Abstract
Vibrio parahaemolyticus can cause acute gastroenteritis, wound infections, and septicemia in humans. The overuse of antibiotics in aquaculture may lead to a high incidence of the multidrug-resistant (MDR) pathogen. Nevertheless, the genome evolution of V. parahaemolyticus in aquatic animals and the mechanism of its antibiotic tolerance remain to be further deciphered. Here, we investigated the molecular basis of the antibiotic tolerance of V. parahaemolyticus isolates (n = 3) originated from shellfish and crustaceans using comparative genomic and transcriptomic analyses. The genome sequences of the V. parahaemolyticus isolates were determined (5.0–5.3 Mb), and they contained 4709–5610 predicted protein-encoding genes, of which 823–1099 genes were of unknown functions. Comparative genomic analyses revealed a number of mobile genetic elements (MGEs, n = 69), antibiotic resistance-related genes (n = 7–9), and heavy metal tolerance-related genes (n = 2–4). The V. parahaemolyticus isolates were resistant to sub-lethal concentrations (sub-LCs) of ampicillin (AMP, 512 μg/mL), kanamycin (KAN, 64 μg/mL), and streptomycin (STR, 16 μg/mL) (p < 0.05). Comparative transcriptomic analyses revealed that there were significantly altered metabolic pathways elicited by the sub-LCs of the antibiotics (p < 0.05), suggesting the existence of multiple strategies for antibiotic tolerance in V. parahaemolyticus. The results of this study enriched the V. parahaemolyticus genome database and should be useful for controlling the MDR pathogen worldwide.
1. Introduction
Vibrio parahaemolyticus, a halophilic Gram-negative bacterium, is typically present in marine and estuarine environments [1]. Consuming raw or improperly cooked seafood contaminated with pathogenic V. parahaemolyticus can result in acute gastroenteritis, and in severe cases, even death [2]. This bacterium was initially recognized as a pathogen in 1950; since then, it has become a major foodborne pathogen worldwide [1,3]. Pathogenic V. parahaemolyticus produces thermostable direct hemolysin (TDH) and TDH-associated hemolysin (TRH), which are molecular markers of its pathogenicity [4,5].
V. parahaemolyticus is commonly detected in fish and shellfish worldwide [6,7,8]. For example, Vu et al. collected 120 seafood samples from different traditional markets in Hanoi, Vietnam between May and October of 2020 [7]. They found that V. parahaemolyticus was present in 58.33% of the samples, with shrimp samples having the highest detection rate at 86.67%, followed by 53.33% of fish samples (n = 30), 53.33% of squid samples (n = 30) and 40% of oyster samples (n = 30) [7]. Antibiotics effectively eliminated infectious diseases in aquaculture caused by pathogenic bacteria [9]. However, the excessive use of antibiotics in the clinical and aquaculture industries have led to the emergence and evolution of antibiotic-resistant V. parahaemolyticus over the past few decades [10]. V. parahaemolyticus is commonly resistant to ampicillin (AMP), kanamycin (KAN), and streptomycin (STR) [8,11]. For example, Lopatek et al. reported that V. parahaemolyticus isolates were found in 595 seafood samples with a detection rate of 17.5% (n = 104). Among these isolates, 75.0% were resistant to AMP, and 68.3% were resistant to STR. Additionally, the majority of the isolates (55.8%) showed resistance to two classes of antimicrobials, primarily AMP and STR [8]. Multidrug-resistant (MDR) pathogens in aquaculture environments have the potential to enter aquatic organisms through the food chain and subsequently pose a significant risk to both aquaculture and human health [12]. The frequent presence of MDR V. parahaemolyticus has become a critical public health concern [13].
Previous studies have shown that mobile genetic elements (MGEs) facilitate the accumulation and spread of antimicrobial resistance genes through horizontal gene transfer (HGT) [14]. With the continuous breakthrough of genome sequencing technology [15], 1740 V. parahaemolyticus isolates have been sequenced so far (GenBank database, https://www.ncbi.nlm.nih.gov/; accession date: 29 January 2022), of which the complete genomes of 64 V. parahaemolyticus isolates are available in the GenBank database. This provided the possibility to obtain insights into antibiotic resistance genes at the whole genome level. However, few studies have been conducted to comparatively analyze V. parahaemolyticus genomes of aquatic animal origins [16,17,18,19,20].
In our previous research, a number of V. parahaemolyticus strains were collected and analyzed from various aquatic animal species [21]. Among these, V. parahaemolyticus B2-28, N9-20, and N2-5 isolates exhibited MDR phenotypes. Hence, we asked what the genome features of the isolates could be and what the molecular mechanism underlying the resistance phenotypes could be. Thus, the primary objectives of this study were (1) to determine draft genome sequences of the three V. parahaemolyticus isolates of aquatic animal origins; (2) to identify MGEs as well as virulence- and resistance-related genes in the V. parahaemolyticus genomes; (3) to investigate the survival of the V. parahaemolyticus strains under different concentrations of antibiotics (AMP, KAN, and STR); and (4) to decipher the molecular mechanism underlying the tolerance of the V. parahaemolyticus strains to the antibiotics through biochemistry, comparative genomics, and transcriptomics analyses (Figure 1). The findings of this study will contribute to the genome data of V. parahaemolyticus and facilitate the control of this foodborne pathogen worldwide.
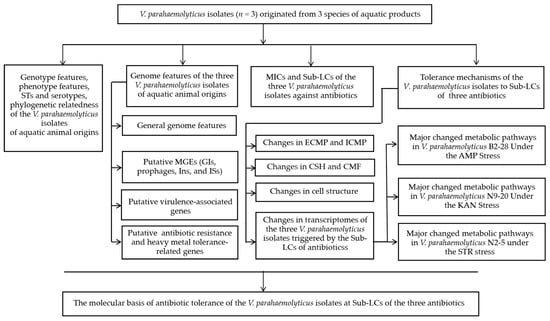
Figure 1.
A flow chart of the investigation strategy in this study.
2. Materials and Methods
2.1. The Characterization of Genome Features of the V. parahaemolyticus Isolates
2.1.1. V. parahaemolyticus Strains and Culture Conditions
V. parahaemolyticus B2-28, N9-20, and N2-5 strains were isolated from two species of shellfish, namely Ruditapes philippinarum and Keenocardium californiense, respectively, and one species of shrimp, Oratosquilla oratori, respectively [21] (Table S1). V. parahaemolyticus strains were routinely incubated in Tryptic Soy Broth (TSB) medium (3% NaCl, pH 8.5) at 37 °C with shaking at 180 rpm. Bacterial growth was measured using the Bioscreen C automated growth analyzer (Lab Systems, Helsinki, Finland), and the OD600 value was used as a related parameter for the bacterial biomass [22].
2.1.2. Genomic DNA Preparation, Sequencing, Assembly, and Annotation
At the middle logarithmic growth phase (mid-LGP), V. parahaemolyticus strains were collected by centrifugation at 8000× g for 1 min. Genomic DNA was extracted using the MiniBEST DNA extraction kit (Japan TaKaRa BIO, Dalian, China). The extracted DNA samples were analyzed using agarose gel electrophoresis, and the DNA concentration and purity were measured [22]. Sequencing of only high-quality DNA samples (a 260/280 nm absorbance ratio of 1.8–2.0) was conducted by Shanghai Majorbio Bio-pharm Technology Co., Ltd., Shanghai, China utilizing the Illumina HiSeq×10 sequencing platform (Illumina, Santiago, CA, USA). The PE150 (pair-end) sequencing (insert size: 400 bp) was performed with a read length of 150 bp. Three independently prepared DNA samples were used for each of the V. parahaemolyticus isolates. The positive and negative controls were routinely included in the sequencing run by Shanghai Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China).
Raw sequencing reads were analyzed using the FastQC software ((https://www.bioinformatics.babraham.ac.uk/projects//fastqc/, accessed on 15 March 2022) [22]. Subsequently, high-quality reads were assembled using the SOAP denovo software (version 2.04) [23]. Coding sequences (CDSs), rRNA, and tRNA genes were predicted using the Glimmer software (version 3.02) [24], the Barrnap tool (https://github.com/tseemann/barrnap, accessed on 16 March 2022), and the tRNAscan-SE software (version 2.0) [25], respectively. The cutoff threshold was set as an 80% sequence identity and 90% coverage at E ≤ 1 × 10−5.
The functional attribution of the predicted coding sequences (CDSs) was inferred using the independent Basic Local Alignment Search Tool (BLAST) (https://www.ncbi.nlm.nih.gov/BLAST, accessed on 16 March 2022) against the NCBI (https://www.ncbi.nlm.nih.gov, accessed on 16 March 2022) database of non-redundant proteins and the database of homologous groups (COG) [26]. In cases where the CDS did not exhibit a match with any COG function, it was designated as an unknown protein. The programs were executed using the default parameters.
2.1.3. Comparative Genome Analysis
Genomic islands (GIs), prophages, integrons (Ins), and insertion sequences (ISs) were predicted using the IslandViewer (version 1.2) [27], Phage_Finder [28], Integron_Finder (version 2.0) [29], and ISEScan software (version 1.7.2.1) [30], respectively. To identify virulence-related genes, we referred to the Virulence Factor Database (https://www.mgc.ac.cn/VFs, accessed on 18 September 2022). The BacMet database (http://bacmet.biomedicine.gu.se/, accessed on 18 September 2022), and Antibiotic Resistance Gene Database (http://ardb.cbcb.umd.edu/, accessed on 18 September 2022) were used to predict heavy metal resistance- and antibiotic resistance-related genes, respectively. The JspeciesWS software (http://jspecies.ribohost.com/jspeciesws/, accessed on 18 September 2022) was employed to determine the average nucleotide identity (ANI) values of the genomes.
2.2. Phylogenetic Relationship of the V. parahaemolyticus Isolates
2.2.1. Serotyping and Multi-Locus Sequence Typing (MLST) Analyses
Serotyping and MLST analyses of the V. parahaemolyticus strains were carried out according to the methods described by Yu et al. [31] and González-Escalona et al. [32], respectively.
2.2.2. Phylogenetic Tree Analysis
The complete genome sequences of 64 V. parahaemolyticus strains were downloaded from the GenBank database. Amino acid data sets of single-copy orthologs present in all the V. parahaemolyticus genomes (n = 67) were inferred and aligned using OrthoFinder (version 2.2.6) [33]. Additionally, the FastTree software (version 2.1.11) [34] was used to construct a phylogenetic tree using the same method and parameters described in our previous report [35].
2.3. Antibiotic Resistance of V. parahaemolyticus Isolates
2.3.1. Determination of Minimum Inhibitory Concentrations (MICs) of Antibiotics
V. parahaemolyticus isolates were individually subjected to broth dilution testing (microdilution) following the guidelines by the Clinical and Laboratory Standards Institute (CLSI, M2-A9, 2006, Berwyn, PA, USA). The MICs of antibiotics against the V. parahaemolyticus isolates were determined, including the AMP, KAN, and STR (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). The MIC is defined as the concentration of a drug that inhibits the visible growth of the bacterium being investigated under defined test conditions. Escherichia coli K-12 (Institute of Industrial Microbiology, Shanghai, China) was used as a quality control strain [21].
2.3.2. Antibiotic Stress Conditions
The V. parahaemolyticus isolates were individually inoculated in the TSB medium supplemented with different concentrations of AMP (0 μg/mL, 512 μg/mL, 2048 μg/mL, 50,000 μg/mL, and 100,000 μg/mL, respectively), KAN (0 μg/mL, 32 μg/mL, 64 μg/mL, 128 μg/mL, and 256 μg/mL, respectively), or STR (0 μg/mL, 16 μg/mL, 32 μg/mL, 64 μg/mL, and 128 μg/mL, respectively). Then, the mixture was incubated at 37 °C for 48 h. Bacterial growth was examined using the Bioscreen C automated growth analyzer (Lab Systems, Helsinki, Finland). The standard colony counting method was used to calculate the bacterial survival rates [36].
2.4. Tolerance Mechanisms of the V. parahaemolyticus Isolates to Sub-LCs of Three Antibiotics
2.4.1. Cell Membrane Permeability (CMP) and Fluidity (CMF) and Cell Surface Hydrophobicity (CSH) Analysis
The V. parahaemolyticus strains were individually incubated in the TSB medium to the mid-LGP at 37 °C. A final concentration of AMP (512 μg/mL), KAN (64 μg/mL), or STR (16 μg/mL) was added into the V. parahaemolyticus B2-28, N9-20, and N2-5 culture, respectively, and then incubated at 37 °C for 2 h. The external CMP (ECMP), internal CMP (ICMP), CSH, and CMF assays were performed using the methods described in our recent report [31] and using N-phenyl-1-naphthylamine (NPN, Shanghai Labtop Bio-Technology Co., Ltd., Shanghai, China), O-nitrophenyl-β-D galactopyranoside (ONPG, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), n-hexadecane (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and 1,6-diphenyl-1,3,5-hexatriene (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) as probes, respectively.
