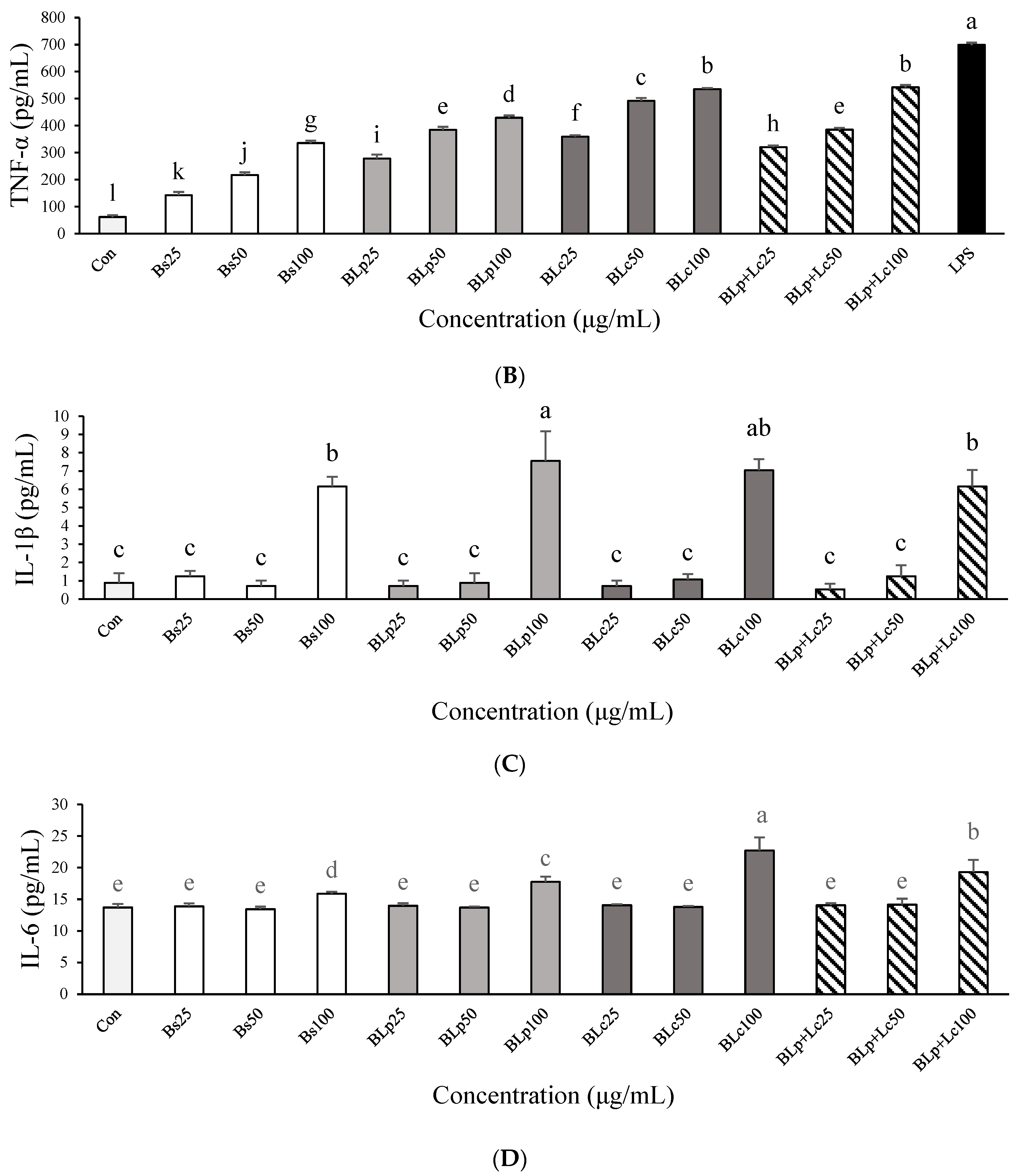
3.3. Immunostimulatory Effects of Fermented Barley Sprout with LAB in RAW264.7 Cells
3.4. Anti-Inflammatory Compound Analysis Using HPLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chen, W.D.; Wang, Y.D. The Relationship between gut microbiota and inflammatory diseases: The role of macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.R.P.; Balachander, N.; Kesav, R.S.K.; Silpa, S.; Rupachandra, S. Anti-inflammatory and wound healing properties of lactic acid bacteria and its peptides. Folia Microbiol. 2023, 68, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.H.; Kim, S.H.; Oh, J.Y.; Seo, W.D.; Kim, K.M.; Jung, J.C.; Jung, Y.S. Barley sprouts extract attenuates alcoholic fatty liver injury in mice by reducing inflammatory response. Nutrients 2016, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kawk, H.W.; Kim, S.H.; Lee, H.J.; Seo, J.W.; Kim, J.T.; Jang, S.H.; Kim, M.J.; Kim, Y.M. Anti-obesity effect of hot water extract of barley sprout through the inhibition of adipocyte differentiation and growth. Metabolites 2021, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Yu, D.S.; Lee, Y.T. The effect of heat processing on chemical composition and antioxidative activity of tea made from barley sprouts and wheat sprouts. J. Food Sci. 2019, 84, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260. [Google Scholar] [CrossRef] [PubMed]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 1486–7432. [Google Scholar] [CrossRef]
- Mahajan, R.; Prasad, S.; Gaikwad, S.; Itankar, P. Antioxidant phenolic compounds from seeds of Hordeum vulgare Linn. ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. J. Tradit. Chin. Med. Sci. 2023, 10, 353–361. [Google Scholar] [CrossRef]
- Aborus, N.E.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T.; Vulić, J.; Ilić, N. Powdered barley sprouts: Composition, functionality and polyphenol digestibility. Int. J. Food Sci. Technol. 2017, 52, 231–238. [Google Scholar] [CrossRef]
- Lam, K.L.; Cheung, P.C.K. Non-digestible long chain beta-glucans as novel prebiotics. Bioact. Carbohydr. Diet. Fibre 2013, 2, 45–64. [Google Scholar] [CrossRef]
- Jeon, B.; Kim, H.R.; Kim, H.; Chung, D.K. In vitro and in vivo downregulation of C3 by lipoteichoic acid isolated from Lactobacillus plantarum K8 suppressed cytokine-mediated complement system activation. FEMS Microbiol. Lett. 2016, 363, 14. [Google Scholar] [CrossRef]
