Preparation of Calcium–Binding Peptides Derived from Mackerel (Scomber japonicus) Protein and Structural Characterization and Stability Analysis of Its Calcium Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MPs
2.3. Determination of DH
2.4. Analysis of MP Characterization
2.4.1. Calcium-Binding Capacity Assay
2.4.2. Determination of MW Distribution
2.4.3. Zeta Potential Measurement
2.4.4. Analysis of Amino Acid (AA) Composition
2.5. Preparation of MP–Calcium Complexes
2.6. Structural Characterization of MPs and MP–Calcium Complexes
2.6.1. Fluorescence Spectroscopy
2.6.2. Fourier Transform Infrared (FTIR) Measurement
2.6.3. Atomic Force Microscopy (AFM)
2.6.4. Scanning Electron Microscopy (SEM) and Energy Dispersion Spectrum (EDS)
2.7. Bio-Accessibility Measurement of the MP–Calcium Complexes
2.7.1. Calcium Solubility Assay in Different Temperature Conditions
2.7.2. Calcium Solubility Assay under Simulated GI Digestion
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the MPs
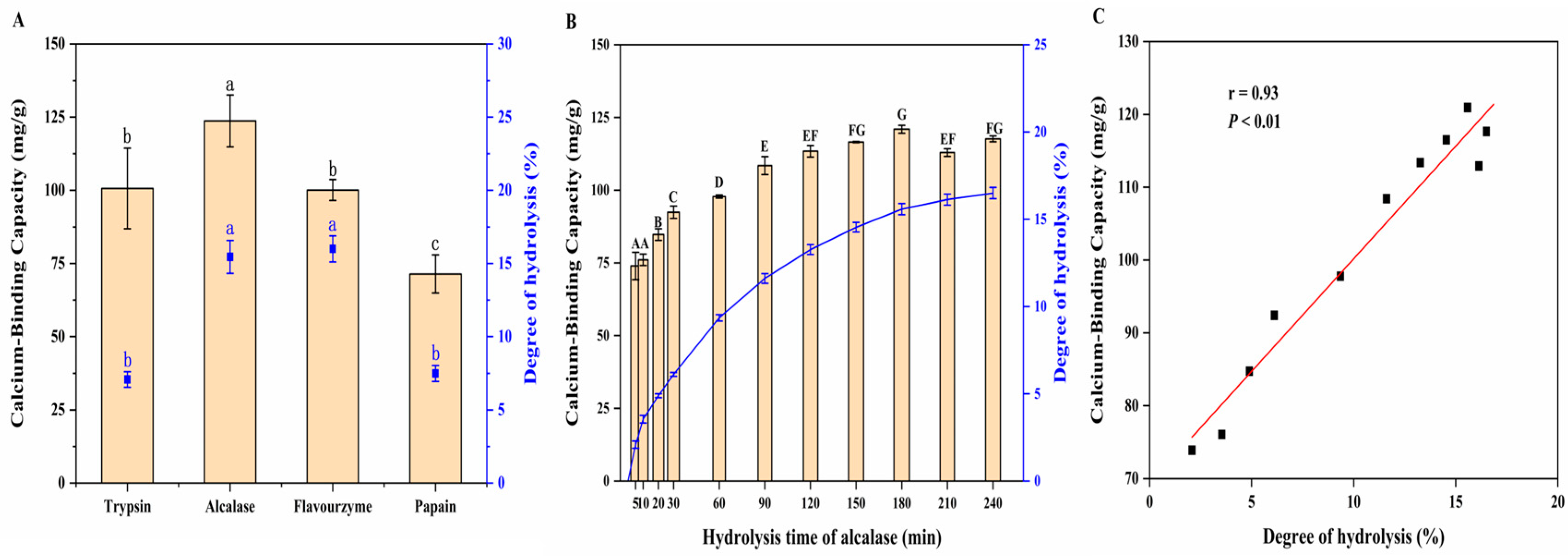
3.1.1. Effect of Proteases on DH and Calcium-Binding Capacity of MPs
3.1.2. Effect of Hydrolysis Time on Calcium-Binding Capacity of MPs
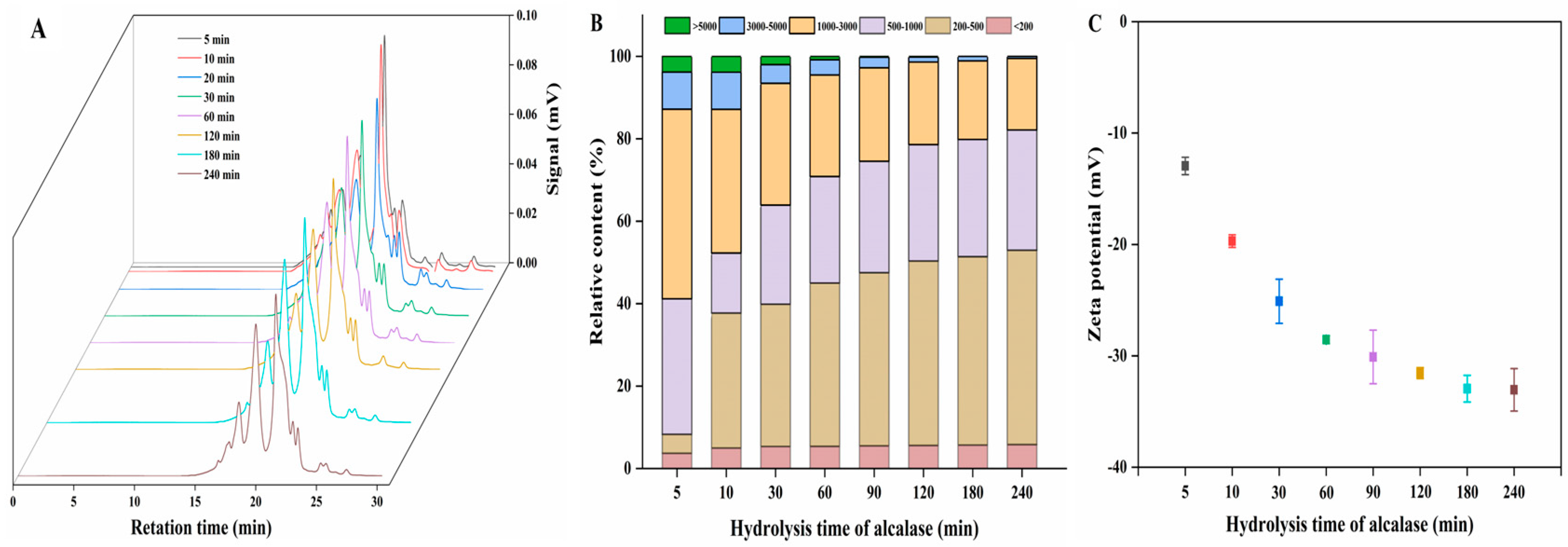
3.1.3. MW Distribution
3.1.4. Zeta Potential Analysis
3.1.5. Amino Acid Composition Analysis
3.2. Structural Characterization of MP–Calcium Complexes
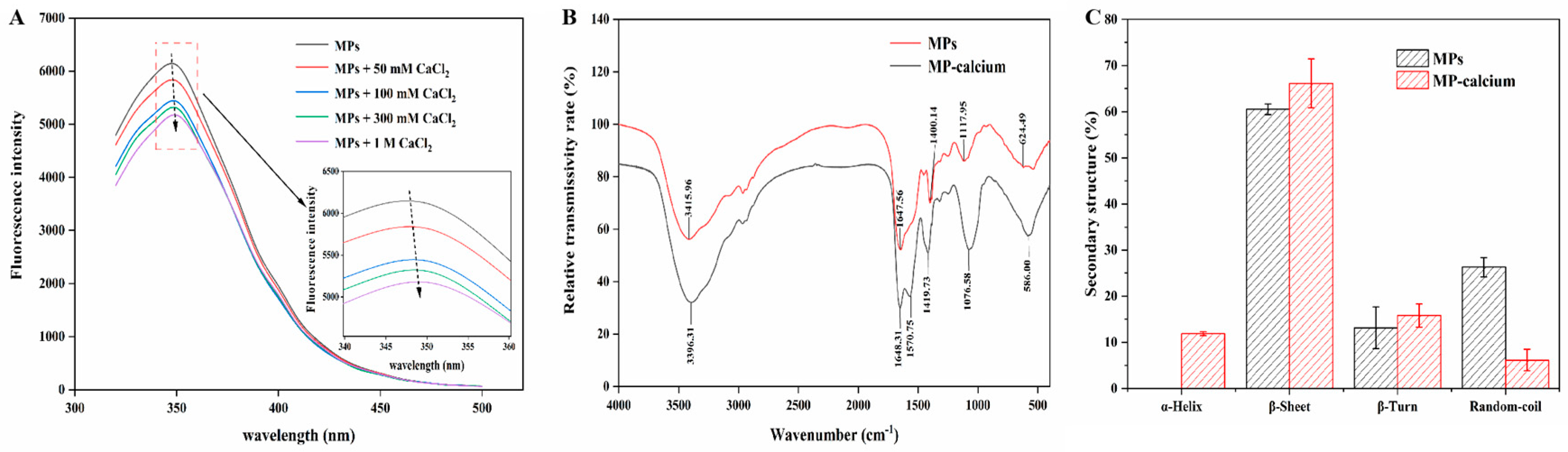
3.2.1. Fluorescence Spectroscopy
3.2.2. FTIR Spectroscopy
3.3. Microstructure Analysis of MP–Calcium Complexes
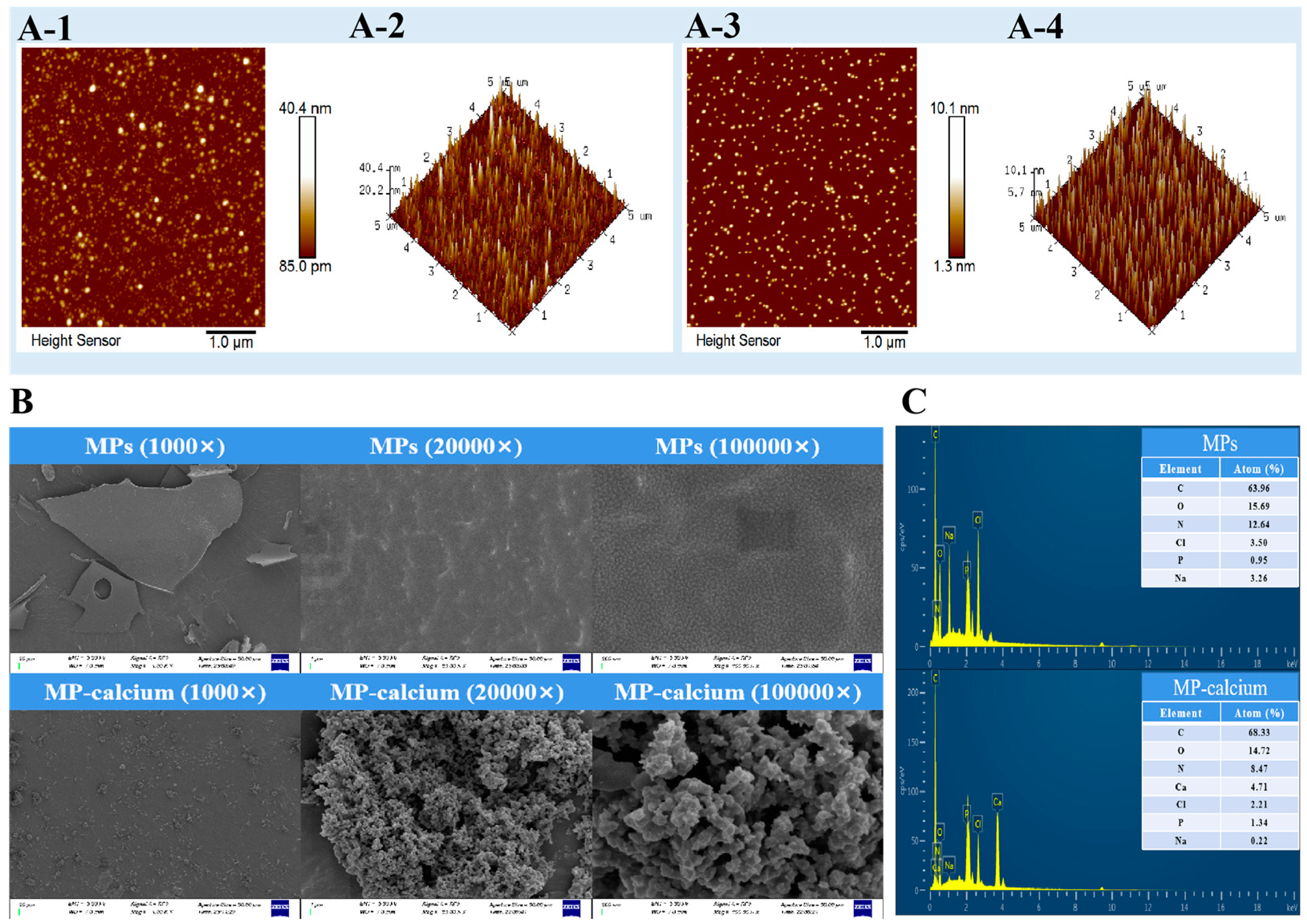
3.3.1. AFM
3.3.2. SEM and EDS Findings
3.4. Bio-Accessibility Measurement of the MP–Calcium Complexes
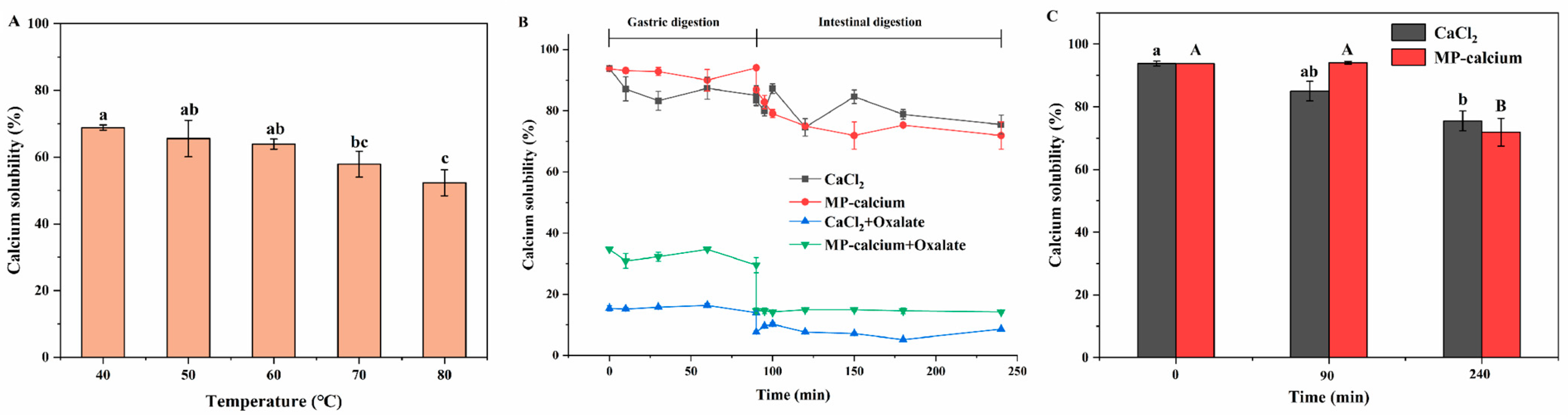
3.4.1. Stability Analysis of MP–Calcium Complexes at Different Temperatures
3.4.2. Stability Analysis of MP–Calcium Complexes under GI Digestion In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Zhao, L.; Shen, Q.; Qi, L.; Jiang, S.; Guo, Y.; Zhang, C.; Richel, A. Preparation of cattle bone collagen peptides-calcium chelate and its structural characterization and stability. LWT 2021, 144, 111264. [Google Scholar] [CrossRef]
