Effect of Inoculation with Autochthonous Lactic Acid Bacteria on Flavor, Texture, and Color Formation of Dry Sausages with NaCl Partly Substituted by KCl
Abstract
1. Introduction
2. Materials and Methods
2.1. Starter Culture Preparation
2.2. Dry Sausage Manufacture
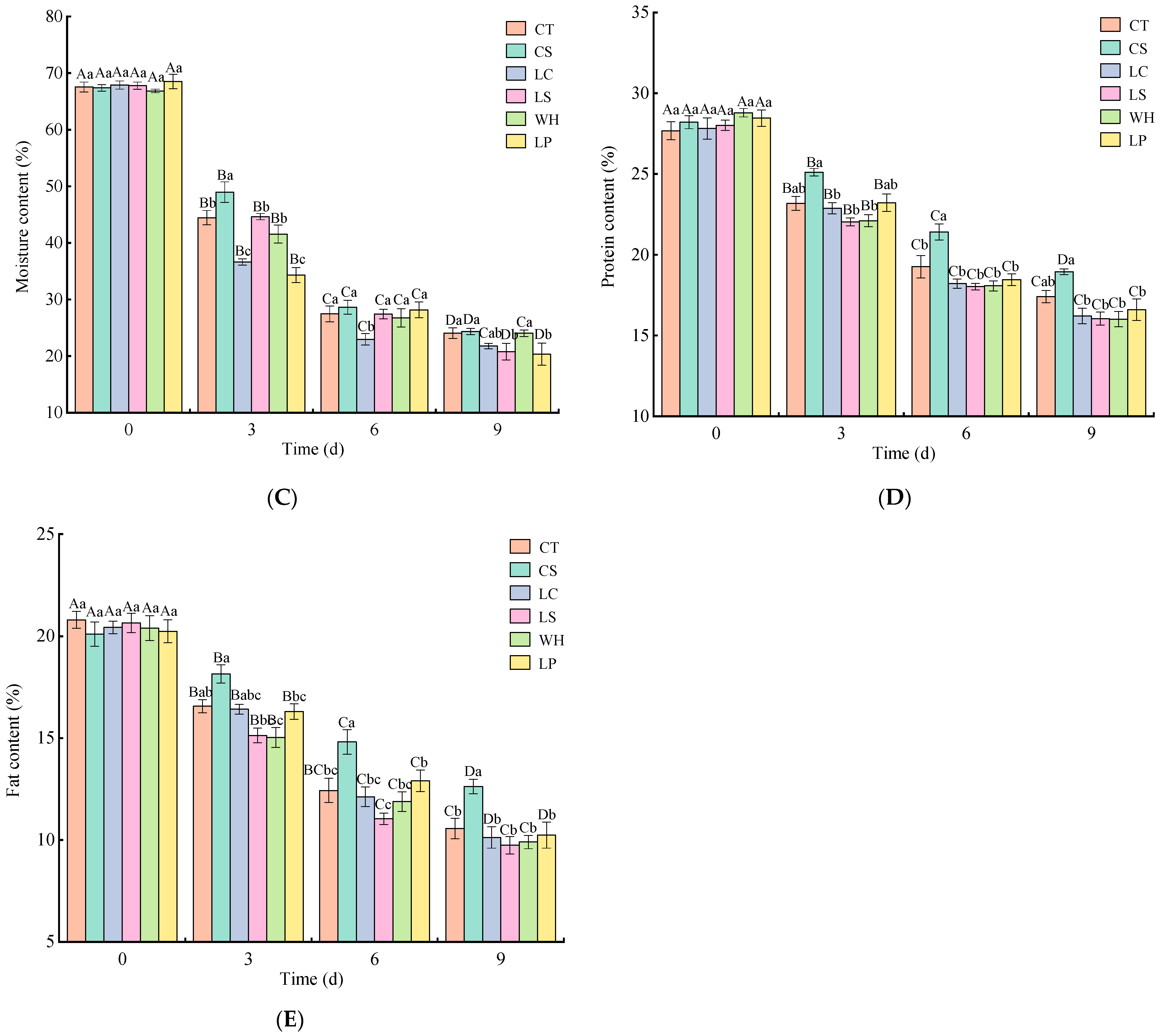
2.3. Determination of Chemical Composition, pH, and Lactic Acid Bacteria Count
2.4. Determination of Color and Shear Force
2.5. Electronic Nose Analysis
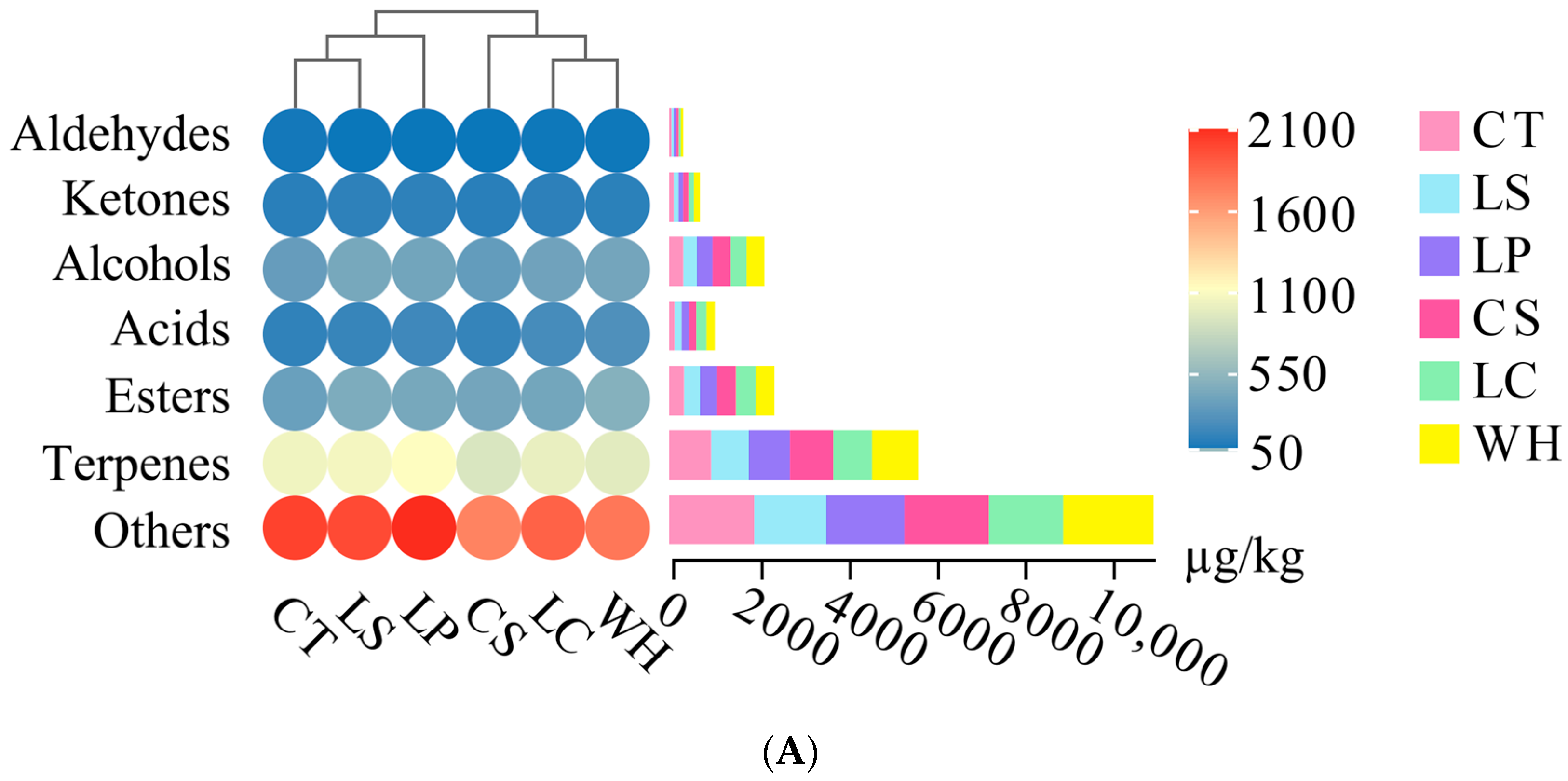
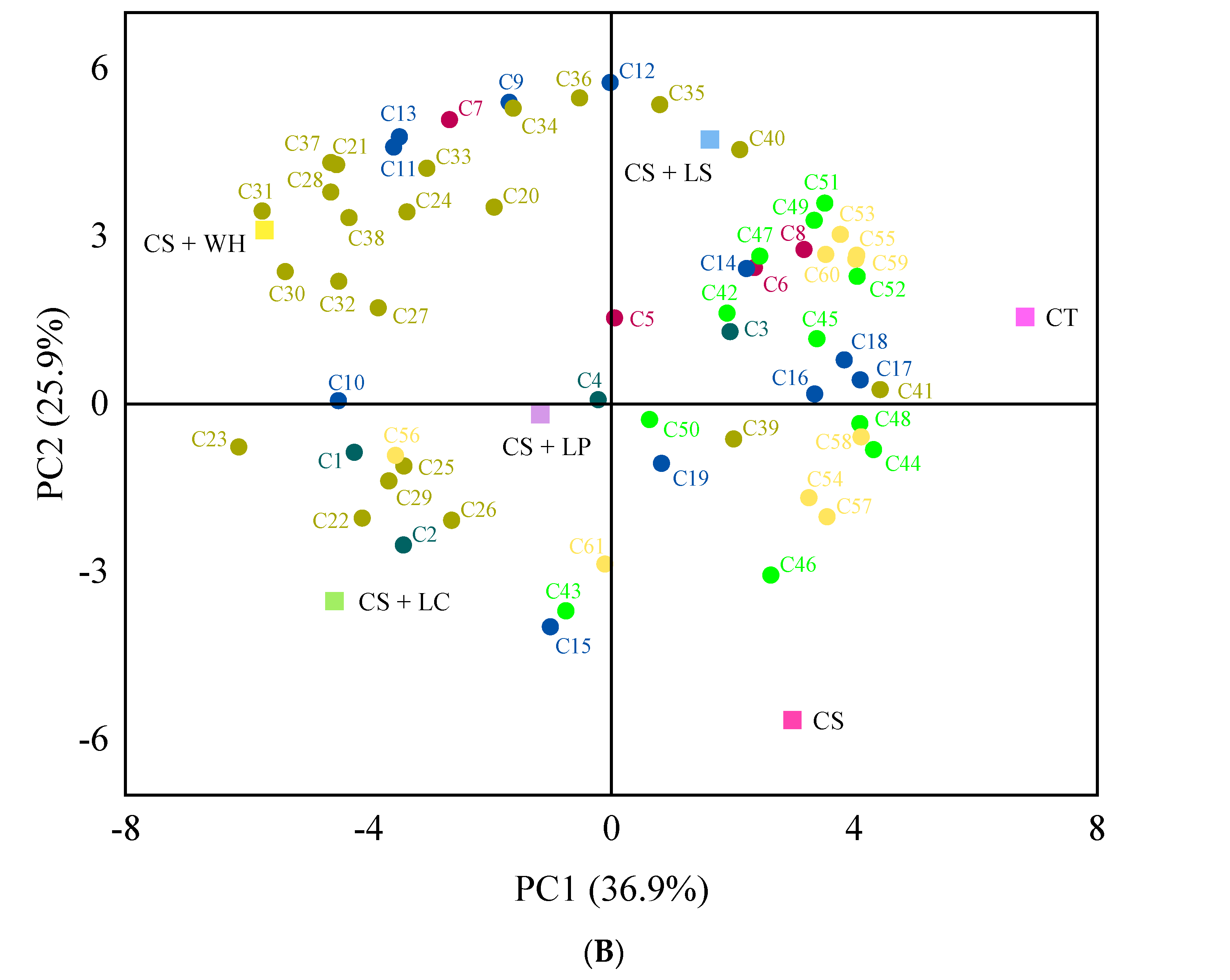
2.6. Volatile Compound Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Chemical Composition, pH, and Lactic Acid Bacteria Count
3.2. Color and Shear Force Analysis
3.3. Electronic Nose Analysis
3.4. Volatile Compound Analysis
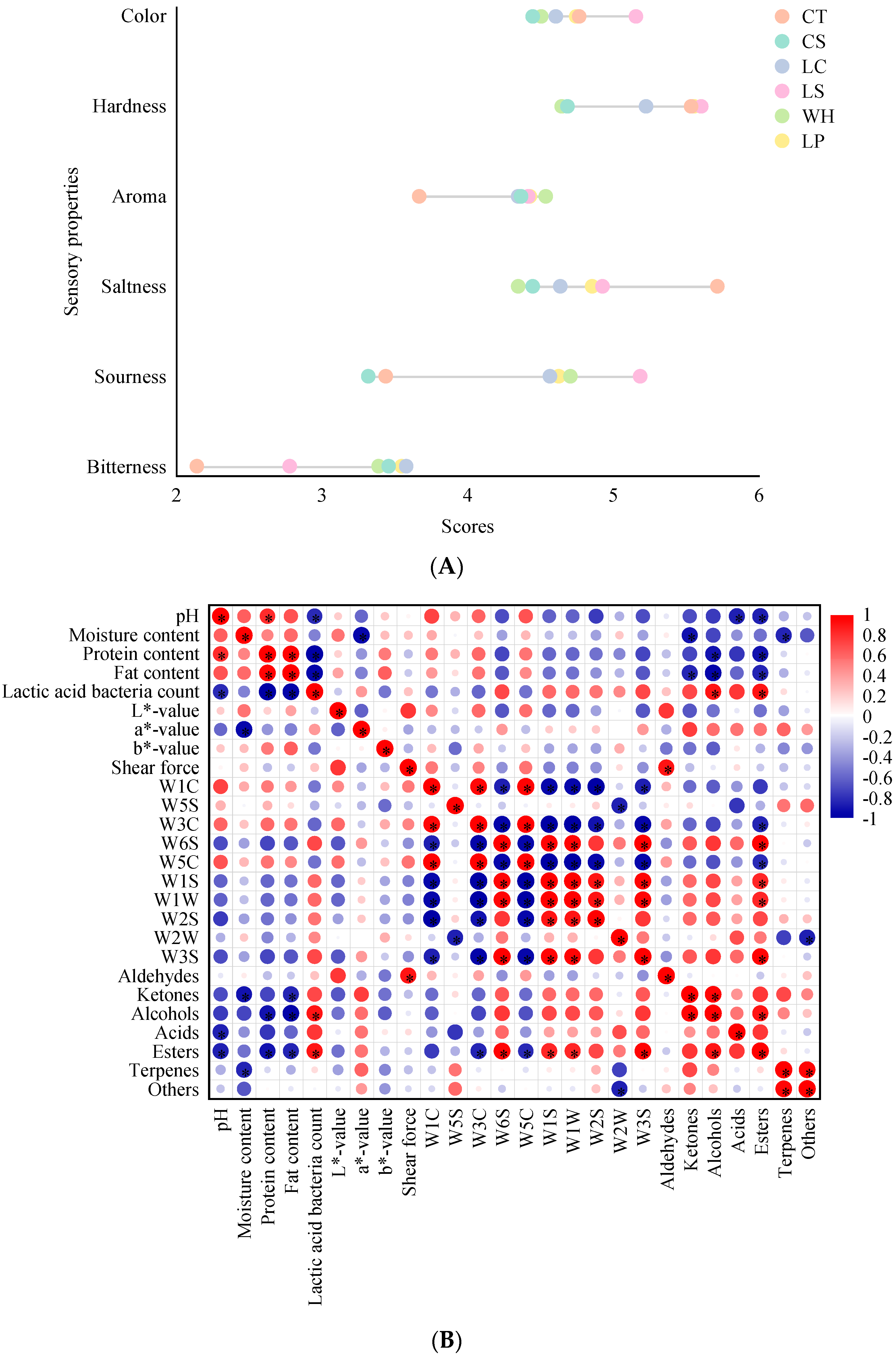
3.5. Sensory Evaluation
3.6. Correlation between Physicochemical Characteristics and Flavor Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.Y.; Zhang, L.; Zhang, H.; Wang, Y.; Chen, Q.; Kong, B.H. Physicochemical properties and flavour profile of fermented dry sausages with a reduction of sodium chloride. LWT-Food Sci. Technol. 2020, 124, 109061. [Google Scholar] [CrossRef]