2.4.2. Scanning Electron Microscope (SEM) Assay
The SEM assay was conducted in accordance with the previously described technique [37]. Briefly, the sub-LC of AMP (512 μg/mL), KAN (64 μg/mL), or STR (16 μg/mL) was added into V. parahaemolyticus B2-28, N9-20, and N2-5 culture at the mid-LGP, respectively. The mixture was incubated at 37 °C for 2 h. An amount of 1.5 mL of each mixture was then collected, washed, dehydrated, dried, and gold-coated through cathodic spraying. Finally, the samples were observed using a thermal field emission SEM (Hitachi, SU5000, Tokyo, Japan) with accelerating voltages ranging from 5 to 10 kV.
2.4.3. Illumina RNA Sequencing
A final concentration of AMP (512 μg/mL), KAN (64 μg/mL), or STR (16 μg/mL) was added into V. parahaemolyticus B2-28, N9-20, and N2-5 culture at the mid-LGP, respectively, and then incubated at 37 °C for 2 h. The bacterial cells were then collected by centrifugation at 12,000 rpm for 2 min. Then, the RNA extraction and quality control processes, as well as the construction of sequencing libraries, were conducted by Shanghai Majorbio Bio-pharm Technology Co., Ltd. in Shanghai, China using the Illumina HiSeq 2500 platform (Illumina, Santiago, CA, USA). To ensure data accuracy, three replicates were performed for every sample.
Gene expression was determined using the RNA-Seq analysis tool called Expectation–Maximization (RSEM, accessible at http://deweylab.github.io/RSEM/, accessed on 11 December 2022). Genes meeting the criteria of fold changes ≥2.0 or ≤0.5 and p-values < 0.05 were classified as differentially expressed genes (DEGs) compared to the untreated control. A gene set enrichment analysis (GSEA) was performed if the enrichment test p-values were less than 0.05 [38].
The expression of representative DEGs was analyzed using real-time reverse transcription PCR (RT-qPCR) assay [38]. Oligonucleotide primers for the RT-qPCR assay were synthesized by Sangon Biotech (Shanghai) Co., Ltd. in Shanghai, China (Table S7).
2.5. Data Analysis
The SPSS software (version 22, IBM, Armonk, NY, USA) was used to analyze the data. A one-way analysis of variance was utilized to compare means and sample changes by employing the least-significant difference (LSD) method and homogeneity of variance test with a significance level of p < 0.05. All experiments in this study were performed in triplicate.
3. Results and Discussion
3.1. The Genotypes, Phenotypes, Sequence Types (STs), and Phylogenetic Relatedness of the V. parahaemolyticus Isolates
3.1.1. Genotypes and Phenotypes
V. parahaemolyticus B2-28, N9-20, and N2-5 isolates were recovered from three species of aquatic animals, including R. philippinarum, K. californiense, and O. oratori, respectively [21]. These isolates tested negative for the toxin-encoding genes tdh and trh, but they tested positive for the species-specific gene tlh. All of the isolates were resistant to the antibiotics AMP and STR; V. parahaemolyticus N9-20 and N2-5 isolates were also resistant to KAN; and V. parahaemolyticus N2-5 was resistant to TET as well. Meanwhile, V. parahaemolyticus N9-20 and N2-5 isolates were tolerant to the heavy metal Cd2+, while V. parahaemolyticus B2-28 was tolerant to Zn2+ (Table S1).
3.1.2. Sequence Types
The BLAST analysis of the antigen gene loci reveled that the V. parahaemolyticus N9-20 genome carried O antigen loci orf16/wvcP and specific loci VPBB0234 for K8 polymorphic sites; the V. parahaemolyticus N2-5 and B2-28 genomes carried O antigen loci VP0208 and wvcN/wvdB/wvcP, indicating that the serotype types of V. parahaemolyticus N9-20, N2-5, and B2-28 isolates were O4/O12:K8, O3: KUT, and O7/O11/O12: KUT, respectively [39,40].
The STs of V. parahaemolyticus N9-20, N2-5, and B2-28 isolates were determined by an MLST analysis, and the results show that the V. parahaemolyticus N9-20 and N2-5 isolates belong to ST-1817 and ST-2192, respectively. Notably, the V. parahaemolyticus B2-28 isolate was a new ST type that has not ever been reported.
3.1.3. Phylogenetic Relatedness
Based on the complete genome sequences of 64 V. parahaemolyticus strains, together with the V. parahaemolyticus B2-28, N9-20, and N2-5 genomes obtained in this study, approximately 1291 homologous single-copy amino acid sequences were identified, by which a phylogenetic tree was constructed (Figure 2). Among the strains analyzed, some were isolated from humans (n = 20), the environment (n = 7), aquatic animals (n = 28), and unknown sources (n = 12) from 1951 to 2021 in Asia, Europe, and the Americas (Table S5). The phylogenetic analysis unveiled four distinct groups, namely Groups 1 to 4. Groups 3 and 4 were further subdivided into two subgroups, namely Groups 3a, 3b, 4a, and 4b (Figure 2).
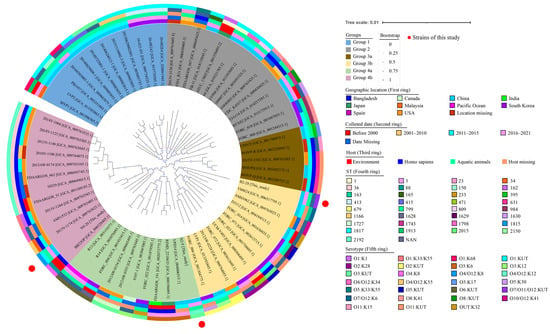
Figure 2.
Genome-wide homologous single-copy gene evolutionary tree with 67 V. parahaemolyticus strains. From the inner circle to the outer circle: isolation location, isolation time, host information, ST type, and serotype of 67 V. parahaemolyticus strains, respectively.
V. parahaemolyticus N9-20 (serotype, O4/O12:K8; ST-1817; GenBank accession no. JALGSD000000000) isolated from K. californiense and V. parahaemolyticus BB22OP (serotype, O4:K8; ST-88; GenBank assembly accession no. GCA_000328405.1) isolated from the environment in Bangladesh in 1980 were placed in Group 4b as they were phylogenetically different from the other V. parahaemolyticus strains. V. parahaemolyticus N2-5 (serotype, O3: KUT; ST-2192; GenBank accession no. JALGSI000000000) isolated from O. oratoria showed the closest distance to V. parahaemolyticus RIMD2210633 (serotype, O3:K6; ST-3; GenBank assembly accession no. GCA_000196095.1) isolated from a human sample, both of which were assigned to Group 4a. V. parahaemolyticus B2-28 (serotype, O7/O11/O12: KUT; ST-NAN; GenBank accession no. JALGSA000000000) isolated from R. philippinarum was assigned to Group 3b together with V. parahaemolyticus CHN25 (serotype, O5:K17; ST-395; GenBank assembly accession no. GCA_001700835.1) isolated from shrimp in Shanghai, China in 2011.
These results demonstrate that the V. parahaemolyticus B2-28, N9-20, and N2-5 isolates originating in edible aquatic animals had considerable genome diversity.
3.2. The Genome Features of the Three V. parahaemolyticus Isolates of Aquatic Animal Origins
3.2.1. General Genome Features
The average nucleotide identity (ANI) values of the three V. parahaemolyticus genomes varied from 98.43% to 98.63%, all surpassing the threshold (94–96%) required for species identification [41]. The draft genome sequences of the three V. parahaemolyticus isolates were determined using the Illumina Hiseq×10 sequencing platform (Figure 3). Approximately 173,337–494,024 clean single reads were obtained. The final assembly yielded 42–324 scaffolds with an average sequencing depth ranging from 235.08-fold to 302.48-fold.
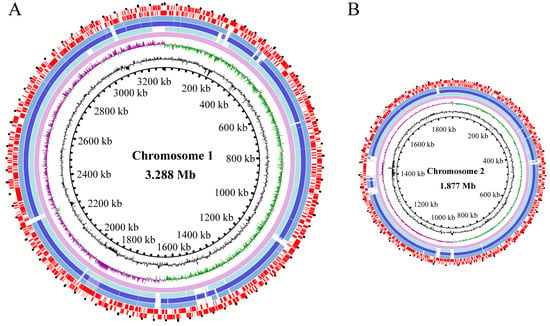
Figure 3.
Genome circle maps of the three V. parahaemolyticus isolates. (A,B) represent the larger and smaller chromosomes of the three V. parahaemolyticus isolates, respectively. Circles from the inside to the outside: the GC contents, the GC skew values (positive in purple and negative in green), the reference genome of V. parahaemolyticus RIMD 2210633 (GenBank accession numbers NC_004603.1 and NC_004605.1), the genomes of V. parahaemolyticus B2-28, N9-20, and N2-5, as well as the CDSs (positioned on the positive and negative strands accordingly), respectively.
The obtained genome sizes ranged from 5.0 Mb to 5.4 Mb with average GC contents ranging from 45.14 to 45.40%. A total of 4709–5610 protein-coding genes were predicted. Among these, approximately 3886–4626 genes were classified into 21 functional catalogues against the COG database. Remarkably, the genes with unknown functions accounted for the highest percentages (17.48–21.17%) (Table 1, Figure 3).

Table 1.
General features of the three V. parahaemolyticus genomes.
The unique 17-mers within the sequencing data of the V. parahaemolyticus N9-20 and N2-5 isolates displayed a clear single peak in frequency. This peak followed a typical Poisson distribution, indicating a lower presence of repetitive DNA in the genomes of V. parahaemolyticus N9-20 and N2-5. Conversely, the V. parahaemolyticus B2-28 genome had a certain degree of heterozygosity with the observed taper at the end of the peak, which suggested relatively more repetitive DNA in its genomes (n = 183) (Figure S1, Table S2). Multiple repetitions were detected towards the terminus of scaffolds (n = 20–183, <1.5 Kb) (Table S2), suggesting that the genome assembly was not fully comprehensive and included numerous gaps. As a result of the limitations of second-generation Illumina short-read sequencing, the unaligned regions between scaffolds consist of recurring sequences [42].
The three V. parahaemolyticus genomes contained various MGEs, including GIs (n = 4–14), prophage gene clusters (n = 0–1), Ins (n = 4–27), and ISs (n = 1–2). This suggests the possibility of HGT, which mediates DNA transmission among the bacterial population, facilitated by these MGEs during the evolution of V. parahaemolyticus genomes. It is worth noting that only one of the identified MGEs (IS002 in V. parahaemolyticus B2-28 genome) was found at the end of one of the scaffolds (Tables S3 and S4), indicating that the assembly of draft genomes did not result in the absence of the identified MGEs (except for IS002).
3.2.2. Putative MGEs
Genomic Islands
GIs are instrumental in shaping the evolutionary trajectory of V. parahaemolyticus genomes as they acquire new biological characteristics via HGT [43]. In this study, 24 GIs (3808–46,379 bp) were identified in the three V. parahaemolyticus genomes, each of which contained 4–14 GIs, carrying 7–49 genes (Figure S2, Table S3).
Interestingly, the genes encoding various functional proteins were found within GIs in three V. parahaemolyticus genomes, e.g., hydrolysis enzymes, chemotaxis proteins, cold shock proteins, stress regulators, transposases, and resistance-related proteins. For example, in the genome of V. parahaemolyticus N2-5, a cold shock protein (Vp_N2-5_0954) was identified in GI3. This protein acts as a global regulator of gene expression and is involved in bacterial growth under various conditions, including adaptation to stress and response to virulence [44].