- Su, J.; Chen, T.; Liao, D.; Wang, Y.; Su, Y.; Liu, S.; Chen, X.; Ruifang, Q.; Jiang, L.; Liu, Z. Novel peptides extracted from Muraenesox cinereus bone promote calcium transport, osteoblast differentiation, and calcium absorption. J. Funct. Foods 2022, 95, 105157. [Google Scholar] [CrossRef]
- Ke, H.; Ma, R.; Liu, X.; Xie, Y.; Chen, J. Highly effective peptide-calcium chelate prepared from aquatic products processing wastes: Stickwater and oyster shells. LWT 2022, 168, 113947. [Google Scholar] [CrossRef]
- Sun, X.; Sarteshnizi, R.A.; Boachie, R.T.; Okagu, O.D.; Abioye, R.O.; Pfeilsticker Neves, R.; Ohanenye, I.C.; Udenigwe, C.C. Peptide-Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods 2020, 9, 1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, K. Calcium supplements and structure-activity relationship of peptide-calcium chelates: A review. Food Sci. Biotechnol. 2022, 31, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, F.; Cai, X.; Wang, S. Glycosylated peptide-calcium chelate: Characterization, calcium absorption promotion and prebiotic effect. Food Chem. 2022, 403, 134335. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Duan, S.; Yuan, P.; Liu, J.; Wang, X.; Liu, Y.; Peng, Y.; Pan, C.; Xia, K. Preparation of casein phosphopeptides calcium complex and the promotion in calcium cellular uptake through transcellular transport pathway. J. Food Biochem. 2021, 45, e14001. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wu, H.; Du, M.; Tang, Y.; Liu, H.; Fu, Y.; Zhu, B. Food protein-derived calcium chelating peptides: A review. Trends Food Sci. Technol. 2016, 58, 140–148. [Google Scholar] [CrossRef]
- Chen, D.; Mu, X.; Huang, H.; Nie, R.; Liu, Z.; Zeng, M. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods 2014, 6, 575–584. [Google Scholar] [CrossRef]