- Campagnol, P.C.B.; Dos Santos, B.A.; Wagner, R.; Terra, N.N.; Pollonio, M.A.R. The effect of yeast extract addition on quality of fermented sausages at low NaCl content. Meat Sci. 2011, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.M.; Nilson, E.; Nieto, C.; Khandpur, N.; Denova, E.; Valero, I.; Barquera, S.; Campos, I. Modelling the impact of sodium intake on cardiovascular disease mortality in Mexico. BMC Public Health 2023, 23, 983. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Gelabert, J.; Gou, P.; Guerrero, L.; Arnau, J. Effect of sodium chloride replacement on some characteristics of fermented sausages. Meat Sci. 2003, 65, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.A.D.; Campagnol, P.C.B.; Morgano, M.A.; Pollonio, M.A.R. Monosodium glutamate, disodium inosinate, disodium guanylate, lysine and taurine improve the sensory quality of fermented cooked sausages with 50% and 75% replacement of NaCl with KCl. Meat Sci. 2014, 96, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Desmond, E.; Vasilopoulos, C. Reducing salt in meat and poultry products. In Reducing Salt Foods; Woodhead Publishing: Sawston, UK, 2019; pp. 159–183. [Google Scholar] [CrossRef]
- Magra, T.; Soultos, N.; Dovas, C.; Papavergou, E.; Lazou, T.; Apostolakos, I.; Dimitreli, G.; Ambrosiadis, I. Dry fermented sausages with total replacement of fat by extra virgin olive oil emulsion and indigenous lactic acid bacteria. Food Technol. Biotech. 2021, 59, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Li, Y.J.; Zhu, J.M.; Kong, B.H.; Liu, Q.; Chen, Q. Improving the taste profile of reduced-salt dry sausage by inoculating different lactic acid bacteria. Food Res. Int. 2021, 145, 110391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Hu, P.; Xie, Y.Y.; Wang, X.Y. Co-fermentation with Lactobacillus curvatus LAB26 and Pediococcus pentosaceus SWU73571 for improving quality and safety of sour meat. Meat Sci. 2020, 170, 108240. [Google Scholar] [CrossRef]
- Qin, L.G.; Li, X.A.; Huang, Y.X.; Li, Y.J.; Chen, Q. Flavour profile of traditional dry sausage prepared with partial substitution of NaCl with KCl. Foods 2023, 12, 388. [Google Scholar] [CrossRef]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanelli, G.; Gardini, F. Effects of the diameter on physicochemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- AOAC. Association of Official Methods of Analysis Methods 925.04, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- ISO 1443:1973; Meat and Meat Products—Determination of Total Fat Content. ISO: Geneva, Switzerland, 1973.
- ISO 937:1978; Meat and Meat Products—Determination of Nitrogen Content. ISO: Geneva, Switzerland, 1978.