- Xiao, M.; Xia, Y.; Chen, Y.; Wang, S.; Zhao, J.; Narbad, A.; Chen, W.; Zhai, Q.; Yu, L.; Tian, F. Therapeutic potential of Latilactobacillus curvatus CCFM1268 in colitis treatment: Insights from in vitro and in vivo studies. Food Biosci. 2024, 59, 103913. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Kim, E.H.; Reaney, M.J.T.; Shim, Y.Y.; Chung, M.J. Immunomodulatory activity of extracellular vesicles of kimchi-derived lactic acid bacteria (Leuconostoc mesenteroides, Latilactobacillus curvatus, and Lactiplantibacillus plantarum). Foods 2022, 11, 313. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, D.H.; Ryu, Y.G.; Lee, S.J.; Bae, Y.R.; Chung, M.J. Immunostimulatory effect of lactic acid bacteria fermentation using mixtures of barley sprout, plant mixed extract, and baechu. J. Korean Soc. Food Sci. Nutr. 2023, 52, 1091–1100. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, H.D.; Lee, S.H.; Lee, A.Y. Taraxacum coreanum Nakai extract attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier dysfunction in Caco-2 cells. J. Ethnopharmacol. 2024, 319, 117105. [Google Scholar] [CrossRef]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic-friend or foe? Curr. Opin. Food Sci. 2020, 32, 45–49. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Hess, J.M.; Gould, T.J.; Slavin, J.L. Prebiotic dietary fiber and gut health: Comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide. Nutrients 2017, 9, 1361. [Google Scholar] [CrossRef]
- Kang, S.J.; Koo, N.G.; Park, M.J.; Nam, J.Y.; Lee, Y.T. Physicochemical and antioxidative properties of pigmented barley cultivars processed by germination and roasting. J. Korean Soc. Food Sci. Nutr. 2023, 52, 186–192. [Google Scholar] [CrossRef]
- Liu, Y.; Alexeeva, S.; Defourny, K.; Smid, E.; Abee, T. Tiny but mighty: Bacterial membrane vesicles in food biotechnological applications. Curr. Opin. Biotechnol. 2018, 49, 179–184. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, K.H.; Kim, S.H.; Lee, S.; Lee, S.H.; Ha, E.S.; Sung, N.J.; Kim, J.G.; Chung, M.J. Lactic acid bacteria directly degrade N-nitrosodimethylamine and increase the nitrite-scavenging ability in kimchi. Food Control 2017, 71, 101–109. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Kang, K.H.; Lee, S.; Kim, S.J.; Kim, J.G.; Chung, M.J. Kimchi probiotic bacteria contribute to reduced amount of N-nitrosodimathylamine in lactic acid bacteria-fortified kimchi. LWT-Food Sci. Technol. 2017, 84, 196–203. [Google Scholar] [CrossRef]