- Chen, M.; Ji, H.; Zhang, Z.; Zeng, X.; Su, W.; Liu, S. A novel calcium-chelating peptide purified from Auxis thazard protien hydrolysate and its binding properties with calcium. J. Funct. Foods 2019, 60, 103447. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, H.; Guo, Y.; Zhang, C.; Xu, Y. A novel calcium-binding peptide from bovine bone collagen hydrolysate and chelation mechanism and calcium absorption activity of peptide-calcium chelate. Food Chem. 2023, 410, 135387. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Lin, S.; Han, W.; Jiang, P.; Zhu, B.; Sun, N. Calcium Delivery System Assembled by a Nanostructured Peptide Derived from the Sea Cucumber Ovum. J. Agric. Food Chem. 2019, 67, 12283–12292. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdes, J.A.; Rodriguez-Herrera, R.; Chavez-Gonzalez, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, Y.; Taga, M.; Yukami, R.; Watanabe, C.; Furuichi, S.; Andersen, K. Intra- and inter-specific density dependence of body condition, growth, and habitat temperature in chub mackerel (Scomber japonicus). ICES J. Mar. Sci. 2021, 78, 3254–3264. [Google Scholar] [CrossRef]
- Motta, C.; Rego, A.; Cardoso, C.; Coelho, I.; Gomes-Bispo, A.; Afonso, C.; Prates, J.A.M.; Castanheira, I.; Bandarra, N.M. Seasonality as experienced in the market and the resulting variation in the amino acid and elemental composition of chub mackerel (Scomber colias). J. Food Compos. Anal. 2021, 104, 104151. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.; Jin, Z.; Wu, H.; Wang, Y.; Lin, S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017, 230, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Sun, N.; Jiang, P.; Wang, D.; Lin, S. Optimised condition for preparing sea cucumber ovum hydrolysate-calcium complex and its structural analysis. Int. J. Food Sci. Technol. 2017, 52, 1914–1922. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.; Lin, S.; Yu, C.; Tang, Y.; Wei, Y.; Xiong, Y.; Wu, H. Characterization of sea cucumber (Stichopus japonicus) ovum hydrolysates: Calcium chelation, solubility and absorption into intestinal epithelial cells. J. Sci. Food Agric. 