- Aro, J.M.A.; Nyam-Osor, P.; Tsuji, K.; Shimada, K.; Fukushima, M.; Sekikawa, M. The effect of starter cultures on proteolytic changes and amino acid content in fermented sausages. Food Chem. 2010, 119, 279–285. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Li, Y.J.; Li, X.A.; Zhang, H.W.; Chen, Q.; Kong, B.H. Application of lactic acid bacteria for improving the quality of reduced-salt dry fermented sausage: Texture, color, and flavor profiles. LWT-Food Sci. Technol. 2022, 154, 112723. [Google Scholar] [CrossRef]
- Shi, J.; Nian, Y.Q.; Da, D.D.; Xu, X.L.; Zhou, G.H.; Zhao, D.; Li, C.B. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography–mass spectrometry and electronic nose. LWT-Food Sci. Technol. 2020, 124, 109182. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Li, C.; Zhao, Y.; Chen, C.; Zhang, Y.; Zhang, J.; Ma, M. Effect of pulsed pressure-assisted brining on lipid oxidation and volatiles development in pork bacon during salting and drying-ripening. LWT-Food Sci. Technol. 2015, 64, 1099–1106. [Google Scholar] [CrossRef]
- Bicchi, A.J.D. Identification of flavour and fragrance constituents. Flavour Fragr. J. 2018, 33, 201–202. [Google Scholar] [CrossRef]
- Jelen, H.H.; Wieczorek, M.N. Commentary: “Quantitative” vs quantitative Headspace Solid-Phase Microextraction (HS-SPME) in food volatile and flavor compounds analysis. J. Food Compos. Anal. 2023, 115, 104955. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Xiao, Y.Q.; Li, P.; Zhou, Y.; Ma, F.; Chen, C. Effect of inoculating Lactobacillus pentosus R3 on N-nitrosamines and bacterial communities in dry fermented sausages. Food Control 2018, 87, 126–134. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Liu, Y.N.; Chen, C.G.; Xie, T.T.; Li, P.J. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef]
- Yoo, S.; Park, S.; Seo, S.; Son, H. Quality characteristics of fermented sausage prepared with soy sauce. Food Sci. Biotechnol. 2016, 25, 533–539. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Z.; Yohannes, K.; Yu, Q.; Yang, Z.; Li, H.; Liu, J.; Wang, J. Functional Characteristics of Lactobacillus and yeast single starter cultures in the ripening process of dry fermented sausage. Front. Microbiol. 2021, 11, 611260. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.C.; Barat, J.M.; Toldrá, F. Biochemical and sensory changes in dry-cured ham salted with partial replacements of NaCl by other chloride salts. Meat Sci. 2012, 90, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Harel, S.; Jaffe, R. Lipid peroxidation of muscle food as affected by NaCl. J. Agric. Food Chem. 1991, 39, 1017–1021. [Google Scholar] [CrossRef]
- Johansson, G.; Berdague, J.; Larsson, M.; Tran, N.; Borch, E. Lipolysis, proteolysis and formation of volatile components during ripening of a fermented sausage with Pediococcus pentosaceus and Staphylococcus xylosus as starter cultures. Meat Sci. 1994, 38, 203–218. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.; Salvador, A.; Flores, M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010, 86, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kauser-Ul-Alam, M.; Toba, Y.; Hioki, S.; Hayakawa, T.; Kumura, H.; Wakamatsu, J. Lactococcus lactis subsp. cremoris produces zinc protoporphyrin IX both aerobically and anaerobically and improves the bright red color of fermented meat products. Foods 2020, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.X.; Wang, S.W.; Zhang, H.; Wang, H.T.; Kong, B.H. Influence of glycated nitrosohaemoglobin prepared from porcine blood cell on physicochemical properties, microbial growth and flavour formation of Harbin dry sausages. Meat Sci. 2019, 148, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Han, K.; Naveen, K.V.; Wang, M. Antioxidant and antibacterial effects of potential probiotics isolated from Korean fermented foods. Int. J. Mol. Sci. 2022, 23, 10062. [Google Scholar] [CrossRef] [PubMed]