- Chung, M.J.; Sohng, J.K.; Choi, D.J.; Park, Y.I. Inhibitory effect of phloretin and biochanin A on IgE-mediated allergic responses in rat basophilic leukemia RBL-2H3 cells. Life Sci. 2013, 93, 401–408. [Google Scholar] [CrossRef]
- Kim, E.H.; Shim, Y.Y.; Lee, H.I.; Lee, S.; Reaney, M.J.T.; Chung, M.J. Astragalin and isolated from Aster scaber suppress LPS-induced neuroinflammatory responses in microglia and mice. Foods 2022, 11, 1505. [Google Scholar] [CrossRef]
- Jeong, I.S.; Kim, E.H.; Park, S.M.; Chung, M.J. Immunostimulatory effect of sweet potato peel-based plant mixture on RAW264.7 macrophages. Korean J Food Presev. 2019, 26, 828–836. [Google Scholar] [CrossRef]
- Uy, N.P.; Lee, H.D.; Byun, D.C.; Lee, S. Comparison of the contents of total polyphenol, total flavonoid, and flavonoids derivatives in unfermented and fermented barley sprouts. J. Appl. Biol. Chem. 2023, 66, 353–358. [Google Scholar] [CrossRef]
- Eun, C.S.; Hwang, E.Y.; Lee, S.O.; Yang, S.A.; Yu, M.H. Antioxidant and anti-inflammatory activities of barley sprout extract. J. Life Sci. 2016, 26, 537–544. [Google Scholar] [CrossRef]
- Chae, K.S.; Ryu, E.H.; Kim, K.D.; Kim, Y.S.; Kwon, J.W. Antioxidant activities of ethanol extracts from barley sprouts. Korean J. Food Sci. Technol. 2019, 51, 486–491. [Google Scholar]
- Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; Sinderen, D.V. Fermented functional foods based on probiotics and their biogenic metabolites. Curr. Opin. Biotechnol. 2005, 16, 198–203. [Google Scholar] [CrossRef]
- Tu, J.; Xu, Y.; Xu, J.; Ling, Y.; Cai, Y. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-κB pathway in Caco-2 cells. Macromolecules 2016, 86, 848–856. [Google Scholar] [CrossRef]
- Seo, J.W.; Lee, H.J.; Lee, C.Y.; Kim, S.H.; Kim, J.T.; Kim, M.J.; Jang, S.H.; Kim, Y.M. Immunity-enhancing effect of hot water extract of barley sprout. Korean Soc. Biotechnol. Bioeng. J. 2022, 37, 25–31. [Google Scholar] [CrossRef]
- Kim, G.S.; Yang, K.H.; Kim, H.K.; Kim, J.E.; Yun, H.N.; Yu, J.W.; Kim, B.S. Antioxidant and immunomodulatory effect of lactic acid bacteria fermented barley sprout hot water extract. J. Korean Soc. Food Sci. Nutr. 2022, 51, 1027–1035. [Google Scholar] [CrossRef]
- Kim, H.W.; Jee, H.S.; Shin, K.S. Polysaccharide isolated from fermented barley extract activates macrophages via the MAPK and NF-κB pathways. Korean J. Food Sci. Technol. 2018, 50, 555–563. [Google Scholar]