2017, 97, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, L.; Wang, X.; Guo, Y.; Liu, J.; Xu, Y.; Liu, C.; Zhang, C.; Richel, A. Preparation of a cattle bone collagen peptide-calcium chelate by the ultrasound method and its structural characterization, stability analysis, and bioactivity on MC3T3-E1 cells. Food Funct. 2023, 14, 978–989. [Google Scholar] [CrossRef]
- Qu, W.; Feng, Y.; Xiong, T.; Li, Y.; Wahia, H.; Ma, H. Preparation of corn ACE inhibitory peptide-ferrous chelate by dual-frequency ultrasound and its structure and stability analyses. Ultrason. Sonochem. 2022, 83, 105937. [Google Scholar] [CrossRef]
- Qu, W.; Li, Y.; Xiong, T.; Feng, Y.; Ma, H.; Dzidzorgbe Kwaku Akpabli-Tsigbe, N. Calcium-chelating improved zein peptide stability, cellular uptake, and bioactivity by influencing the structural characterization. Food Res. Int. 2022, 162, 112033. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, I.B.; Kelly, P.M.; Murray, B.A.; FitzGerald, R.J.; Brodkorb, A. Molecular characterization of whey protein hydrolysate fractions with ferrous chelating and enhanced iron solubility capabilities. J. Agric. Food Chem. 2015, 63, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Zhao, H.; Zhao, R.; Xu, Y.; Lyu, X. Preparation of grape seed polypeptide and its calcium chelate with determination of calcium bioaccessibility and structural characterisation. Int. J. Food Sci. Technol. 2020, 56, 166–177. [Google Scholar] [CrossRef]
- Hu, G.; Wang, D.; Su, R.; Corazzin, M.; Liu, X.; Sun, X.; Dou, L.; Liu, C.; Yao, D.; Sun, L.; et al. Calcium-binding capacity of peptides obtained from sheep bone and structural characterization and stability of the peptide-calcium chelate. J. Food Meas. Charact. 2022, 16, 4934–4946. [Google Scholar] [CrossRef]