Notably, there were six identified GIs carrying virulence-related genes, including the GI5 in V. parahaemolyticus N9-20 and GI2, GI4, GI7, GI8, and GI9 in V. parahaemolyticus N2-5. For example, in the V. parahaemolyticus N2-5 genome, approximately 10 virulence-related genes were detected on five GIs (GI2, GI4, GI7, GI8, and GI9), e.g., the Tol–Pal system (TolABQR and Pal, Vp_N2-5_2805, Vp_N2-5_2806, Vp_N2-5_2803, Vp_N2-5_2804, and Vp_N2-5_2807), the haem utilization system (HutZXW, Vp_N2-5_1072, Vp_N2-5_1073, and Vp_N2-5_1074), and the AraC family transcriptional regulators (Vp_N2-5_1706 and Vp_N2-5_3004). Of these, AraC family regulators usually attach to the DNA target and control the virulence of bacteria by detecting small molecule inducers (such as urea, bicarbonate, or cellobiose) that are plentiful at the locations where the bacterial pathogen colonizes and harms its host [45]. The Tol–Pal system is necessary for maintaining the outer membrane integrity of Gram-negative bacteria, functions in cell morphology, sensitivity to bile salts, and bacterial virulence [46]. The haem utilization system plays an important role in bacterial adversity adaptation and pathogenicity [47].
Additionally, there were several GIs carrying phage-related genes, including G2 in V. parahaemolyticus B2-28, GI1 in V. parahaemolyticus N2-5, and GI2 in V. parahaemolyticus N9-20.
Prophages
Prophages play a vital role in shaping the characteristics and disease-causing abilities of bacteria [38,48]. In this study, two prophage gene clusters (17,702–26,733 bp) were identified in V. parahaemolyticus B2-28 and N2-5 genomes, but they were absent from the V. parahaemolyticus N9-20 genome (Figure S3, Table S4). They showed sequence similarity with the Enterobacteria phage N15 (46,375 bp, NCBI accession number: NC_001901) and Enterobacteria phage Mu (36,717 bp, NCBI accession number: NC_000929) in E. coli, respectively. The two prophage homologues contained a total of 64 genes, approximately 60.9% of which encoded unknown proteins.
In 2002, Chamblee et al. sequenced and annotated the complete phage Mu genome that is a temperate phage of a rather wide host range among enteric bacteria [49]. Recently, Xu et al. reported three prophages, namely Vibrio phage K139, Pseudomonas phage D3, and Vibrio phage fs2, in the V. parahaemolyticus L7-7, N1-22, N3-33, N4-46, N8-42, and Q8-15 strains isolated from six species of edible aquatic animals [35]. In this study, our results coupled with those of a previous study [35] that demonstrated prophage diversity in V. parahaemolyticus genomes. V. parahaemolyticus may have acquired phages from different genera via HGT. Notably, in the genome of V. parahaemolyticus N2-5, ABC transporters were derived from the Enterobacteria phage Mu homologue. These transporter proteins utilize ATP to facilitate the absorption of a wide range of molecules, including nutrients, drugs, antibiotics, and a variety of other compounds [50].
Integrons
Ins enable bacteria to acquire, store, and interchange antibiotic resistance genes [51]. Mobile Ins were widely observed in environments where there was a protracted interaction with selective factors like detergents, antibiotics, and heavy metals [52]. In this study, all three V. parahaemolyticus genomes contained Ins (n = 4–27) ranging from 662 bp to 227,599 bp. Among them, there were only two complete Ins and a number of gene cassettes (n = 36) (Figure S4, Table S3).
Interestingly, the Vp_B2-28_2997 gene in In3 of V. parahaemolyticus B2-28 and the Vp_N2-5_0496 gene in In1 of V. parahaemolyticus N2-5 had high sequence identity (89.94% and 99.4%) with the super In IntI4 (NR reference sequence: AHI99301.1) [53]. The super In was initially identified in V. cholerae in 1999, harboring genes associated with both antibiotic resistance and pathogenicity [52,54].
Although approximately 72.35% of integron-carrying genes (n = 369) encode unknown proteins, the identified Ins may have complex and diverse effects on the adaptation of V. parahaemolyticus isolates to their environment and host. For example, genes encoding an antibiotic biosynthesis monooxygenase (Vp_B2-28_2523, In2; Vp_B2-28_5243, In9; Vp_N2-5_5207, In7) were identified in the V. parahaemolyticus B2-28 and N2-5 genomes, and an arginine–aspartate–aspartate (RDD) family protein (Vp_B2-28_5313, In11; Vp_N9-20_4731, In2) was identified in the V. parahaemolyticus B2-28 and N9-20 genomes. Antibiotic biosynthesis monooxygenase was exclusively expressed only in response to arsenic and chromium in Brevibacterium casei [55]. The RDD protein family acts as a unique antiporter for Na+ (Li+, K+)/H+ [56], and it has a crucial function in decreasing the cytoplasmic levels of harmful alkali cations [57].
In addition, virulence-related genes were also observed in Ins in the three V. parahaemolyticus genomes. For example, genes encoding an RelE toxin [58] (Vp_B2-28_4871, In5; Vp_N2-5_5202, In7), a ccdB family protein [59] (ccdB, Vp_N2-5_5091, In4), and a plasmid maintenance protein [59] (ccdA, Vp_N2-5_5092, In4) were identified in the V. parahaemolyticus N2-5 genome. The ccd toxin–antitoxin system plays a role in plasmid preservation and bacterial endurance [59]. Initially discovered in plasmids, toxin–antitoxin gene (e.g., RelE toxin) cassettes play a crucial role in bacterial programmed cell death and assist free-living prokaryotes in adapting to nutritional stress [58].
Insertion Sequences
In Gram-negative bacteria, ISs can transmit multiple genes associated with resistance, ultimately impacting the phenotype of bacterial resistance [60]. In this study, five ISs were identified in the V. parahaemolyticus B2-28, N9-20, and N2-5 genomes (n = 1–2) (Table S3).
The V. parahaemolyticus N2-5 genome had two ISs: an ISVa6 (1079 bp) encoding a IS30 family transposase and an IS110 (1227 bp) encoding an IS110 family transposase.
The V. parahaemolyticus B2-28 genome had two ISs, namely an IS3 (1228 bp) encoding two IS3 family transposases and a partial IS5 (962 bp) encoding an AraC family transcriptional regulator. The latter was also found in the V. parahaemolyticus N9-20 genomes.
Taken together, the identified MGEs (n = 69) in this study carrying many genes may have been important drivers of genome evolution and speciation in V. parahaemolyticus.
3.2.3. Putative Virulence-Associated Genes
Previous studies have indicated that V. parahaemolyticus isolates that do not have the tdh and/or trh genes also exhibit significant cytotoxicity towards human gastrointestinal cells, suggesting the presence of alternative virulence-associated genes [61]. In this study, several putative genes related to virulence (n = 43–45) were identified in the three V. parahaemolyticus genomes (Table S6).
Such genes are involved in bacterial adhesion, colonization, and secretion systems, e.g., 34 genes encoding type III secretion system 1 (T3SS1)-related proteins, katb [62], gmd [63], ilpA [64], gmhA [65], multivalent adhesion molecule 7 (MAM7) [66], and kdsA genes [67]. For example, T3SSs are important determinants of the pathogenicity of V. parahaemolyticus [68]. The ilpA encodes an adhesion and immune stimulator [64], while MAM7 facilitates Gram-negative pathogens in forming strong attachment to host cells during the initial phases of infection, playing a vital role in the transmission of virulence factors to hosts [66]. These results suggest that there are possible health risk in consuming R. philippinarum, K. californiense, and O. oratoria contaminated by V. parahaemolyticus isolates.
3.2.4. Heavy Metal Resistance- and Antibiotic Resistance-Associated Genes
In this study, putative antibiotic resistance genes (n = 7–9) were also identified in the three V. parahaemolyticus genomes (Table 2). All of the genomes contained tuf [69], crp [70], rpoB [71], uhpT [72], tet (34, 35), and β-lactamase (blaCARB-17 and blaCARB-21) genes. Moreover, the acrA gene (Vp_N2-5_2823, GI8) and acrB gene (Vp_N2-5_2824, GI8) were also identified in the V. parahaemolyticus N2-5 genome, which encoded the acriflavine resistance protein A and the multidrug efflux RND transporter permease subunit, respectively. The acrA/B–tolC efflux pumps can confer TET resistance [73], which is consistent with the bacterial TET resistance phenotype.
The BLAST search for heavy metal resistance-associated genes revealed that V. parahaemolyticus B2-28, N9-20, and N2-5 contained the fecE and pfr genes, which were responsible for the tolerance to nickel (Ni), copper (Cu), manganese (Mn), iron (Fe), and cobalt (Co). Notably, the PA0320 gene (Vp_N2_5_0188), which is responsible for the tolerance to Cd2+ and Hg2+, was identified in the V. parahaemolyticus N2-5 genome (Table 2). The gene (Vp_N9-20_4075, GI 6) encoding a short-chain dehydrogenase/reductase SDR family member was identified in V. parahaemolyticus N9-20, which was involved in the response to Cd2+ stress in Pleurotus eryngii [74], which is consistent with the resistance phenotype to the Cd2+ of V. parahaemolyticus N9-20.
Variations in resistance genes, genetic diversity, and environmental pressures [75], can lead to distinctions between resistance phenotypes and genotypes.

Table 2.
The antimicrobial resistance- and heavy metal resistance-associated genes identified in the three V. parahaemolyticus genomes.
Table 2.
The antimicrobial resistance- and heavy metal resistance-associated genes identified in the three V. parahaemolyticus genomes.
| Antibiotic and Heavy Metal | Resistance Gene | V. parahaemolyticus Isolate | Reference |
|---|---|---|---|
| Antibiotic | |||
| Beta-lactamases | blaCARB-17 | B2-28, N9-20, N2-5 | [76] |
| blaCARB-21 | N2-5 | [76] | |
| Elfamycin | tuf | B2-28, N9-20, N2-5 | [69] |
| Fluoroquinolone | crp | B2-28, N9-20, N2-5 | [70] |
| mfd | B2-28 | [77] | |
| gyrA | B2-28 | [78] | |
| Fosfomycin | uhpT | B2-28, N9-20, N2-5 | [72] |
| Peptide, rifamycin | rpoB | B2-28, N9-20, N2-5 | [71] |
| Tetracycline | tet (34) | B2-28, N9-20, N2-5 | [79] |
| tet (35) | B2-28, N9-20, N2-5 | [79] | |
| Heavy metal | |||
| Ni, Co | fecE | B2-28, N9-20, N2-5 | [80] |
| Fe, Cu, Mn | pfr | B2-28, N9-20, N2-5 | [81] |
| Co, Ni, Fe | rcnR/yohL | N2-5 | [81] |
| Cd, Hg | PA0320 | N2-5 | [81] |
3.3. The MICs and Sublethal Concentrations (Sub-LCs) of the Three V. parahaemolyticus Isolates against Antibiotics
The MIC values of the antibiotics against the V. parahaemolyticus B2-28, N9-20, and N2-5 isolates were determined (Table 3). Remarkably, the observed MICs of V. parahaemolyticus B2-28 against AMP and STR were 100,000 μg/mL and 128 μg/mL, respectively; the MIC values of V. parahaemolyticus N9-20 against AMP, KAN, and STR were 50,000 μg/mL, 256 μg/mL, and 128 μg/mL, respectively; the MIC values of V. parahaemolyticus N2-5 against AMP, KAN, and STR were 50,000 μg/mL, 128 μg/mL, and 128 μg/mL, respectively.

Table 3.
The MIC values and sub-LCs of the V. parahaemolyticus isolates against antibiotics.
The growth curves of the V. parahaemolyticus B2-28, N9-20, and N2-5 isolates were determined at different concentrations of AMP, KAN, and STR in the TSB medium (3% NaCl, pH 8.5) at 37 °C (Figure 4). The growth of V. parahaemolyticus B2-28 was completely inhibited at a concentration of 100,000 μg/mL of AMP. The growth of bacteria was inhibited at concentrations of 50,000 μg/mL and 2048 μg/mL of AMP, resulting in extended lag phases of 10 h and 4 h, respectively. The bacterial biomass reached its peak with OD600 values of 1.12 and 1.22 after 44 h and 40 h, respectively. Only a minor decline in growth was observed at 512 μg/mL of AMP when compared to the control group (0 μg/mL of AMP) (Figure 4A). Under the AMP (512 μg/mL) condition, the observed fatality rate of V. parahaemolyticus B2-28 was 10.38% (Table 3).
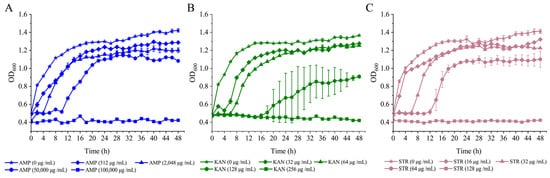
Figure 4.
Survival of V. parahaemolyticus isolates under different concentrations of antibiotics AMP, KAN, and STR; (A–C) V. parahaemolyticus B2-28, N9-20, and N2-5. Three replicates were assessed at each concentration.