- Zhang, J.; Wang, P.; Tan, C. Effects of L. plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley β-glucan in vitro. Curr. Res. Food Sci. 2022, 5, 125–130. [Google Scholar] [CrossRef]
- Yang, J.Y.; Woo, S.Y.; Lee, M.J.; Kim, H.Y.; Lee, J.H.; Kim, S.H.; Seo, W.D. Lutonarin from barley seedlings inhibits the lipopolysaccharide-stimulated inflammatory response of RAW264.7 macrophages by suppressing nuclear factor-kB signaling. Molecules 2021, 26, 1571. [Google Scholar] [CrossRef]
- Seo, K.H.; Park, M.J.; Ra, J.E.; Han, S.I.; Nam, M.H.; Kim, J.H.; Lee, J.H.; Seo, W.D. Saponarin from barley sprouts inhibits NF-κB and MAPK on LPS-induced RAW264.7 cells. Food Funct. 2014, 5, 3005–3013. [Google Scholar] [CrossRef]



| Group 1 | Ingredient | ||||
|---|---|---|---|---|---|
| Barley Sprout Powder (g) | Water (mL) | Lp | Lm | Lc | |
| F-Bs | 7 | 35 | |||
| F-Lp | 7 | 35 | 104 CFU/g | ||
| F-Lm | 7 | 35 | 104 CFU/g | ||
| F-Lc | 7 | 35 | 104 CFU/g | ||
| F-Lp+Lm | 7 | 35 | 104 CFU/g | 104 CFU/g | |
| F-Lp+Lc | 7 | 35 | 104 CFU/g | 104 CFU/g | |
| F-Lm+Lc | 7 | 35 | 104 CFU/g | 104 CFU/g | |
| F-Lp+Lm+Lc | 7 | 35 | 104 CFU/g | 104 CFU/g | 104 CFU/g |
| Samples 1 | Concentration (mg/g) | |
|---|---|---|
| Lutonarin | Saponarin | |
| Bs | 4.92 ± 0.05 c | 2.17 ± 0.01 c |
| BLp | 5.49 ± 0.05 a | 2.44 ± 0.04 b |
| BLc | 5.23 ± 0.06 b | 2.57 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Shim, Y.Y.; Kim, Y.J.; Reaney, M.J.T.; Chung, M.J. Anti-Inflammatory Effects of Barley Sprout Fermented by Lactic Acid Bacteria in RAW264.7 Macrophages and Caco-2 Cells. Foods 2024, 13, 1781. https://doi.org/10.3390/foods13111781
Kim S-H, Shim YY, Kim YJ, Reaney MJT, Chung MJ. Anti-Inflammatory Effects of Barley Sprout Fermented by Lactic Acid Bacteria in RAW264.7 Macrophages and Caco-2 Cells. Foods. 2024; 13(11):1781. https://doi.org/10.3390/foods13111781
Chicago/Turabian StyleKim, Sang-Hyun, Youn Young Shim, Young Jun Kim, Martin J. T. Reaney, and Mi Ja Chung. 2024. "Anti-Inflammatory Effects of Barley Sprout Fermented by Lactic Acid Bacteria in RAW264.7 Macrophages and Caco-2 Cells" Foods 13, no. 11: 1781. https://doi.org/10.3390/foods13111781
APA StyleKim, S.-H., Shim, Y. Y., Kim, Y. J., Reaney, M. J. T., & Chung, M. J. (2024). Anti-Inflammatory Effects of Barley Sprout Fermented by Lactic Acid Bacteria in RAW264.7 Macrophages and Caco-2 Cells. Foods, 13(11), 1781. https://doi.org/10.3390/foods13111781