- He, J.; Guo, H.; Zhang, M.; Wang, M.; Sun, L.; Zhuang, Y. Purification and Characterization of a Novel Calcium-Binding Heptapeptide from the Hydrolysate of Tilapia Bone with Its Osteogenic Activity. Foods 2022, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, M.; Zhang, L.; Law, C.L.; Wang, Y.; Liu, K. Preparation of enzymatic hydrolysate using edible fungi by-products of soup seasoning: Effect of different enzymes on enzymatic hydrolysis. Food Biosci. 2022, 49, 101844. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Yang, T.; Zheng, D.; Liu, X.; Zhang, J.; Zheng, M. Preparation of immobilized Alcalase based on metal affinity for efficient production of bioactive peptides. LWT 2022, 162, 113505. [Google Scholar] [CrossRef]
- Karami, Z.; Butkinaree, C.; Yingchutrakul, Y.; Simanon, N.; Duangmal, K. Comparative study on structural, biological and functional activities of hydrolysates from Adzuki bean (Vigna angularis) and mung bean (Vigna radiata) protein concentrates using Alcalase and Flavourzyme. Food Res. Int. 2022, 161, 111797. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Wang, X.-P.; Guo, X.-N. Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J. Funct. Foods 2015, 12, 23–32. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhao, Y.; Zeng, M. Enzymatic preparation and characterization of iron-chelating peptides from anchovy (Engraulis japonicus) muscle protein. Food Res. Int. 2012, 48, 435–441. [Google Scholar] [CrossRef]
- Bao, X.; Yuan, X.; Feng, G.; Zhang, M.; Ma, S. Structural characterization of calcium-binding sunflower seed and peanut peptides and enhanced calcium transport by calcium complexes in Caco-2 cells. J. Sci. Food Agric. 2021, 101, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Blank-Shim, S.A.; Scheifele, I.; Fraga-Garcia, P.; Berensmeier, S. Peptide binding to metal oxide nanoparticles. Faraday Discuss. 2017, 204, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Kelimu, A.; Korma, S.A.; Cacciotti, I.; Xiang, H.; Cui, C. Novel iron-chelating peptide from egg yolk: Preparation, characterization, and iron transportation. Food Chem. X 2023, 18, 100692. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Qian, Y.; Fan, X.; Cao, H.; Tian, Y.; Chen, L. Nutritional Properties of Europen-Eel (Anguilla anguilla) Bone Peptide-calcium and Its Apoptosis Effect on Caco-2 cells. Food Sci. Hum. Wellness 2022, 11, 1482–1490. [Google Scholar] [CrossRef]