At a concentration of KAN of 256 μg/mL, the complete inhibition of V. parahaemolyticus N9-20 was observed (Figure 4B). The bacteria displayed a delayed growth pattern at 128 μg/mL and 64 μg/mL of KAN, with the lag phase being extended to 14 h and 6 h, respectively. Furthermore, the maximum OD600 values were observed at 48 h, reaching 0.91 and 1.25 at 128 μg/mL and 64 μg/mL of KAN, respectively. Only a slight decrease in growth was observed at 64 μg/mL of KAN compared to the control group (0 μg/mL of KAN). Under the KAN (64 μg/mL) condition, V. parahaemolyticus N9-20 showed a fatality rate of 10.02% (Table 3).
The growth of V. parahaemolyticus N2-5 was completely inhibited at a concentration of 128 μg/mL of STR (Figure 4C). Bacterial growth was inhibited at 64 μg/mL and 32 μg/mL of STR with extended lag phases of 12 h and 6 h, respectively. Additionally, the bacterial biomass reached its maximum with OD600 values of 1.10 and 1.25 at 48 h and 30 h, respectively. Growth only slightly decreased at 16 μg/mL of STR compared to the control (0 μg/mL STR). Under the STR (16 μg/mL) condition, the observed mortality rate of V. parahaemolyticus N2-5 was 10.45% (Table 3).
3.4. The Tolerance Mechanisms of V. parahaemolyticus Isolates from Aquatic Animals to the Sub-LCs of the Three Antibiotics
3.4.1. The Cell Membrane Permeability and Fluid and Cell Surface Hydrology of the V. parahaemolyticus Isolates under the Sub-LCs of Antibiotics
External Cell Membrane Permeability
Based on the above results, the effects of the sub-LCs of AMP (512 μg/mL), KAN (64 μg/mL), and STR (16 μg/mL) were further determined on the CMP, CMF, and CSH of the V. parahaemolyticus isolates.
The permeation of antibiotics through the outer membrane of Gram-negative bacteria is facilitated by porin channels [82]. Specifically, hydrophilic molecules like β-lactams, TET, and some fluoroquinolones are highly influenced by the changes in the permeability of the outer membrane, as they rely on porins to cross this barrier [83]. In this study, N-phenyl-1-naphthylamine was used as a probe to detect the external cell membrane permeability.
As shown in Figure 5A, after being treated with AMP (512 μg/mL) for 1.5 h, a consistent decrease in fluorescence intensity was observed. The ECMP of V. parahaemolyticus B2-28 was significantly increased by 1.04- to 1.09-fold when compared with the control group (p < 0.05).
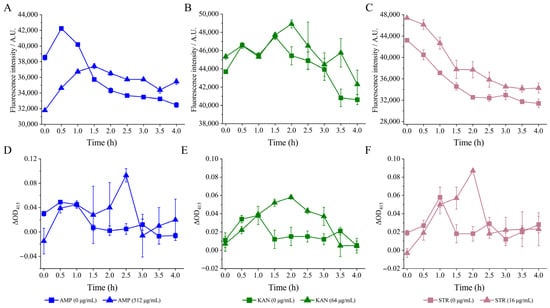
Figure 5.
The changes in the CMP of the three V. parahaemolyticus isolates under AMP (512 μg/mL), KAN (64 μg/mL), and STR (16 μg/mL) stresses, respectively. (A–C) The ECMP of V. parahaemolyticus B2-28, N9-20, and N2-5, respectively. (D–F) The ICMP of V. parahaemolyticus B2-28, N9-20, and N2-5, respectively.
After the treatment of KAN (64 μg/mL), the fluorescence intensity consistently decreased over a period of 4 h. The ECMP of V. parahaemolyticus N9-20 was significantly increased by 1.01- and 1.12-fold at 2 h and 3.5 h compared with the control group (p < 0.05) (Figure 5B).
Similarly, the fluorescence intensity showed an overall downward trend after the treatment with STR (16 μg/mL) for 0 h to 4 h. The ECMP of V. parahaemolyticus N2-5 was significantly increased by 1.05- to 1.16-fold compared with the control group (Figure 5C) (p < 0.05).
Internal Cell Membrane Permeability
In this study, the O-nitrophenyl-β-D galactopyranoside was used as a probe to monitor internal cell membrane permeability. As shown in Figure 5D, no significant difference in the ICMP of V. parahaemolyticus B2-28 was observed after being treated with AMP (512 μg/mL) for 0 h to 2.0 h and for 3.0 h to 4.0 h compared with the control group (p > 0.05). However, its ICMP increased by 18.60-fold at 2.5 h (p < 0.05).
Similarly, no significant difference in the ICMP of V. parahaemolyticus N9-20 was observed after being treated with KAN (64 μg/mL) for 0 h to 1.0 h compared with the control group (p > 0.05) (Figure 5E). However, during the longer treatment time (1.5 to 3.0 h), its CIMP increased by 2.95- to 4.22-fold (p < 0.05).
Likewise, after being treated with STR (16 μg/mL) for 0 h to 1.0 h and for 2.5 h to 4.0 h, the ICMP of V. parahaemolyticus N2-5 showed no significant change compared with the control group (p < 0.05) (Figure 5F). However, at 1.5 to 2.0 h, its ICMP increased by 3.13- and 4.87-fold (p < 0.05).
Cell Surface Hydrology and Cell Membrane Fluid
The CSH plays an important role in bacterial adhesion to abiotic and biotic surfaces as well as penetration into host tissues [84]. As shown in Figure 6A, when compared with the control group, the CSH of V. parahaemolyticus B2-28 was significantly increased by 4.20-fold after the treatment with AMP (512 μg/mL) for 2 h (p < 0.001). In contrast, the treatment with KAN (64 μg/mL) for 2 h significantly decreased the CSH of V. parahaemolyticus N9-20 by 1.89-fold compared with the control group (p < 0.001). Similarly, the treatment with STR (16 μg/mL) for 2 h significantly reduced the CSH of V. parahaemolyticus N2-5 by 1.53-fold compared to the control group (p < 0.05).
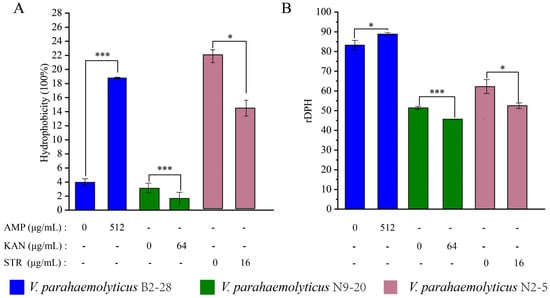
Figure 6.
The changes in the CSH and CMF of the three V. parahaemolyticus isolates under AMP (512 μg/mL), KAN (64 μg/mL), and STR (16 μg/mL) stresses. (A,B) The CSH and CMH of V. parahaemolyticus B2-28, N9-20, and N2-5, respectively. * p < 0.05. *** p < 0.001.
The CMF plays a key role in the action of membrane-active antibiotics [85]. As shown in Figure 6B, when compared with the control group, the CMF of V. parahaemolyticus N9-20 was significantly increased by 1.12-fold (p < 0.001) after the treatment with KAN (64 μg/mL) for 2 h. The treatment with STR (16 μg/mL) for 2 h significantly increased the CMF of V. parahaemolyticus N2-5 by 1.19-fold (p < 0.05). In contrast, the treatment with AMP (512 μg/mL) for 2 h significantly decreased the CMF of V. parahaemolyticus B2-28 by 1.07-fold (p < 0.05).
Taken together, under the sub-LCs of antibiotics, the ECMP, ICMP, CSH, and CMF of the V. parahaemolyticus B2-28, N9-20, and N2-5 isolates were significantly altered (p < 0.05), suggesting that there was a possible change in the bacterial cellular structure.
3.4.2. The Cell Structure Change of the Three V. parahaemolyticus Isolates under the Sub-LCs of Antibiotics
As shown in Figure 7, the control groups displayed intact rod cells with a flat and transparent surface structure, whereas the treatment groups exhibited a slight contraction. Some of the cells in the treatment groups appeared to be deformed, showing folds on their surfaces. For example, when compared with the control groups, after the treatment with AMP (512 μg/mL) for 2 h, the cell surface of V. parahaemolyticus B2-28 shrunk slightly and was subtly depressed (Figure 7A); for V. parahaemolyticus N9-20, the treatment for 2 h using KAN (64 μg/mL) resulted in the slight shrinking in the bacterial cell surface (Figure 7B). A similar case was observed for V. parahaemolyticus N2-5 after the treatment with STR (16 μg/mL) for 2 h (Figure 7C).
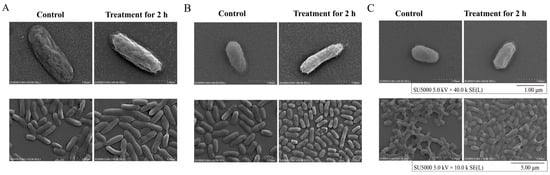
Figure 7.
The SEM observations of the cell surface structure of the V. parahaemolyticus isolates. (A) V. parahaemolyticus B2-28 treated with AMP (512 μg/mL); (B) V. parahaemolyticus N9-20 treated with KAN (64 μg/mL); (C) V. parahaemolyticus N2-5 treated with STR (16 μg/mL) for 2 h at 37 °C (observed by ×40.0 k, and ×10.0 k).
3.4.3. The Differential Transcriptomes of the Three V. parahaemolyticus Isolates Induced by the Sub-LCs of Antibiotics
Based on the obtained results, V. parahaemolyticus B2-28, N9-20, and N2-5 grown at mid-LGP in the TSB medium at 37 °C were treated with AMP (512 μg/mL), KAN (64 μg/mL), and STR (16 μg/mL) for 2 h, respectively, and gene expression changes at the global genome level were investigated using Illumina HiSeq 2500 sequencing technology.
The Major Changed Metabolic Pathways in V. parahaemolyticus B2-28 under AMP Stress
Approximately 16.9% (947 of 5610 genes) of the bacterial genes were differentially expressed in V. parahaemolyticus B2-28 after being treated by the sub-LC of AMP (512 μg/mL) for 2 h compared to the control group. Of these, 123 DEGs showed lower transcriptional levels (fold change ≤ 0.5), while 824 DEGs were up-regulated (fold change ≥ 2.0) (Figure 8A,D). Approximately eight significantly altered metabolic pathways were identified in V. parahaemolyticus B2-28, including sulfur metabolism; fatty acid biosynthesis; methane metabolism; fructose and mannose metabolism (FMM); peptidoglycan biosynthesis; the phosphotransferase system (PTS); glycine, serine, and threonine metabolism (GSTM); and tropane, piperidine, and pyridine alkaloid biosynthesis (TPPAB) (Table S8).
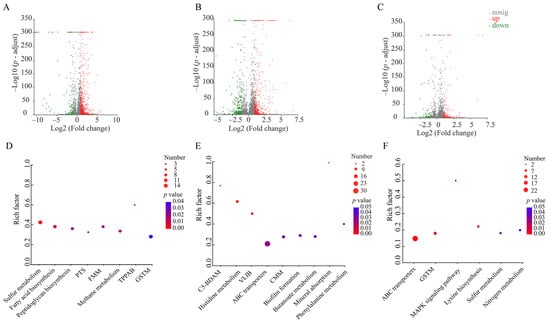
Figure 8.
The major changed metabolic pathways in the three V. parahaemolyticus isolates under AMP (512 μg/mL), KAN (64 μg/mL), and STR (16 μg/mL) stresses. (A–C) The volcano plots of the DEGs in the V. parahaemolyticus B2-28, N9-20, and N2-5 isolates, respectively. (D–F) The significantly altered metabolic pathways in the V. parahaemolyticus B2-28, N9-20, and N2-5 isolates, respectively. PTS, phosphotransferase system; FMM, fructose and mannose metabolism; TPPAB, tropane, piperidine, and pyridine alkaloid biosynthesis; GSTM, serine and threonine metabolism; C5-BDAM, C5-branched dibasic acid metabolism; VLIB, valine, leucine, and isoleucine biosynthesis; CMM, cysteine and methionine metabolism.
For example, remarkably, 14 DEGs in the sulfur metabolism were significantly up-regulated (2.294- to 8.058-fold) in V. parahaemolyticus B2-28 under AMP (512 μg/mL) stress (p < 0.05), including the cysCDNHIJ genes (Vp_B2_28_1508, Vp_B2_28_1509, Vp_B2_28_1513, and Vp_B2_28_2815 to Vp_B2_28_2819) in the assimilation of sulfate. Of these, the cysDN genes were highly up-regulated (6.807 and 8.058-fold) (p < 0.05), which encoded an adenylyl transferase CysD (Vp_B2_28_1508) and a GTPase CysN (Vp_B2_28_1509) [86]. Sulfur metabolism is associated with virulence, antibiotic resistance, and antioxidant defense in Mycobacterium tuberculosis [86].