- Aliño, M.; Grau, R.; Baigts, D.; Barat, J.M. Influence of sodium replacement on the salting kinetics of pork loin. J. Food Eng. 2009, 95, 551–557. [Google Scholar] [CrossRef]
- Flores, M.; Corral, S.; Cano-Garcia, L.; Salvador, A.; Belloch, C. Yeast strains as potential aroma enhancers in dry fermented sausages. Int. J. Food Microbiol. 2015, 212, 16–24. [Google Scholar] [CrossRef]
- Tian, X.; Li, Z.; Chao, Y.; Wu, Z.; Zhou, M.; Xiao, S.; Zeng, J.; Zhe, J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Res. Int. 2020, 137, 109456. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.Q.; Wang, H.B.; Xi, B.; He, X.N.; Yang, X.L.; Li, W.H. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.X.; Sun, F.D.; Li, X.A.; Chen, Q.; Kong, B.H. The potential correlations between the fungal communities and volatile compounds of traditional dry sausages from Northeast China. Food Microbiol. 2021, 98, 103787. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.Y.; He, L.P.; Li, C.Q. Determination of bacterial community and its correlation to volatile compounds in Guizhou Niuganba, a traditional Chinese fermented dry-cured beef. LWT-Food Sci. Technol. 2022, 161, 113380. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; Montero, R.; Belloch, C.; Flores, M. Microbial changes and aroma profile of nitrate reduced dry sausages during vacuum storage. Meat Sci. 2019, 147, 100–107. [Google Scholar] [CrossRef]
- Toyomizu, M.; Hanaoka, K.; Yamaguchi, K. Effect of release of free fatty acids by enzymatic hydrolysis of phospholipids on lipid oxidation during storage of fish muscle at −5 °C. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 615–620. [Google Scholar] [CrossRef]
- Brendel, S.; Hofmann, T.; Granvogl, M. Dry-hopping to modify the aroma of alcohol-free beer on a molecular level-loss and transfer of odor-active compounds. J. Agric. Food Chem. 2020, 68, 8602–8612. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on the evolution of flavor compounds in probiotic dry-fermented sausages during ripening. Meat Sci. 2015, 100, 41–51. [Google Scholar] [CrossRef]
- Rai, K.P.; Zhang, C.H.; Xia, W.S. Effects of pure starter cultures on physico-chemical and sensory quality of dry fermented Chinese-style sausage. J. Food Sci. Technol. 2010, 47, 188–194. [Google Scholar] [CrossRef]
- Li, X.A.; Sui, Y.M.; Lu, J.S.; Ren, J.; Kong, B.H.; Li, Y.J.; Chen, Q.; Yang, W.W. Compensative role of autochthonous lactic acid bacteria in physical properties and taste profiles of dry sausage with partial substitution of NaCl by KCl. LWT-Food Sci. Technol. 2024, 199, 116115. [Google Scholar] [CrossRef]
- Eymard, S.; Baron, C.P.; Jacobsen, C. Oxidation of lipid and protein in horse mackerel (Trachurus trachurus) mince and washed minces during processing and storage. Food Chem. 2009, 114, 57–65. [Google Scholar] [CrossRef]
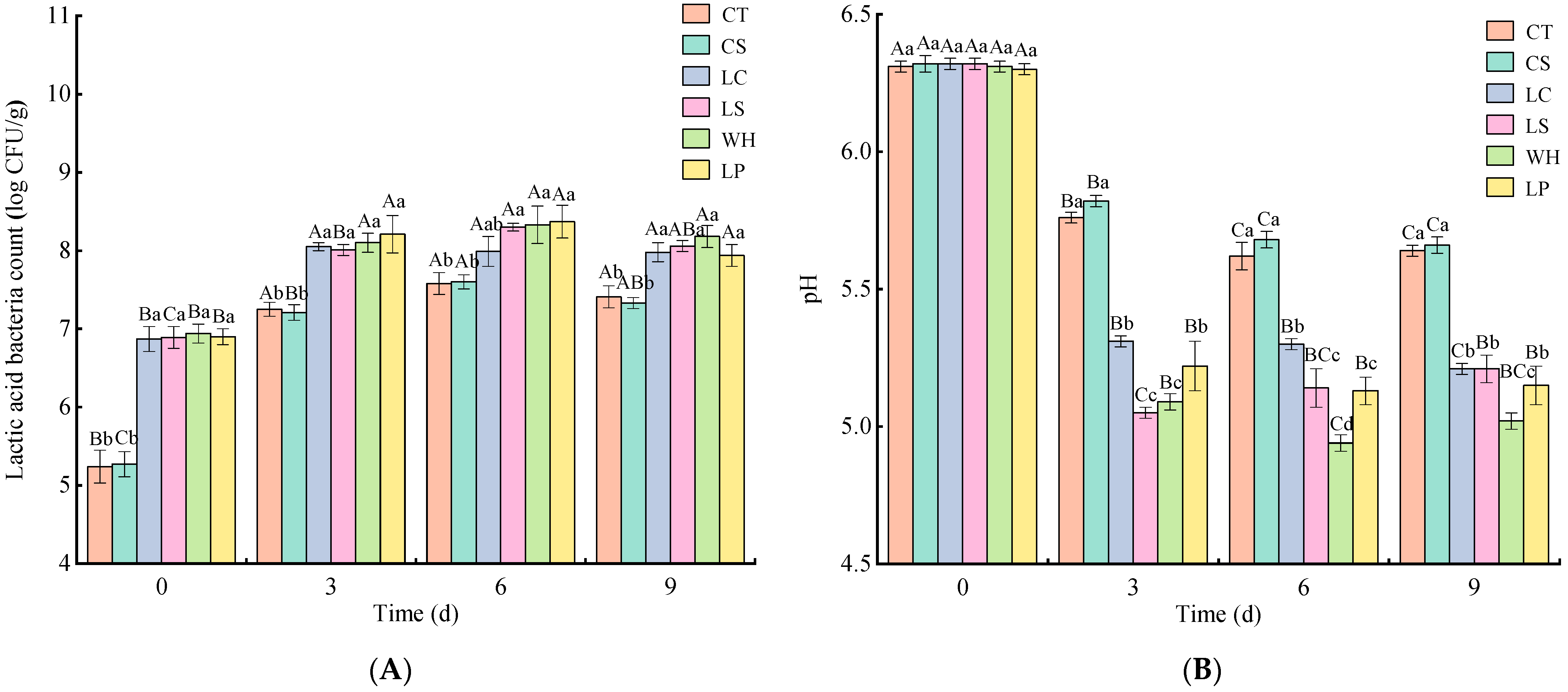

| Time (d) | CT | CS | LC | LS | WH | LP | |
|---|---|---|---|---|---|---|---|
| L*-value | 0 | 44.96 ± 0.99 Aa | 45.81 ± 2.04 Aa | 45.23 ± 2.19 Aa | 44.73 ± 0.54 Aa | 45.17 ± 0.96 Aa | 46.19 ± 1.20 Aa |
| 3 | 39.90 ± 3.18 Bb | 39.65 ± 0.81 Bb | 37.69 ± 1.17 Bb | 44.23 ± 2.07 Aa | 43.47 ± 0.48 Ba | 39.13 ± 1.38 Bb | |
| 6 | 36.62 ± 1.35 Cbc | 38.01 ± 1.26 BCb | 35.75 ± 0.51 Cc | 37.06 ± 1.08 Bbc | 40.81 ± 0.78 Ca | 34.16 ± 1.05 Cd | |
| 9 | 38.38 ± 0.81 BCa | 37.00 ± 0.69 Cb | 37.41 ± 0.60 BCab | 33.78 ± 1.05 Cd | 37.17 ± 0.66 Db | 35.21 ± 1.08 Cc | |
| a*-value | 0 | 12.42 ± 1.62 Ca | 11.68 ± 0.81 Da | 11.92 ± 0.60 Ca | 11.88 ± 1.17 Ca | 12.55 ± 0.93 Ca | 12.01 ± 0.90 Da |