- Wang, T.; Lin, S.; Cui, P.; Bao, Z.; Liu, K.; Jiang, P.; Zhu, B.; Sun, N. Antarctic krill derived peptide as a nanocarrier of iron through the gastrointestinal tract. Food Biosci. 2020, 36, 100657. [Google Scholar] [CrossRef]
- Ramesh, L.; Latha, B.V.; Kumar Mukunda, C. Identification and characterization of metal-chelating bioenhancer peptide derived from fermented Citrullus lanatus seed milk. J. Food Biochem. 2022, 46, e14102. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, Y.; Zhang, X.; Li, Y.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Chen, Z. Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chem. 2018, 239, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Zhang, P.; Sun, N.; Lin, S. Elucidating the Calcium-Binding Site, Absorption Activities, and Thermal Stability of Egg White Peptide-Calcium Chelate. Foods 2021, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lan, Y.; Liao, W.; Lin, L.; Liu, G.; Xu, H.; Xue, J.; Guo, B.; Cao, Y.; Miao, J. Preparation, characterization and biological activities of egg white peptides-calcium chelate. LWT 2021, 149, 112035. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Q.; Li, M.; Liu, H.; Wang, Q.; Wu, Y.; Niu, L.; Liu, Z. Isolation of a novel calcium-binding peptide from phosvitin hydrolysates and the study of its calcium chelation mechanism. Food Res. Int. 2021, 141, 110169. [Google Scholar] [CrossRef]
- Wang, X.; Gao, A.; Chen, Y.; Zhang, X.; Li, S.; Chen, Y. Preparation of cucumber seed peptide-calcium chelate by liquid state fermentation and its characterization. Food Chem. 2017, 229, 487–494. [Google Scholar] [CrossRef] [PubMed]
| AAs | MPs | MP–Calcium | ||
|---|---|---|---|---|
| Content (g/100 g) | Relative Content (%) | Content (g/100 g) | Relative Content (%) | |
| Asp + Asn | 6.02 | 8.58 | 9.86 | 14.82 |
| Thr | 2.67 | 4.52 | 3.25 | 4.88 |