The DEGs (n = 11) that were related to the FAS II pathway [87,88] in the fatty acid biosynthesis were all significantly up-regulated (2.018- to 2.803-fold) (p < 0.05). Of these, the FabF protein (Vp_B2_28_0092) was extensively proven as the desired focus in Gram-positive bacteria targeted by natural compounds such as platensimycin, platencin, and fasamycin A and B. [87]. The accC (Vp_B2_28_4804) and accA (Vp_B2_28_1017) genes encoded an ACCase transferase subunit alpha and an ACCase biotin carboxylase subunit, respectively. The ACCase subunit is highly conserved in Gram-positive and Gram-negative bacteria, suggesting that it is possible to find ACCase inhibitors with broad-spectrum antibacterial activity [88]. Moreover, the necessity of ACCase activity for bacterial growth and survival has been clearly established [88].
The DEGs (n = 9) in peptidoglycan biosynthesis were significantly up-regulated (2.003- to 2.545-fold) (p < 0.05) in V. parahaemolyticus B2-28. For example, the mrcAB and mrdA genes (Vp_B2_28_2783, Vp_B2_28_0568, and Vp_B2_28_3910) encoded PBP1A family penicillin-binding proteins (PBPs), a PBP1B and a PBP2, respectively. β-lactam antibiotics disrupt the assembly of peptidoglycan within the bacterial cell wall by inhibiting the enzymatic activity of PBPs [89]. In response to the injury of AMP, the murACEG gene (Vp_B2_28_1892) was significantly up-regulated (2.003- to 2.249-fold) (p < 0.05), which encoded an UDP-N-acetylglucosamine 1-carboxyvinyltransferase, an UDP-N-acetylmuramate-L-alanine ligase, an UDP-N-acetylmuramoyl-L-alanyl-D-glutamate-2,6-diaminopimelate ligase, and an undecaprenyldiphospho-muramoylpentapeptide Beta-N-acetylglucosaminyltransferase, which are related to peptidoglycan biosynthesis.
AMP stress also triggered significant changes in the other three metabolic pathways in V. parahaemolyticus B2-28. All DEGs (n = 14) were significantly up-regulated (2.028- to 12.837-fold) in the GSTM (p < 0.05). For instance, the ectABC genes (Vp_B2_28_3088, Vp_B2_28_3089, and Vp_B2_28_3090) encoding a diaminobutyrate acetyltransferase were diaminobutyrate-2-oxoglutarate transaminase genes, and an ectoine synthase in the ectoine biosynthesis in the GSTM was significantly up-regulated (2.028- to 2.065-fold) (p < 0.05). Ectoines, which are extensively employed by microorganisms, are noteworthy compatible solutes. They effectively safeguard protein functionality when faced with diverse challenges, impact membrane fluidity, and stabilize lipid bilayers [90]. This was consistent with the increased CMF of the V. parahaemolyticus isolates treated by AMP in this study.
In the TPPAB, the ldcC genes (Vp_B2_28_3828 and Vp_B2_28_3829) encoding a cadaverine-producing lysine decarboxylase and non-antibiotic-induced endogenous cadaverine were significantly up-regulated (by 3.930- and 7.173-fold) in V. parahaemolyticus B2-28 (p < 0.05), which contributed to its tolerance to β-lactams, fluoroquinolones, and aminoglycosides [91].
Taken together, under the sub-LC of AMP stress, V. parahaemolyticus B2-28 significantly up-regulated the enzymatic activity of PBPs to eliminate AMP’s binding ability and enhanced the assimilation of sulfate, fructose transport, ectoines, and endogenous cadaverine biosynthesis to reduce AMP damage to cells.
The Major Changed Metabolic Pathways in V. parahaemolyticus N9-20 under KAN Stress
Approximately 15.5% (731 of 4705 genes) of the bacterial genes were differentially expressed in V. parahaemolyticus N9-20 after being treated with the sub-LC of KAN (64 μg/mL) for 2 h compared to the control group. Of these, 236 DEGs showed lower transcriptional levels (fold change ≤ 0.5), while 495 DEGs were up-regulated (fold change ≥ 2.0) (Figure 8B,E). Approximately nine significantly altered metabolic pathways were identified in V. parahaemolyticus N9-20, including the C5-branched dibasic acid metabolism (C5-BDAM); histidine metabolism; valine, leucine, and isoleucine biosynthesis (VLIB); mineral absorption; ABC transporters; biofilm formation; cysteine and methionine metabolism (CMM); butanoate metabolism; and phenylalanine metabolism (Table S9).
For example, the expression of 30 DEGs involved in the ABC transporters were all significantly regulated (0.174- to 5.311-fold) (p < 0.05) in V. parahaemolyticus N9-20. For instance, the choXVW (Vp_N9_20_3749, Vp_N9_20_3750, and Vp_N9_20_3751), proXVW (Vp_N9_20_2771, Vp_N9_20_2772, and Vp_N9_20_2773), oppBC (Vp_N9_20_1525, and Vp_N9_20_1526), araHGF (Vp_N9_20_1268, Vp_N9_20_1269, and Vp_N9_20_1270), rbsBC (Vp_N9_20_3775 and Vp_N9_20_3776), and tupABC (Vp_N9_20_2585, Vp_N9_20_2586, and Vp_N9_20_2587) genes were all significantly down-regulated (0.205- to 0.486-fold) (p < 0.05), and they were related to choline, glycine betaine/L-proline, oligopeptide, L-arabinose, ribose, and tungstate, respectively. Of these, OppABCDF are required for the function of the oligopeptide osmotic transporter, two of which (OppB and OppC) are highly hydrophilic integral membrane proteins responsible for mediating peptide passage across the cytoplasmic membrane [92]. This may be related to the reduced CSH of V. parahaemolyticus N9-20 under the sub-LC of KAN (64 μg/mL) in this study. Moreover, the potD, pstS, and livK genes (Vp_N9_20_0227, Vp_N9_20_1054, and Vp_N9_20_3709), encoding a spermidine/putrescine ABC transporter substrate-binding protein, a phosphate ABC transporter substrate-binding protein, and a ABC transporter substrate-binding protein in the periplasmic binding proteins (PPBPs) [93], were also significantly down-regulated (0.174- to 0.380-fold) (p < 0.05). PPBPs serve as receptors for various water-soluble ligands in ATP-binding cassette transport systems; they sense solutes and play key roles in nutrient uptake [94]. Osmolytes play a crucial role in balancing the internal osmolarity of the cell [95]. Various classes of compounds, such as sugars, polyols, free amino acids, amino acid derivatives (e.g., ectoine), and quaternary amines (e.g., glycine betaine, choline, and l-carnitine), are classified as osmolytes [95].
In contrast, most DEGs (n = 22 of 25) were significantly up-regulated in amino acid metabolism (2.010 to 7.057-fold) (p < 0.05), such as the hisABCDFGH(IE) (Vp_N9_20_1730 to Vp_N9_20_1737) and leuABCD (Vp_N9_20_2872 to Vp_N9_20_2875) genes, which were related to L-histidine and L-leucine synthesis, respectively. Also, the cysEK genes (Vp_N9_20_4215 and Vp_N9_20_1922) [96,97] encoding a serine O-acetyltransferase and a PLP-dependent cysteine synthase family protein related to cysteine were significantly up-regulated (2.222 to 2.508-fold) (p < 0.05). The preservation of bacterial cells from redox harm and the development of antibiotic resistance (such as amphotericin B, KAN, and chloramphenicol) are closely tied to cysteine and its associated metabolites [97].
In biofilm formation, six DEGs encoding the type VI secretion system (T6SS) were significantly down-regulated (0.289 to 0.5-fold) (p < 0.05). The T6SS of V. cholerae and Pseudomonas aeruginosa has been shown to be involved in the export of hemolysin-coregulated proteins and valine–glycine repeat proteins [54]. In this study, the T3SS transcriptional regulator ExsA (Vp_N9_20_2745) was significantly up-regulated by 7.082-fold (p < 0.05). The majority of T3SS1 genes require the master regulator ExsA for their expression [98].
Taken together, V. parahaemolyticus N9-20 employed multiple strategies to cope with the sub-LC of KAN (64 μg/mL) stress: (1) it reduced soluble ligand PPBPs and the transmembrane transport of choline, betaine, tungstate, L-arabinose, and ribose; and (2) it decreased carbon source utilization to increase the CMP; (3) it promoted the accumulation of amino acid metabolites, such as L-histidine, L-leucine, glutathione, and cystine, to improve cell survivability.
The Major Changed Metabolic Pathways in V. parahaemolyticus N2-5 under STR Stress
Approximately 5.6% (293 of 5191 genes) of the bacterial genes were differentially expressed in V. parahaemolyticus N2-5 after being treated by the sub-LC of STR (16 μg/mL) for 2 h compared to the control group. Of these, 90 DEGs showed lower transcriptional levels (fold change ≤ 0.5), while 203 DEGs were up-regulated (fold change ≥ 2.0) (Figure 8C,F). Approximately six significantly altered metabolic pathways were identified in V. parahaemolyticus N2-5, including ABC transporters, GSTM, lysine biosynthesis, the MAPK signaling pathway, sulfur metabolism, and nitrogen metabolism (Table S10).
For instance, approximately 22 DEGs involved in ABC transporters were all significantly regulated (0.157- to 5.130-fold) (p < 0.05). For example, the rbsAD (Vp_N2_5_3922 and Vp_N2_5_3923), znuB (Vp_N2_5_1340), malEG (Vp_N2_5_2242 and Vp_N2_5_2244), araH (Vp_N2_5_1948), pstS (Vp_N2_5_0091), and proW (Vp_N2_5_3899) genes encoding ribose, metal, sugar, phosphonate, and choline transporters, respectively, were all significantly up-regulated (2.039- to 3.866-fold) (p < 0.05).
The DEGs (n = 3) in the lysine biosynthesis were also up-regulated (2.156- to 3.935-fold) (p < 0.05), such as the lysC gene (Vp_N2_5_4367) encoding a Lysine-sensitive aspartokinase 3, and the thrA gene (Vp_N2_5_3440) encoding a bifunctional aspartate kinase/homoserine dehydrogenase I. Lysine plays a crucial role in the adaptation and tolerance to environmental stresses in diverse organisms [99].
In terms of energy metabolism, the DEGs (n = 7) involved in sulfur metabolism and nitrogen metabolism were significantly up-regulated (2.03- to 3.044-fold) (p < 0.05), such as the metA gene (Vp_N2_5_3773) encoding a homoserine O-succinyltransferase, the fccA gene (Vp_N2_5_2651) encoding a C-type cytochrome, and the gltBD genes (Vp_N2_5_3431 and Vp_N2_5_3428) encoding a glutamate synthase large and small subunits.
Taken together, V. parahaemolyticus N2-5 employed multiple strategies to cope with the sub-LC of STR (16 μg/mL) stress: (1) it reduced the membrane transport of arginine and the biosynthesis of ectoine (2) and promoted sugar, choline, ribose, and phosphate membrane transport and energy metabolism to reduce STR damage to cells.
Additionally, the expressions of representative DEGs were examined using an RT-PCR assay, and the resulting data were consistent with the transcriptomic analysis (Table S11).
3.4.4. The Molecular Basis Underlying the Antibiotic Tolerance of the V. parahaemolyticus Isolates at the Sub-LCs of the Antibiotics
A comparative transcriptomic analysis revealed nine significantly altered metabolic pathways in V. parahaemolyticus B2-28 under the sub-LC of AMP (512 μg/mL). Remarkably, the DEG (blaCARB-17) encoding carbenicillin-hydrolyzing class A beta-lactamase was highly up-regulated by 6.409-fold (p < 0.05). Interestingly, PBPs (mrcAB and mrdA) in peptidoglycan biosynthesis were significantly up-regulated by 2.003- to 2.472-fold (p < 0.05). In Gram-negative bacteria, the most prevalent mechanism for the development of β-lactam resistance is the production of β-lactamases, followed by altered permeability, the extrusion of efflux pumps, and altered PBPs [100], which is consistent with the mechanism of the β-lactam resistance of V. parahaemolyticus B2-28 in this study. In sulfur metabolism, DEGs participate in the assimilation of sulfate (cysCDNHIJ), and tetrathionate reductase (ttrBCA) was significantly up-regulated (p < 0.05). The DEGs in fructose and mannose metabolism and PTS (fruABK), the ectoine biosynthesis (ectABC) in the GSTM, and the endogenous cadaverine (ldcC, cadA) in the TPPAB were also significantly up-regulated (p < 0.05). These results indicated the increased substance synthesis for energy conservation and stringent response regulation in V. parahaemolyticus B2-28 under AMP (512 μg/mL) stress.