| 3 | 13.67 ± 1.11 BCbc | 14.03 ± 0.66 Cb | 15.72 ± 0.96 Ba | 12.57 ± 1.29 Cc | 14.12 ± 0.48 Bb | 13.66 ± 1.44 Cbc | |
| 6 | 14.77 ± 1.20 ABd | 17.36 ± 0.81 Aab | 16.91 ± 0.96 Aab | 18.10 ± 1.02 Aa | 16.15 ± 0.69 Abc | 15.11 ± 0.30 Bcd | |
| 9 | 16.13 ± 1.02 Aa | 16.23 ± 0.84 Ba | 16.53 ± 0.54 ABa | 16.53 ± 0.72 Ba | 16.25 ± 0.57 Aa | 16.59 ± 1.38 Aa | |
| b*-value | 0 | 16.70 ± 1.56 Aa | 17.90 ± 0.75 Aa | 17.35 ± 0.99 Aa | 17.70 ± 0.93 Aa | 17.15 ± 1.08 Aa | 17.12 ± 0.48 Aa |
| 3 | 12.20 ± 1.05 Cb | 15.01 ± 0.54 Ba | 14.17 ± 0.75 Ca | 11.19 ± 0.90 Cb | 13.95 ± 1.11 Ca | 11.95 ± 0.84 Cb | |
| 6 | 13.02 ± 0.51 Cb | 15.39 ± 1.74 Ba | 14.83 ± 0.93 BCa | 14.55 ± 0.33 Ba | 15.06 ± 0.81 BCa | 10.97 ± 0.81 Dc | |
| 9 | 14.63 ± 0.42 Bc | 16.08 ± 0.42 Ba | 15.70 ± 0.39 Bab | 13.78 ± 0.36 Bd | 15.41 ± 0.33 Bb | 15.70 ± 0.39 Bab | |
| Shear force (N) | 0 | 2.03 ± 0.17 Da | 2.36 ± 0.17 Ca | 2.23 ± 0.24 Da | 2.32 ± 0.28 Da | 2.36 ± 0.28 Ca | 2.20 ± 0.14 Ca |
| 3 | 8.52 ± 1.25 Cab | 7.56 ± 0.43 Bab | 7.58 ± 1.30 Cab | 5.84 ± 0.62 Cb | 9.72 ± 1.20 Ba | 8.63 ± 1.21 Bab | |
| 6 | 11.65 ± 1.06 Ba | 11.46 ± 0.78 Aa | 12.17 ± 1.07 Ba | 9.48 ± 1.35 Ba | 11.29 ± 0.73 Ba | 11.79 ± 1.84 ABa | |
| 9 | 16.10 ± 1.06 Aa | 12.58 ± 1.28 Abc | 16.12 ± 0.52 Aa | 14.68 ± 1.04 Aabc | 15.32 ± 1.28 Aab | 12.08 ± 1.37 Ac |
| Number | Volatile Compound | CT | CS | LC | LS | WH | LP |
|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||
| C1 | Hexanal | 6.26 ± 0.35 a | 5.73 ± 0.31 ab | 5.13 ± 0.26 bc | 5.79 ± 0.36 ab | 6.08 ± 0.38 a | 4.38 ± 0.12 c |
| C2 | Nonanal | 28.56 ± 1.40 a | 24.55 ± 1.30 ab | 27.90 ± 2.60 a | 21.93 ± 1.85 b | 26.68 ± 1.28 a | 21.72 ± 1.11 b |
| C3 | Cinnamaldehyde | 11.76 ± 0.94 b | 13.65 ± 0.90 ab | 11.46 ± 1.44 b | 13.48 ± 0.76 ab | 11.07 ± 1.04 b | 14.72 ± 0.90 a |
| C4 | 10-Undecenal | 12.32 ± 1.21 a | 4.06 ± 0.21 c | 11.22 ± 1.11 a | 7.82 ± 0.80 b | 10.32 ± 0.89 a | 7.72 ± 0.69 b |
| Total | 58.90 ± 3.90 a | 47.99 ± 2.72 b | 55.71 ± 5.41 a | 49.02 ± 3.77 b | 54.15 ± 3.59 a | 48.54 ± 2.82 b | |
| Ketones | |||||||
| C5 | 2-Nonanone | 3.35 ± 0.35 b | 3.82 ± 0.17 b | 4.65 ± 0.35 a | 3.98 ± 0.31 ab | 3.26 ± 0.14 b | 4.03 ± 0.31 ab |
| C6 | 3-hydroxy-2-butanone | 21.34 ± 1.33 b | 20.35 ± 1.30 b | 16.28 ± 0.68 c | 24.66 ± 1.35 a | 20.24 ± 0.88 b | 24.88 ± 1.39 a |
| C7 | Sulcatone | 10.26 ± 0.43 c | 10.46 ± 0.38 c | 18.36 ± 0.31 b | 18.69 ± 0.66 b | 25.65 ± 1.11 a | 17.38 ± 1.06 b |
| C8 | Fenchone | 72.48 ± 3.97 ab | 69.47 ± 4.26 ab | 75.27 ± 4.31 a | 76.89 ± 3.43 a | 69.41 ± 4.90 ab | 75.90 ± 4.80 a |
| Total | 107.43 ± 6.08 c | 104.10 ± 6.11 d | 114.56 ± 5.65 b | 124.22 ± 5.75 a | 118.56 ± 7.03 ab | 122.19 ± 7.56 a | |
| Alcohols | |||||||
| C9 | Ethanol | 106.87 ± 7.90 e | 140.04 ± 7.40 c | 126.80 ± 5.06 d | 188.41 ± 10.50 a | 162.43 ± 10.27 b | 150.37 ± 9.72 c |
| C10 | 2,3-Butanediol | 14.70 ± 1.00 c | 9.15 ± 0.62 d | 19.73 ± 1.18 b | 4.44 ± 0.12 f | 23.95 ± 0.57 a | 6.48 ± 0.29 e |
| C11 | 2-Ethylhexanol | 12.10 ± 0.28 b | 11.91 ± 0.40 b | 12.74 ± 0.23 ab | 13.56 ± 0.17 a | 13.83 ± 0.21 a | 11.94 ± 0.31 b |
| C12 | 2-Heptanol | n.d. | n.d. | 2.05 ± 0.21 b | 3.19 ± 0.19 a | 2.35 ± 0.05 b | 2.48 ± 0.23 b |
| C13 | 2-Nonanol | n.d. | n.d. | 18.39 ± 1.02 b | 18.66 ± 1.21 b | 25.63 ± 1.28 a | 4.58 ± 0.19 c |
| C14 | Benzyl alcohol | 8.60 ± 0.31 c | 9.34 ± 0.42 c | 11.93 ± 0.97 b | 9.94 ± 0.73 c | 9.73 ± 0.38 c | 15.37 ± 1.13 a |
| C15 | Phenethyl alcohol | 19.53 ± 1.54 a | 19.64 ± 1.06 a | 20.18 ± 0.80 a | 15.31 ± 0.90 b | 15.38 ± 0.73 b | 17.45 ± 1.16 ab |
| C16 | 3-phenylpropanol | 22.61 ± 0.50 c | 2.82 ± 0.29 e | 27.52 ± 1.30 a | 17.15 ± 0.83 d | 2.83 ± 0.17 e | 24.77 ± 0.68 b |
| C17 | Terpinen-4-ol | 60.67 ± 3.34 ab | 52.53 ± 2.03 b | 59.56 ± 4.07 ab | 57.81 ± 2.18 ab | 54.21 ± 4.26 ab | 62.55 ± 3.57 a |