| Ser | 2.60 | 3.70 | 3.01 | 4.52 |
| Glu + Gln | 8.63 | 12.29 | 14.84 | 22.30 |
| Gly | 3.27 | 4.64 | 3.25 | 4.90 |
| Ala | 3.67 | 5.23 | 2.63 | 3.97 |
| Cys | 0.63 | 0.89 | 0.63 | 0.93 |
| Val | 4.39 | 6.25 | 2.10 | 3.17 |
| Met | 5.42 | 7.72 | 2.60 | 3.94 |
| Ile | 5.42 | 7.72 | 3.89 | 5.86 |
| Leu | 6.52 | 9.28 | 3.50 | 5.29 |
| Tyr | 2.67 | 3.80 | 2.07 | 3.09 |
| Phe | 2.95 | 4.19 | 1.81 | 2.71 |
| His | 2.92 | 4.16 | 2.35 | 3.54 |
| Lys | 5.94 | 8.46 | 4.99 | 7.51 |
| Arg | 2.46 | 3.50 | 2.77 | 4.14 |
| Pro | 3.56 | 5.07 | 9.86 | 4.44 |
| Negatively charged AAs | 14.65 | 20.87 | 24.70 | 37.12 |
| Positively charged AAs | 11.32 | 16.12 | 10.10 | 15.19 |
| Hydrophobic AAs | 31.91 | 45.45 | 22.72 | 34.27 |
| Essential AAs | 33.80 | 48.14 | 22.14 | 33.36 |
| Aromatic AAs | 5.61 | 7.99 | 3.87 | 5.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, P.; Liang, J.; Cheng, T.; Zhang, J. Preparation of Calcium–Binding Peptides Derived from Mackerel (Scomber japonicus) Protein and Structural Characterization and Stability Analysis of Its Calcium Complexes. Foods 2024, 13, 1652. https://doi.org/10.3390/foods13111652
Cui P, Liang J, Cheng T, Zhang J. Preparation of Calcium–Binding Peptides Derived from Mackerel (Scomber japonicus) Protein and Structural Characterization and Stability Analysis of Its Calcium Complexes. Foods. 2024; 13(11):1652. https://doi.org/10.3390/foods13111652
Chicago/Turabian StyleCui, Pengbo, Jianqin Liang, Tianyu Cheng, and Jianyou Zhang. 2024. "Preparation of Calcium–Binding Peptides Derived from Mackerel (Scomber japonicus) Protein and Structural Characterization and Stability Analysis of Its Calcium Complexes" Foods 13, no. 11: 1652. https://doi.org/10.3390/foods13111652
APA StyleCui, P., Liang, J., Cheng, T., & Zhang, J. (2024). Preparation of Calcium–Binding Peptides Derived from Mackerel (Scomber japonicus) Protein and Structural Characterization and Stability Analysis of Its Calcium Complexes. Foods, 13(11), 1652. https://doi.org/10.3390/foods13111652