Interestingly, DEGs (fabAFGHV, fadD, and accACD) that participate in the FAS II pathway in the fatty acid biosynthesis were up-regulated in V. parahaemolyticus B2-28 under AMP stress (p < 0.05). These results suggest that AMP may target the FAS II pathway and thus affect cell membrane synthesis.
A comparative transcriptomic analysis revealed nine significantly altered metabolic pathways in V. parahaemolyticus N9-20 under the sub-LC of KAN (64 μg/mL). Remarkably, the DEGs encoding the molecular chaperone DnaK (dnaK), DnaJ (dnaJ), HtpG (htpG), and the Hsp20 family protein (ibpA) were greatly up-regulated by 15.337-, 16.871-, 16.065, and 87.874-fold (p < 0.05), respectively. KAN, a type of aminoglycoside antibiotic, disrupts the process of protein synthesis by binding to the 16S rRNA located in the A site of the 30S ribosomal subunit. This interaction hinders the accurate selection of cognate tRNAs, leading to the synthesis of abnormal proteins [101]. Zhang et al. reported that stress-related proteins (GroS, DnaK, GroL, HtpG, ClpB, HslU, and DnaJ) are hub proteins that significantly increase to reduce the pressure from the misreading of mRNA caused by KAN [102]. In this study, interestingly, hisABCDFGH(IE) in the histidine metabolism, leuABCD in the VLIB, and gshAB and cysEK in the CMM were significantly up-regulated (p < 0.05). The sucCD in the C5-BDAM and PHA (phbBC and atoB) in the butanoate metabolism were significantly regulated (p < 0.05). These results indicate that V. parahaemolyticus N9-20 utilized PHA for carbon storage and promoted the accumulation of amino acid metabolites under KAN (64 μg/mL) stress.
In contrast, all DEGs in the PPBPs (potD, pstS, livG, and livK), choline (choXVW), glycine betaine/L-proline (proXVW), tungstate (tupABC), L-arabinose (araHGF), ribose (rbsBC), and oligopeptide (oppBC) were significantly down-regulated (p < 0.05) in V. parahaemolyticus N9-20 under KAN stress, indicating that water-soluble ligands and PPBPs were reduced to decrease the transmembrane transport of choline, betaine, tungstate, L-arabinose, and ribose.
A comparative transcriptomic analysis revealed six significantly altered metabolic pathways in V. parahaemolyticus N2-5 under the sub-LC of STR (16 μg/mL). Remarkably, the DEGs encoding an efflux RND transporter permease subunit (cusA, Vp_N2_5_1025), an efflux RND transporter periplasmic adaptor subunit (cusB, Vp_N2_5_1024), and a multidrug efflux MFS transporter (Vp_2_5_0473) were up-regulated by 2.241-, 3.568-, and 2.804-fold, respectively (p < 0.05). The MFS efflux family are involved in the transport of anions, drugs (e.g., macrolides and TET), metabolites (e.g., bile salts), and sugars [103]. The DEGs encoding ectoine (ectABC) in the GSTM were significantly down-regulated by 0.394- to 0.434-fold (p < 0.05), suggesting that damage was caused to the cell membrane, resulting in osmotic changes.
The DEGs encoding a homoserine O-succinyltransferase (metA) and a c-type cytochrome (fccA) in the sulfur metabolism and the large (gltB) and small (gltD) subunits of glutamate synthase in the nitrogen metabolism were significantly up-regulated by 2.030- to 3.044-fold (p < 0.05) in V. parahaemolyticus N2-5. In the ABC transporter, the DEGs encoding arginine (artIMP) were significantly down-regulated (p < 0.05). On the other hand, the DEGs encoding ribose (rbsAD), sugar (malEG, araH), phosphate (pstS and afuB), and choline (proW) were significantly up-regulated (p < 0.05). These results suggest the possible altered energy metabolism for the transport of ribose, sugar, choline, and phosphate in V. parahaemolyticus N2-5 under STR stress.
The results of this study offer valuable insights into the adaptation of V. parahaemolyticus to the sub-LCs of AMP, KAN, and STR and facilitate the effective control of this seafood-borne pathogen in edible aquatic animals.
4. Conclusions
In this study, the genome sequences of the three V. parahaemolyticus isolates, namely B2-28, N9-20, and N2-5, were determined (5.0–5.4 Mb), which were isolated from R. philippinarum, K. californiense, and O. oratori, respectively. Approximately 4709–5610 protein-encoding genes were predicted, of which 823–1099 were of unknown functions. Comparative genomic analyses revealed a number of MGEs, including GIs (n = 4–14), prophage gene clusters (n = 0–1), Ins (n = 4–27), and ISs (n = 1–2). Antibiotic-resistant genes (n = 7–9), heavy metal-resistant genes (n = 2–4), and virulence-associated genes (n = 43–45) were also identified in the three V. parahaemolyticus genomes. The V. parahaemolyticus isolates were resistant to the sub-LCs of AMP (512 μg/mL), KAN (64 μg/mL), and STR (16 μg/mL) (p < 0.05). Comparative transcriptomics revealed significantly altered metabolic pathways elicited by the sub-LCs of the antibiotics (p < 0.05), suggesting the existence of multiple strategies for antibiotic tolerance. Overall, the results of this study enrich the V. parahaemolyticus genome data and should be useful for controlling the MDR pathogen worldwide.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/foods13111674/s1, Figure S1: Sequencing depth of three V. parahaemolyticus genomes; Figure S2: The gene organizations of the GIs identified in the three V. parahaemolyticus genomes; Figure S3. A structure diagram of the prophage gene clusters identified in the V. parahaemolyticus genomes; Figure S4. A structure diagram of the INs identified in the three V. parahaemolyticus genomes; Table S1: The features of the V. parahaemolyticus isolates used in this study; Table S2: The identified repeats in the three V. parahaemolyticus genomes; Table S3: The identified GIs, ISs, and Ins in the three V. parahaemolyticus genomes; Table S4: The identified prophages in the three V. parahaemolyticus genomes; Table S5: The genome features of the 67 V. parahaemolyticus strains analyzed in the phylogenetic tree; Table S6: The virulence-associated genes identified in the three V. parahaemolyticus genomes; Table S7: The oligonucleotide primers used in this study; Table S8: The major altered metabolic pathways in V. parahaemolyticus B2-28; Table S9: The major altered metabolic pathways in V. parahaemolyticus N9-20; Table S10: The major altered metabolic pathways in V. parahaemolyticus N2-5; Table S11: The expression of representative DEGs by the RT-PCR assay. References [38,62,63,64,65,66,67,104,105,106,107,108,109] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, L.C.; Investigation, L.Y. and P.Y.; Data Curation, L.Y., P.Y., J.W. and T.Z.; Supervision and Discussion, Y.Z. and Y.P.; Funding Acquisition, L.C.; Writing—Original Draft, L.Y. and P.Y.; Writing—Review and Editing, L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality, grant number 17050502200, and the National Natural Science Foundation of China, grant number 31671946.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The draft genomes of V. parahaemolyticus B2-28, N9-20, and N2-5 are available in the GenBank database under the accession numbers JALGSA000000000, JALGSD000000000, and JALGSI000000000. A complete list of DEGs in the V. parahaemolyticus isolates under AMP, KAN, and STR stresses are available in the NCBI (https://www.ncbi.nlm.nih.gov, accessed on 31 May 2023) and SRA databases under the accession number PRJNA825334. Other data are contained within the article or Supplementary Materials.
Acknowledgments
We would like to express gratitude to Zhengke Shen from Shanghai Ocean University for her help in the data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, L.; Wang, J.; Chen, J.; Zhang, R.; Zhang, H.; Qi, X.; He, Y. Epidemiological characteristics of Vibrio parahaemolyticus outbreaks, Zhejiang, China, 2010–2022. Front. Microbiol. 2023, 14, 1171350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yan, H.; Cui, Z.; Li, H.; Zhou, W.; Liu, Z.; Zhang, H.; Manoli, T.; Mo, H.; Hu, L. Ultrasound-assisted blue light killing Vibrio parahaemolyticus to improve salmon preservation. Ultrason. Sonochemistry 2023, 95, 106389. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Okuno, Y.; Nakada, D.; Aoyama, A.; Fukai, K.; Mukai, T.; Ueho, T. On the bacteriological examination of shirasu-food poisoning. Med. J. Osaka Univ. 1953, 4, 299–304. [Google Scholar]
- Saito, S.; Iwade, Y.; Tokuoka, E.; Nishio, T.; Otomo, Y.; Araki, E.; Konuma, H.; Nakagawa, H.; Tanaka, H.; Sugiyama, K. Epidemiological evidence of lesser role of thermostable direct hemolysin (TDH)–related hemolysin (TRH) than TDH on Vibrio parahaemolyticus pathogenicity. Foodborne Pathog. Dis. 2015, 12, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Saetang, J.; Sukkapat, P.; Palamae, S.; Singh, P.; Senathipathi, D.N.; Buatong, J.; Benjakul, S. Multiplex PCR-lateral flow dipstick method for detection of thermostable direct hemolysin (TDH) producing V. parahaemolyticus. Biosensors 2023, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Yu, Q.; Tang, X.; Zhao, J.; He, X. Prevalence, antibiotic susceptibility and characterization of Vibrio parahaemolyticus isolates in China. FEMS Microbiol. Lett. 2020, 367, fnaa136. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.T.; Hoang, T.T.H.; Fleischmann, S.; Pham, H.N.; Lai, T.L.H.; Cam, T.T.H.; Truong, L.O.; Le, V.P.; Alter, T. Quantification and antimicrobial resistance of Vibrio parahaemolyticus in retail seafood in Hanoi, Vietnam. J. Food Prot. 2022, 85, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Lopatek, M.; Wieczorek, K.; Osek, J. Antimicrobial resistance, virulence factors, and genetic profiles of Vibrio parahaemolyticus from seafood. Appl. Environ. Microbiol. 2018, 84, e00537-18. [Google Scholar] [CrossRef] [PubMed]
- Stratev, D.; Fasulkova, R.; Krumova-Valcheva, G. Incidence, virulence genes and antimicrobial resistance of Vibrio parahaemolyticus isolated from seafood. Microb. Pathog. 2023, 177, 106050. [Google Scholar] [CrossRef]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne pathogenic Vibrios: Antimicrobial resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef]
- Tan, X.; Qiao, J.; Wang, J.; Li, H.; Wang, X. Characterization of ampicillin-resistant genes in Vibrio parahaemolyticus. Microb. Pathog. 2022, 168, 105573. [Google Scholar] [CrossRef]
- Li, Z.; Junaid, M.; Chen, G.; Wang, J. Interactions and associated resistance development mechanisms between microplastics, antibiotics and heavy metals in the aquaculture environment. Rev. Aquac. 2022, 14, 1028–1045. [Google Scholar] [CrossRef]