| C18 | α-Terpineol | 32.62 ± 1.97 ab | 25.14 ± 1.51 c | 28.55 ± 1.66 bc | 29.07 ± 2.03 bc | 28.88 ± 1.11 bc | 35.37 ±1.73 a |
| C19 | Linalool | 42.34 ± 3.19 ab | 43.66 ± 1.11 a | 35.46 ± 2.23 b | 40.41 ± 2.46 ab | 39.50 ± 1.47 ab | 43.92 ± 1.96 a |
| Total | 320.04 ± 20.03 d | 314.23 ± 14.84 e | 362.91 ± 18.73 c | 397.95 ± 21.32 a | 378.72 ± 20.50 b | 375.28 ± 20.97 b | |
| Acids | |||||||
| C20 | Acetic acid | 84.72 ± 7.01 cd | 71.23 ± 6.50 d | 124.61 ± 8.04 b | 90.13 ± 5.70 c | 146.31 ± 8.00 a | 119.47 ± 5.99 b |
| C21 | Butanoic acid | 4.65 ± 0.21 e | 12.06 ± 0.57 d | 17.23 ± 0.71 b | 14.21 ± 0.80 c | 19.17 ± 1.04 a | 10.85 ± 0.54 d |
| C22 | Hexanoic acid | 9.98 ± 0.23 a | 7.60 ± 0.16 b | 3.62 ± 0.21 c | 3.59 ± 0.12 c | 10.00 ± 0.40 a | 2.96 ± 0.16 d |
| C23 | Heptanoic acid | 15.16 ± 1.45 c | 19.46 ± 0.66 a | 18.83 ± 0.50 ab | 15.47 ± 0.48 c | 17.12 ± 0.42 bc | 11.98 ± 0.31 d |
| C24 | Octanoic acid | 2.12 ± 0.07 d | 23.45 ± 1.28 b | 27.46 ± 1.45 a | 17.24 ± 1.70 c | 26.16 ± 1.65 ab | 24.52 ± 1.21 ab |
| C25 | Nonanoic acid | 8.31 ± 0.24 a | 5.46 ± 0.23 c | 8.00 ± 0.53 a | 6.50 ± 0.40 b | 6.57 ± 0.31 b | 2.42 ± 0.10 d |
| C26 | 3-Hydroxybutyric acid | 4.69 ± 0.12 a | 4.67 ± 0.16 a | n.d. | n.d. | 4.98 ± 0.07 a | 3.15 ± 0.12 b |
| Total | 129.63 ± 9.33 e | 143.93 ± 9.56 d | 199.75 ± 11.44 b | 147.14 ± 9.20 d | 230.31 ± 11.89 a | 175.35 ± 8.43 c | |
| Esters | |||||||
| C27 | Methyl acetate | n.d. | 4.56 ± 0.43 a | n.d. | 2.45 ± 0.09 bc | 2.89 ± 0.12 b | 2.02 ± 0.19 c |
| C28 | Methyl lactate | n.d. | n.d. | 2.30 ± 0.10 b | 2.23 ± 0.07 b | 6.54 ± 0.42 a | n.d. |
| C29 | Methyl butyrate | 12.32 ± 1.18 b | 13.88 ± 1.13 b | 16.28 ± 0.57 a | 2.23 ± 0.07 c | 17.34 ± 0.68 a | 12.50 ± 0.33 b |
| C30 | Methyl hexanoate | 45.85 ± 2.13 d | 73.91 ± 3.93 b | 59.97 ± 3.15 c | 55.37 ± 2.81 cd | 87.15 ± 6.08 a | 62.33 ± 5.84 c |
| C31 | Methyl octanoate | n.d. | 3.83 ± 0.17 c | 2.40 ± 0.09 d | 4.61 ± 0.29 b | 5.63 ± 0.31 a | n.d. |
| C32 | Methyl 3-phenylpropionate | 4.70 ± 0.16 d | 9.01 ± 0.45 c | 12.20 ± 0.94 b | 4.64 ± 0.14 d | 16.50 ± 1.75 a | 9.63 ± 0.66 c |
| C33 | Ethyl acetate | 30.41 ± 1.14 d | 38.16 ± 2.79 c | 39.48 ± 2.58 c | 71.70 ± 4.33 a | 51.31 ± 2.25 b | 25.85 ± 1.11 d |
| C34 | Ethyl butyrate | 4.53 ± 0.17 d | 4.72 ± 0.21 d | 7.96 ± 0.26 b | 10.12 ± 0.61 a | 7.82 ± 0.61 b | 6.06 ± 0.59 c |
| C35 | Ethyl hexanoate | 67.80 ± 6.48 b | 60.75 ± 5.30 b | 70.33 ± 7.01 b | 94.06 ± 5.98 a | 88.22 ± 5.18 a | 90.83 ± 5.80 a |
| C36 | Ethyl heptanoate | 3.39 ± 0.40 b | 3.09 ± 0.09 b | 4.39 ± 0.17 a | 4.72 ± 0.21 a | 5.00 ± 0.26 a | 4.69 ± 0.33 a |
| C37 | Ethyl caprylate | 10.13 ± 0.88 c | 13.89 ± 0.74 b | 13.06 ± 0.73 b | 14.00 ± 0.99 b | 17.92 ± 1.11 a | 13.67 ± 0.61 b |
| C38 | Ethyl caprate | 7.40 ± 0.33 c | 9.35 ± 0.50 bc | 9.43 ± 0.43 b | 8.63 ± 0.59 bc | 14.24 ± 1.16 a | 10.03 ± 0.97 b |
| C39 | γ-Butyrolactone | 7.72 ± 0.40 b | 9.30 ± 0.10 a | 4.75 ± 0.43 c | 8.65 ± 0.64 ab | 3.73 ± 0.28 c | 9.33 ± 0.36 a |
| C40 | 4-Terpinenyl acetate | 3.32 ± 0.21 b | 3.45 ± 0.16 ab | 3.69 ± 0.23 ab | 3.98 ± 0.35 ab | 3.56 ± 0.33 ab | 4.13 ± 0.40 a |
| C41 | Bornyl acetate | 142.18 ± 7.98 a | 131.47 ± 10.08 a | 134.47 ± 5.66 a | 137.36 ± 6.46 a | 132.00 ± 8.00 a | 147.51 ± 7.88 a |
| Total | 339.75 ± 21.46 e | 379.37 ± 26.08 d | 380.71 ± 22.35 d | 424.75 ± 23.63 b | 459.85 ± 28.54 a | 398.58 ± 25.07 c | |
| Terpenes | |||||||
| C42 | α-Cubebene | 18.94 ± 1.42 a | 15.07 ± 0.68 b | 17.77 ± 0.83 ab | 17.32 ± 2.08 ab | 18.75 ± 0.73 a | 19.23 ± 1.77 a |
| C43 | α-Muurolene | 21.41 ± 0.94 ab | 23.27 ± 1.97 a | 19.08 ± 1.00 b | 17.46 ± 2.04 b | 18.51 ± 1.73 b | 21.30 ± 1.33 ab |
| C44 | α-Curcumene | 98.57 ± 6.50 a | 83.37 ± 16.71 a | 88.34 ± 4.52 a | 89.88 ± 7.27 a | 82.30 ± 10.25 a | 98.68 ± 7.15 a |