- Jeong, H.-W.; Kim, J.-a.; Jeon, S.-J.; Choi, S.-S.; Kim, M.-K.; Yi, H.-J.; Cho, S.-J.; Kim, I.-Y.; Chon, J.-W.; Kim, D.-H. Prevalence, antibiotic-resistance, and virulence characteristics of Vibrio parahaemolyticus in restaurant fish tanks in Seoul, South Korea. Foodborne Pathog. Dis. 2020, 17, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Liang, W. The application of the CRISPR-Cas system in antibiotic resistance. Infect. Drug Resist. 2022, 15, 4155–4168. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.E.; Boone, B.E. Next-generation sequencing strategies. Cold Spring Harb. Perspect. Med. 2019, 9, a025791. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Weimer, B.C.; Timme, R.; Lüdeke, C.H.M.; Pettengill, J.B.; Bandoy, D.D.; Weis, A.M.; Kaufman, J.; Huang, B.C.; Payne, J.; et al. Phylogenetic and biogeographic patterns of Vibrio parahaemolyticus strains from North America inferred from whole-genome sequence data. Appl. Environ. Microbiol. 2021, 87, e01403-20. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Duque, A.; Gonzalez-Muñoz, A.; Arboleda-Valencia, J.; Vivas-Aguas, L.J.; Córdoba-Meza, T.; Rodriguez-Rey, G.T.; Díaz-Guevara, P.; Martinez-Urtaza, J.; Wiesner-Reyes, M. Comparative genomics of clinical and environmental Isolates of Vibrio spp. of Colombia: Implications of traits associated with virulence and resistance. Pathogens 2021, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Prithvisagar, K.S.; Krishna Kumar, B.; Kodama, T.; Rai, P.; Iida, T.; Karunasagar, I.; Karunasagar, I. Whole genome analysis unveils genetic diversity and potential virulence determinants in Vibrio parahaemolyticus associated with disease outbreak among cultured Litopenaeus vannamei (Pacific white shrimp) in India. Virulence 2021, 12, 1936–1949. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, X.; Xie, G.; Fu, S.; Zou, P.; Sun, J.; Wang, Y.; Huang, J. Comparative genomic analysis unravels the transmission pattern and intra-species divergence of acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus strains. Mol. Genet. Genom. 2019, 294, 1007–1022. [Google Scholar] [CrossRef]
- Yu, L.H.; Teh, C.S.J.; Yap, K.P.; Ung, E.H.; Thong, K.L. Comparative genomic provides insight into the virulence and genetic diversity of Vibrio parahaemolyticus associated with shrimp acute hepatopancreatic necrosis disease. Infect. Genet. Evol. 2020, 83, 104347. [Google Scholar] [CrossRef]
- Su, C.; Chen, L. Virulence, resistance, and genetic diversity of Vibrio parahaemolyticus recovered from commonly consumed aquatic products in Shanghai, China. Mar. Pollut. Bull. 2020, 160, 111554. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Yang, L.; Shao, Z.; Xie, L.; Chen, L. Identification of salt tolerance-related genes of Lactobacillus plantarum D31 and T9 strains by genomic analysis. Ann. Microbiol. 2020, 70, 10. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. Erratum: SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2015, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA genes in genomic sequences. In Gene Prediction; Kollmar, M., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 1962, pp. 1–14. [Google Scholar] [CrossRef]
- Jensen, L.J.; Julien, P.; Kuhn, M.; von Mering, C.; Muller, J.; Doerks, T.; Bork, P. eggNOG: Automated construction and annotation of orthologous groups of genes. Nucleic Acids Res. 2008, 36, D250–D254. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E. Phage_Finder: Automated identification and classification of prophage regions in complete bacterial genome sequences. Nucleic Acids Res. 2006, 34, 5839–5851. [Google Scholar] [CrossRef] [PubMed]
- Néron, B.; Littner, E.; Haudiquet, M.; Perrin, A.; Cury, J.; Rocha, E.P.C. IntegronFinder 2.0: Identification and analysis of integrons across bacteria, with a focus on antibiotic resistance in Klebsiella. Microorganisms 2022, 10, 700. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, H. ISEScan: Automated identification of insertion sequence elements in prokaryotic genomes. Bioinformatics 2017, 33, 3340–3347. [Google Scholar] [CrossRef]
- Yu, P.; Yang, L.; Wang, J.; Su, C.; Qin, S.; Zeng, C.; Chen, L. Genomic and transcriptomic analysis reveal multiple strategies for the cadmium tolerance in Vibrio parahaemolyticus N10-18 isolated from aquatic animal Ostrea gigas Thunberg. Foods 2022, 11, 3777. [Google Scholar] [CrossRef]
- González-Escalona, N.; Martinez-Urtaza, J.; Romero, J.; Espejo, R.T.; Jaykus, L.A.; DePaola, A. Determination of molecular phylogenetics of Vibrio parahaemolyticus strains by multilocus sequence typing. J. Bacteriol. 2008, 190, 2831–2840. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, C.; Abuín, J.M.; Pichel, J.C. Very Fast Tree: Speeding up the estimation of phylogenies for large alignments through parallelization and vectorization strategies. Bioinformatics 2020, 36, 4658–4659. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Peng, X.; Xie, L.; Chen, L. Survival and genome diversity of Vibrio parahaemolyticus isolated from edible aquatic animals. Diversity 2022, 14, 350. [Google Scholar] [CrossRef]
- Buck, J. The plate count in aquatic microbiology. Nativ. Aquat. Bact. Enumer. Act. Ecol. 1979, 1, 19–28. [Google Scholar]
- Fu, J.; Wang, Y.; Sun, M.; Xu, Y.; Chen, L. Antibacterial activity and components of the methanol-phase extract from rhizomes of pharmacophagous plant Alpinia officinarum Hance. Molecules 2022, 27, 4308. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Yu, P.; Ren, S.; Zhu, Z.; Jin, Y.; Yan, J.; Peng, X.; Chen, L. Prophage-related gene VpaChn25_0724 contributes to cell membrane integrity and growth of Vibrio parahaemolyticus CHN25. Front. Cell. Infect. Microbiol. 2020, 10, 595709. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zeng, W.; Li, Q.; Li, Y.; Wong, N.K.; Jiang, M.; Zuo, L.; Hu, Q.; Li, L. Genetic structure, function, and evolution of capsule biosynthesis loci in Vibrio parahaemolyticus. Front. Microbiol. 2020, 11, 546150. [Google Scholar] [CrossRef]
- Pang, Y.; Guo, X.; Tian, X.; Liu, F.; Wang, L.; Wu, J.; Zhang, S.; Li, S.; Liu, B. Developing a novel molecular serotyping system based on capsular polysaccharide synthesis gene clusters of Vibrio parahaemolyticus. Int. J. Food Microbiol. 2019, 309, 108332. [Google Scholar] [CrossRef]
- Ciufo, S.; Kannan, S.; Sharma, S.; Badretdin, A.; Clark, K.; Turner, S.; Brover, S.; Schoch, C.L.; Kimchi, A.; DiCuccio, M. Using average nucleotide identity to improve taxonomic assignments in prokaryotic genomes at the NCBI. Int. J. Syst. Evol. Microbiol. 2018, 68, 2386–2392. [Google Scholar] [CrossRef]
- Ahsan, M.U.; Liu, Q.; Perdomo, J.E.; Fang, L.; Wang, K. A survey of algorithms for the detection of genomic structural variants from long-read sequencing data. Nat Methods 2023, 20, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wang, Q.; Wang, R.; Zhang, Y.; Lan, R.; He, F.; Yang, Q. Horizontal transfer of antibiotic resistance genes within the bacterial communities in aquacultural environment. Sci. Total Environ. 2022, 820, 153286. [Google Scholar] [CrossRef] [PubMed]
- Muchaamba, F.; Wambui, J.; Stephan, R.; Tasara, T. Cold shock proteins promote nisin tolerance in Listeria monocytogenes through modulation of cell envelope modification responses. Front. Microbiol. 2021, 12, 811939. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Liu, L.; Zhang, Y.; Yang, H.; Yan, Y.; Ding, X.; Han, Y.; Zhou, D.; Yang, R. BfvR, an AraC-family regulator, controls biofilm formation and pH6 antigen production in opposite ways in Yersinia pestis biovar Microtus. Front. Cell. Infect. Microbiol. 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z.; Fei, X.; Tian, Y.; Zhou, G.; Hu, Y.; Wang, S.; Shi, H. The role of TolA, TolB, and TolR in cell morphology, OMVs production, and virulence of Salmonella Choleraesuis. AMB Express 2022, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Fang, Q.J.; Huang, H.Q.; Gong, C.G.; Hu, Y.H. HutZ is required for biofilm formation and contributes to the pathogenicity of Edwardsiella piscicida. Veter Res. 2019, 50, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, J.; Ren, J.; Liang, L.; Li, J.; Niu, S.; Wu, X.; Zhao, Y.; Gao, S.; Yan, F.; et al. RAP44 phage integrase-guided 50K genomic island integration in Riemerella anatipestifer. Front. Veter Sci. 2022, 9, 961354. [Google Scholar] [CrossRef] [PubMed]
- Chamblee, J.S.; Ramsey, J.; Chen, Y.; Maddox, L.T.; Ross, C.; To, K.H.; Cahill, J.L.; Young, R. Endolysin regulation in phage Mu lysis. mBio 2022, 13, e0081322. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, C.; Su, S.; Panmanee, W.; Lau, G.W.; Browne, T.; Cox, K.; Paul, A.T.; Ko, S.H.; Mortensen, J.E.; Lam, J.S.; et al. A Putative ABC transporter permease is necessary for resistance to acidified nitrite and EDTA in Pseudomonas aeruginosa under aerobic and anaerobic planktonic and biofilm conditions. Front. Microbiol. 2016, 7, 291. [Google Scholar] [CrossRef]
- Baltazar, M.; Bourgeois-Nicolaos, N.; Larroudé, M.; Couet, W.; Uwajeneza, S.; Doucet-Populaire, F.; Ploy, M.C.; Da Re, S. Activation of class 1 integron integrase is promoted in the intestinal environment. PLoS Genet. 2022, 18, e1010177. [Google Scholar] [CrossRef]
- An, X.L.; Chen, Q.L.; Zhu, D.; Zhu, Y.G.; Gillings, M.R.; Su, J.Q. Impact of wastewater treatment on the prevalence of integrons and the genetic diversity of integron gene cassettes. Appl. Environ. Microbiol. 2018, 84, e02766-17. [Google Scholar] [CrossRef] [PubMed]
- Mazel, D.; Dychinco, B.; Webb, V.A.; Davies, J. A distinctive class of integron in the Vibrio cholerae genome. Science 1998, 280, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Sarris, P.F.; Zoumadakis, C.; Panopoulos, N.J.; Scoulica, E.V. Distribution of the putative type VI secretion system core genes in Klebsiella spp. Infect. Genet. Evol. 2011, 11, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Damare, S. Cellular response of Brevibacterium casei #NIOSBA88 to arsenic and chromium—A proteomic approach. Braz. J. Microbiol. 2020, 51, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Abdel-Motaal, H.; Chen, J.; Chen, H.; Xu, T.; Meng, L.; Zhang, Z.; Meng, F.; Jiang, J. Characterization of a functionally unknown arginine-aspartate-aspartate family protein from Halobacillus andaensis and functional analysis of its conserved arginine/aspartate residues. Front. Microbiol. 2018, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Wang, L.; Song, N.; Yang, L.; Chen, J.; Yan, M.; Chen, H.; Zhang, R.; Li, J.; Abdel-Motaal, H.; et al. A UPF0118 family protein with uncharacterized function from the moderate halophile Halobacillus andaensis represents a novel class of Na(+)(Li(+))/H(+) antiporter. Sci. Rep. 2017, 7, 45936. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, K.; Christensen, S.K.; Løbner-Olesen, A. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 2005, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Aghera, N.K.; Prabha, J.; Tandon, H.; Chattopadhyay, G.; Vishwanath, S.; Srinivasan, N.; Varadarajan, R. Mechanism of CcdA-Mediated rejuvenation of DNA gyrase. Structure 2020, 28, 562–572.e4. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Alarcón Elvira, F.; Pardío-Sedas, V.T.; Martínez Herrera, D.; Quintana Castro, R.; Oliart Ros, R.M.; López Hernández, K.; Flores Primo, A.; Ramírez Elvira, K. Comparative survival and the cold-induced gene expression of pathogenic and nonpathogenic Vibrio Parahaemolyticus from tropical eastern oysters during cold storage. Int. J. Environ. Res. Public Health 2020, 17, 1836. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Steinman, H.M. Legionella pneumophila catalase-peroxidases: Cloning of the katB gene and studies of KatB function. J. Bacteriol. 1998, 180, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, T.; Langelier, M.F.; Pascal, J.M. Structural and functional analysis of parameters governing tankyrase-1 interaction with telomeric repeat-binding factor 1 and GDP-mannose 4,6-dehydratase. J. Biol. Chem. 2019, 294, 14574–14590. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Y.; Yan, L.; Yang, C.; Wu, Y.R.; Qin, J.L.; Hao, T.Y.; Yang, D.J.; Guo, Y.C.; Pei, X.Y.; Zhao, T.Y.; et al. Population genomics study of Vibrio alginolyticus. Hereditas 2021, 43, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.K.; Wang, C.J.; Chew, Y.; Wang, P.C.; Yin, H.S.; Kao, M.C. Functional characterization of Helicobacter pylori 26695 sedoheptulose 7-phosphate isomerase encoded by hp0857 and its association with lipopolysaccharide biosynthesis and adhesion. Biochem. Biophys. Res. Commun. 2016, 477, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Krachler, A.M.; Ham, H.; Orth, K. Outer membrane adhesion factor multivalent adhesion molecule 7 initiates host cell binding during infection by gram-negative pathogens. Proc. Natl. Acad. Sci. USA 2011, 108, 11614–11619. [Google Scholar] [CrossRef]
- Cesur, M.F.; Siraj, B.; Uddin, R.; Durmuş, S.; Çakır, T. Network-based metabolism-centered screening of potential drug targets in Klebsiella pneumoniae at genome scale. Front. Cell. Infect. Microbiol. 2019, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Meparambu Prabhakaran, D.; Ramamurthy, T.; Thomas, S. Genetic and virulence characterisation of Vibrio parahaemolyticus isolated from Indian coast. BMC Microbiol. 2020, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Che, R.X.; Xing, X.X.; Liu, X.; Qu, Q.W.; Chen, M.; Yu, F.; Ma, J.X.; Chen, X.R.; Zhou, Y.H.; God’Spower, B.O.; et al. Analysis of multidrug resistance in Streptococcus suis ATCC 700794 under tylosin stress. Virulence 2019, 10, 58–67. [Google Scholar] [CrossRef]
- Kary, S.C.; Yoneda, J.R.K.; Olshefsky, S.C.; Stewart, L.A.; West, S.B.; Cameron, A.D.S. The global regulatory cyclic AMP receptor protein (CRP) controls multifactorial fluoroquinolone susceptibility in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 2017, 61, e01666-17. [Google Scholar] [CrossRef]
- Wang, B.W.; Zhu, J.H.; Javid, B. Clinically relevant mutations in mycobacterial LepA cause rifampicin-specific phenotypic resistance. Sci. Rep. 2020, 10, 8402. [Google Scholar] [CrossRef]
- Ortiz-Padilla, M.; Portillo-Calderón, I.; Maldonado, N.; Rodríguez-Martínez, J.; de Gregorio-Iaria, B.; Merino-Bohórquez, V.; Rodríguez-Baño, J.; Pascual, Á.; Docobo-Pérez, F. Role of inorganic phosphate concentrations in in vitro activity of fosfomycin. Clin. Microbiol. Infect. 2022, 28, 302.e1–302.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Mao, D.; Gao, H.; Zheng, L.; Chen, Z.; Gao, Y.; Duan, Y.; Guo, J.; Luo, Y.; Ren, H. Colonization of gut microbiota by plasmid-carrying bacteria is facilitated by evolutionary adaptation to antibiotic treatment. Isme J. 2021, 16, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bao, Z.; Feng, H.; Chen, L.; Li, Q. Nitric oxide enhances resistance of Pleurotus eryngii to cadmium stress by alleviating oxidative damage and regulating of short-chain dehydrogenase/reductase family. Environ. Sci. Pollut. Res. Int. 2022, 29, 53036–53049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Huang, Y.; Liu, P.; Yan, L.; Zhou, Y.; Yang, C.; Wu, Y.; Qin, J.; Guo, Y.; Pei, X.; et al. Population genomics of the food-borne pathogen Vibrio fluvialis reveals lineage associated pathogenicity-related genetic elements. Microb. Genom. 2022, 8, 000769. [Google Scholar] [CrossRef] [PubMed]
- Decré, D.; Arlet, G.; Bergogne-Bérézin, E.; Philippon, A. Identification of a carbenicillin-hydrolyzing beta-lactamase in Alcaligenes denitrificans subsp. xylosoxydans. Antimicrob. Agents Chemother. 1995, 39, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sahin, O.; Barton, Y.W.; Zhang, Q. Key role of Mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLoS Pathog. 2008, 4, e1000083. [Google Scholar] [CrossRef] [PubMed]
- Yakout, M.A.; Ali, G.H. A novel parC mutation potentiating fluoroquinolone resistance in Klebsiella pneumoniae and Escherichia coli clinical isolates. J. Infect. Dev. Ctries. 2022, 16, 314–319. [Google Scholar] [CrossRef] [PubMed]
- El-Razik, K.A.A.; Arafa, A.A.; Hedia, R.H.; Ibrahim, E.S. Tetracycline resistance phenotypes and genotypes of coagulase-negative Staphylococcal isolates from bubaline mastitis in Egypt. Veter World 2017, 10, 702–710. [Google Scholar] [CrossRef]
- Jabeen, S.; Yap, H.Y.; Abdullah, F.F.J.; Zakaria, Z.; Isa, N.M.; Tan, Y.C.; Joo, Y.S.; Satharasinghe, D.A.; Omar, A.R. Complete genome sequence analysis and characterization of selected iron regulation genes of Pasteurella multocida serotype A Strain PMTB2.1. Genes 2019, 10, 81. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Rensing, C.; Kristiansson, E.; Larsson, D.G. BacMet: Antibacterial biocide and metal resistance genes database. Nucleic Acids Res. 2014, 42, D737–D743. [Google Scholar] [CrossRef]
- Bartsch, A.; Llabrés, S.; Pein, F.; Kattner, C.; Schön, M.; Diehn, M.; Tanabe, M.; Munk, A.; Zachariae, U.; Steinem, C. High-resolution experimental and computational electrophysiology reveals weak β-lactam binding events in the porin PorB. Sci. Rep. 2019, 9, 1264. [Google Scholar] [CrossRef]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Evaluation of membrane fluidity of multidrug-resistant isolates of Escherichia coli and Staphylococcus aureus in presence and absence of antibiotics. J. Photochem. Photobiol. B 2018, 181, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Liu, P.; Jin, Y.; Qin, S.; Chen, L. Identification of antibacterial components in the methanol-phase extract from edible herbaceous plant Rumex madaio Makino and their antibacterial action Modes. Molecules 2022, 27, 660. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Vischer, N.O.E.; Strahl, H.; Hamoen, L.W. Assessing membrane fluidity and visualizing fluid membrane domains in bacteria using fluorescent membrane dyes. Bio Protoc. 2018, 8, e3063. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Tang, Q.X.; Britton, W.J.; Leyh, T.S.; Triccas, J.A. The Mycobacterium tuberculosis cysD and cysNC genes form a stress-induced operon that encodes a tri-functional sulfate-activating complex. Microbiology 2004, 150, 1681–1686. [Google Scholar] [CrossRef]
- Espeland, L.O.; Georgiou, C.; Klein, R.; Bhukya, H.; Haug, B.E.; Underhaug, J.; Mainkar, P.S.; Brenk, R. An experimental toolbox for structure-based hit discovery for P. aeruginosa FabF, a promising target for antibiotics. ChemMedChem 2021, 16, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Westling, L.; Peters, E.C.; Harris, J.L.; Trauger, J.W. Cloning, expression, and enzymatic activity of Acinetobacter baumannii and Klebsiella pneumoniae acetyl-coenzyme A carboxylases. Anal. Biochem. 2011, 417, 103–111. [Google Scholar] [CrossRef]
- Lobritz, M.A.; Andrews, I.W.; Braff, D.; Porter, C.B.M.; Gutierrez, A.; Furuta, Y.; Cortes, L.B.G.; Ferrante, T.; Bening, S.C.; Wong, F.; et al. Increased energy demand from anabolic-catabolic processes drives β-lactam antibiotic lethality. Cell Chem. Biol. 2022, 29, 276–286.e4. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.A.; Kobus, S.; Czech, L.; Hoeppner, A.; Zarzycki, J.; Erb, T.J.; Lauterbach, L.; Dickschat, J.S.; Bremer, E.; Smits, S.H.J. The architecture of the diaminobutyrate acetyltransferase active site provides mechanistic insight into the biosynthesis of the chemical chaperone ectoine. J. Biol. Chem. 2020, 295, 2822–2838. [Google Scholar] [CrossRef]
- Akhova, A.; Nesterova, L.; Shumkov, M.; Tkachenko, A. Cadaverine biosynthesis contributes to decreased Escherichia coli susceptibility to antibiotics. Res. Microbiol. 2021, 172, 103881. [Google Scholar] [CrossRef]
- Pearce, S.R.; Mimmack, M.L.; Gallagher, M.P.; Gileadi, U.; Hyde, S.C.; Higgins, C.F. Membrane topology of the integral membrane components, OppB and OppC, of the oligopeptide permease of Salmonella typhimurium. Mol. Microbiol. 1992, 6, 47–57. [Google Scholar] [CrossRef]
- Fukami-Kobayashi, K.; Tateno, Y.; Nishikawa, K. Domain dislocation: A change of core structure in periplasmic binding proteins in their evolutionary history. J. Mol. Biol. 1999, 286, 279–290. [Google Scholar] [CrossRef]
- Kröger, P.; Shanmugaratnam, S.; Ferruz, N.; Schweimer, K.; Höcker, B. A comprehensive binding study illustrates ligand recognition in the periplasmic binding protein PotF. Structure 2021, 29, 433–443.e4. [Google Scholar] [CrossRef]
- Gregory, G.J.; Dutta, A.; Parashar, V.; Boyd, E.F. Investigations of dimethylglycine, glycine betaine, and ectoine uptake by a betaine-carnitine-choline transporter family transporter with diverse substrate specificity in Vibrio Species. J. Bacteriol. 2020, 202, e00314-20. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, C.P.; Encinas, M.V.; Araya, M.A.; Pérez, J.M.; Tantaleán, J.C.; Fuentes, D.E.; Calderón, I.L.; Pichuantes, S.E.; Vásquez, C.C. Biochemical characterization of a thermostable cysteine synthase from Geobacillus stearothermophilus V. Biochimie 2004, 86, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Rosa, B.; Marchetti, M.; Paredi, G.; Amenitsch, H.; Franko, N.; Benoni, R.; Giabbai, B.; De Marino, M.G.; Mozzarelli, A.; Ronda, L.; et al. Combination of SAXS and protein painting discloses the three-dimensional organization of the bacterial cysteine synthase complex, a potential target for enhancers of antibiotic action. Int. J. Mol. Sci. 2019, 20, 5219. [Google Scholar] [CrossRef]
- Getz, L.J.; Thomas, N.A. The Transcriptional regulator HlyU positively regulates expression of exsA, leading to Type III secretion system 1 activation in Vibrio parahaemolyticus. J. Bacteriol. 2018, 200, e00653-17. [Google Scholar] [CrossRef]
- Isogai, S.; Takagi, H. Enhancement of lysine biosynthesis confers high-temperature stress tolerance to Escherichia coli cells. Appl. Microbiol. Biotechnol. 2021, 105, 6899–6908. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef]
- Carter, A.P.; Clemons, W.M.; Brodersen, D.E.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 2000, 407, 340–348. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Wang, G.; Yao, J.; Wei, J.; Liu, Z.; Lin, X.; Liu, Y. Quantitative proteomics reveals the antibiotics adaptation mechanism of Aeromonas hydrophila under kanamycin stress. J. Proteom. 2022, 264, 104621. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Almuhaideb, E.; Chintapenta, L.K.; Abbott, A.; Parveen, S.; Ozbay, G. Assessment of Vibrio parahaemolyticus levels in oysters (Crassostrea virginica) and seawater in Delaware Bay in relation to environmental conditions and the prevalence of molecular markers to identify pathogenic Vibrio parahaemolyticus strains. PLoS ONE 2020, 15, e0242229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Y.; Feng, J.; Guo, Z.; Chen, H.; Wang, B.; Hu, J.; Lin, Z.; Su, Y. Functional characterization of VscCD, an important component of the type III secretion system of Vibrio harveyi. Microb. Pathog. 2021, 157, 104965. [Google Scholar] [CrossRef] [PubMed]
- Abushattal, S.; Vences, A.; Osorio, C.R. A virulence gene typing scheme for Photobacterium damselae subsp. piscicida, the causative agent of fish photobacteriosis, reveals a high prevalence of plasmid-encoded virulence factors and of type III secretion system genes. Aquaculture 2020, 521, 735057. [Google Scholar] [CrossRef]
- Akeda, Y.; Okayama, K.; Kimura, T.; Dryselius, R.; Kodama, T.; Oishi, K.; Iida, T.; Honda, T. Identification and characterization of a type III secretion-associated chaperone in the type III secretion system 1 of Vibrio parahaemolyticus. FEMS Microbiol Lett 2009, 296, 18–25. [Google Scholar] [CrossRef]
- Tam, V.C.; Suzuki, M.; Coughlin, M.; Saslowsky, D.; Biswas, K.; Lencer, W.I.; Faruque, S.M.; Mekalanos, J.J. Functional analysis of VopF activity required for colonization in Vibrio cholerae. mBio 2010, 1, e00289-10. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Qi, X.; Zhou, B.; Wang, J.; Chen, Y.; Zhang, H. Epidemiology of foodborne disease outbreaks caused by Vibrio parahaemolyticus during 2010–2014 in Zhejiang Province, China. Food Control 2017, 77, 110–115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).