| C45 | β-Caryophyllene | 167.83 ± 7.67 a | 125.27 ± 5.06 a | 151.95 ± 7.79 b | 152.49 ± 11.71 a | 151.80 ± 8.23 a | 171.79 ± 9.01 a |
| C46 | β-Phellandrene | 28.45 ± 1.37 a | 23.28 ± 2.51 b | 24.94 ± 1.30 ab | 21.80 ± 1.70 b | 21.99 ± 1.68 b | 26.65 ± 2.39 ab |
| C47 | β-Ocimene | 4.27 ± 0.12 bc | 4.00 ± 0.17 c | 4.70 ± 0.21 b | 4.36 ± 0.24 bc | 4.59 ± 0.35 bc | 5.67 ± 0.36 a |
| C48 | β-Muurolene | 38.29 ± 2.56 a | 29.20 ± 1.28 d | 33.27 ± 1.40 bcd | 34.16 ± 1.61 abc | 30.68 ± 1.52 cd | 37.22 ± 2.04 ab |
| C49 | β-Myrcene | 52.51 ± 3.60 a | 51.65 ± 2.81 a | 52.50 ± 2.48 a | 58.44 ± 3.62 a | 50.75 ± 2.23 a | 58.44 ± 2.08 a |
| C50 | Camphene | 5.93 ± 0.21 b | 4.14 ± 0.07 c | 7.07 ± 0.16 a | 5.79 ± 0.26 b | 3.64 ± 0.12 d | 4.45 ± 0.10 c |
| C51 | D-Limonene | 453.40 ± 19.66 ab | 432.63 ± 12.45 b | 455.85 ± 25.60 ab | 503.47 ± 41.85 ab | 442.96 ± 18.69 ab | 511.86 ± 31.80 a |
| C52 | γ-Terpinene | 75.45 ± 7.00 ab | 62.53 ± 5.68 b | 70.30 ± 4.19 ab | 77.70 ± 4.28 a | 68.17 ± 3.05 ab | 79.53 ± 4.85 a |
| Total | 965.05 ± 51.05 b | 854.41 ± 49.39 e | 925.77 ± 49.48 c | 982.87 ± 76.66 b | 894.14 ± 48.58 d | 1034.82 ± 62.88 a | |
| Others | |||||||
| C53 | D-Camphor | 227.93 ± 10.36 bc | 203.23 ± 14.72 c | 227.97 ± 12.12 bc | 240.98 ± 14.24 ab | 222.69 ± 8.78 bc | 266.82 ± 7.98 a |
| C54 | Octadecane | 58.14 ± 2.63 a | 46.03 ± 3.00 c | 51.31 ± 2.04 abc | 47.89 ± 2.88 bc | 48.09 ± 2.36 bc | 55.71 ± 3.59 ab |
| C55 | P-Cymene | 23.40 ± 1.77 ab | 20.39 ± 0.54 b | 21.81 ± 1.77 ab | 24.84 ± 2.15 a | 21.47 ± 1.44 ab | 25.30 ± 1.54 a |
| C56 | Safrole | 56.49 ± 3.12 b | 63.35 ± 3.22 b | 81.08 ± 4.31 a | 44.71 ± 2.72 c | 63.01 ± 2.59 b | 56.16 ± 3.95 b |
| C57 | Eugenol | 495.10 ± 22.71 a | 419.62 ± 16.21 b | 428.27 ± 22.53 b | 407.10 ± 12.12 b | 399.21 ± 15.64 b | 501.71 ± 26.33 a |
| C58 | 4-Allylanisole | 106.83 ± 6.63 ab | 79.72 ± 4.52 c | 90.31 ± 5.23 bc | 89.67 ± 4.50 bc | 83.76 ± 6.51 c | 109.06 ± 11.02 a |
| C59 | 1,8-Cineole | 114.17 ± 5.13 ab | 107.60 ± 6.20 b | 115.89 ± 9.02 ab | 121.35 ± 9.11 ab | 108.58 ± 6.30 b | 128.99 ± 8.61 a |
| C60 | Anethole | 804.94 ± 27.85 b | 654.40 ± 15.17 d | 724.48 ± 18.83 c | 892.66 ± 25.84 a | 704.53 ± 31.54 cd | 841.78 ± 29.06 ab |
| C61 | Methyl eugenol | 47.72 ± 2.44 a | 41.29 ± 2.18 ab | 48.47 ± 3.59 a | 33.39 ± 2.60 b | 41.16 ± 4.02 ab | 45.01 ± 2.27 a |
| Total | 1934.72 ± 82.64 b | 1635.63 ± 65.76 d | 1789.59 ± 79.44 c | 1902.59 ± 76.16 b | 1692.50 ± 79.18 d | 2030.54 ± 94.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lu, J.; Zhang, X.; Kong, B.; Li, Y.; Chen, Q.; Wen, R. Effect of Inoculation with Autochthonous Lactic Acid Bacteria on Flavor, Texture, and Color Formation of Dry Sausages with NaCl Partly Substituted by KCl. Foods 2024, 13, 1747. https://doi.org/10.3390/foods13111747
Wang J, Lu J, Zhang X, Kong B, Li Y, Chen Q, Wen R. Effect of Inoculation with Autochthonous Lactic Acid Bacteria on Flavor, Texture, and Color Formation of Dry Sausages with NaCl Partly Substituted by KCl. Foods. 2024; 13(11):1747. https://doi.org/10.3390/foods13111747
Chicago/Turabian StyleWang, Jiawang, Jiasheng Lu, Xin Zhang, Baohua Kong, Yongjie Li, Qian Chen, and Rongxin Wen. 2024. "Effect of Inoculation with Autochthonous Lactic Acid Bacteria on Flavor, Texture, and Color Formation of Dry Sausages with NaCl Partly Substituted by KCl" Foods 13, no. 11: 1747. https://doi.org/10.3390/foods13111747
APA StyleWang, J., Lu, J., Zhang, X., Kong, B., Li, Y., Chen, Q., & Wen, R. (2024). Effect of Inoculation with Autochthonous Lactic Acid Bacteria on Flavor, Texture, and Color Formation of Dry Sausages with NaCl Partly Substituted by KCl. Foods, 13(11), 1747. https://doi.org/10.3390/foods13111747






