The Effects of Crataegus pinnatifida and Wolfiporia extensa Combination on Diet-Induced Obesity and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experimental Design
2.3. GTT and ITT
2.4. Biochemical Analysis
2.5. Histological Analysis
2.6. 16S rRNA Gene Sequencing and Analyses
2.7. Statistic Analysis
3. Results
3.1. Effects of CP and WE on HFD-Induced Obesity
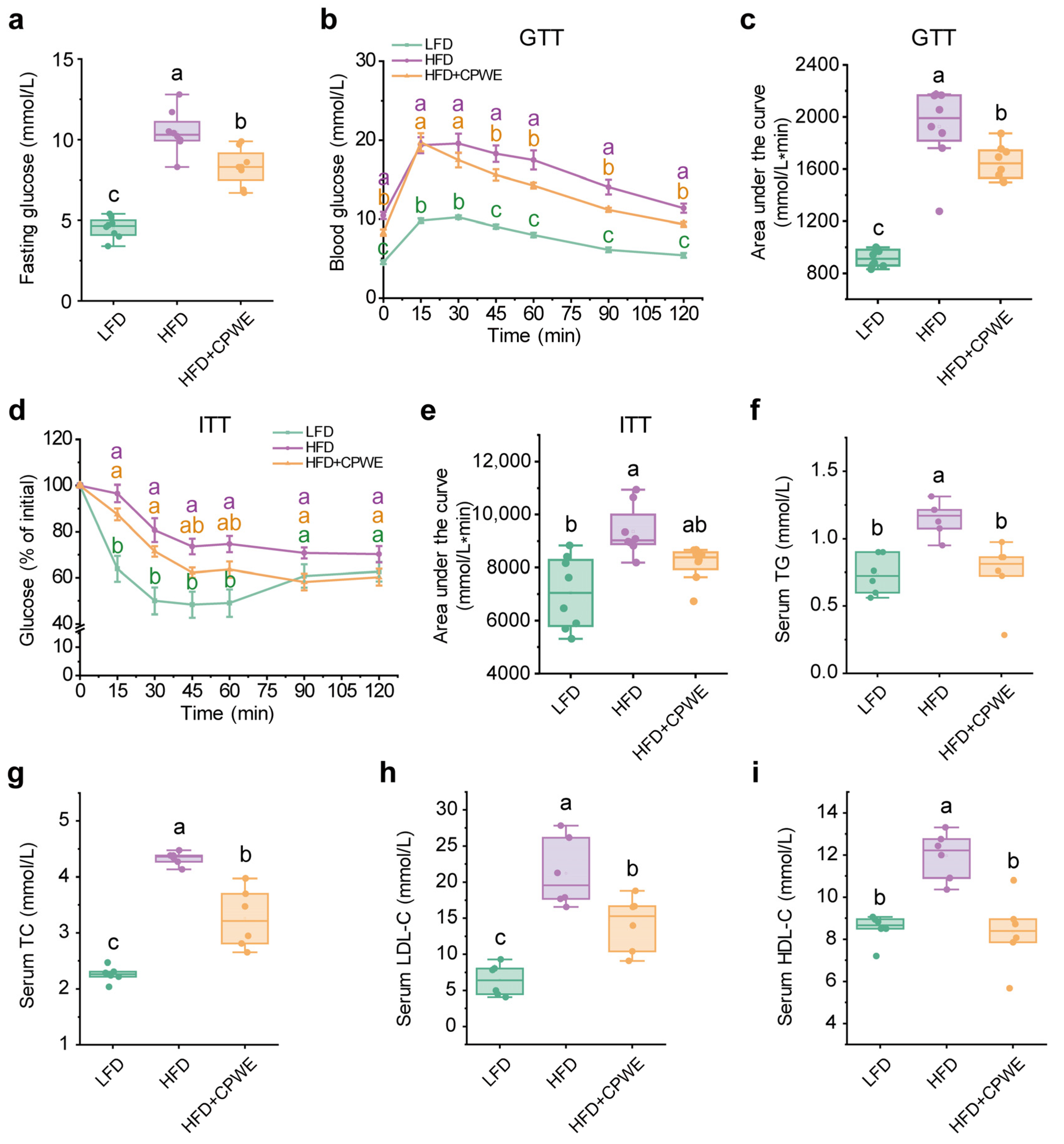
3.2. Effects of CPWE on Glucose Intolerance and Hyperlipidemia in HFD-Fed Mice
3.3. Effects of CPWE on Liver Lipid Deposition in HFD-Fed Mice
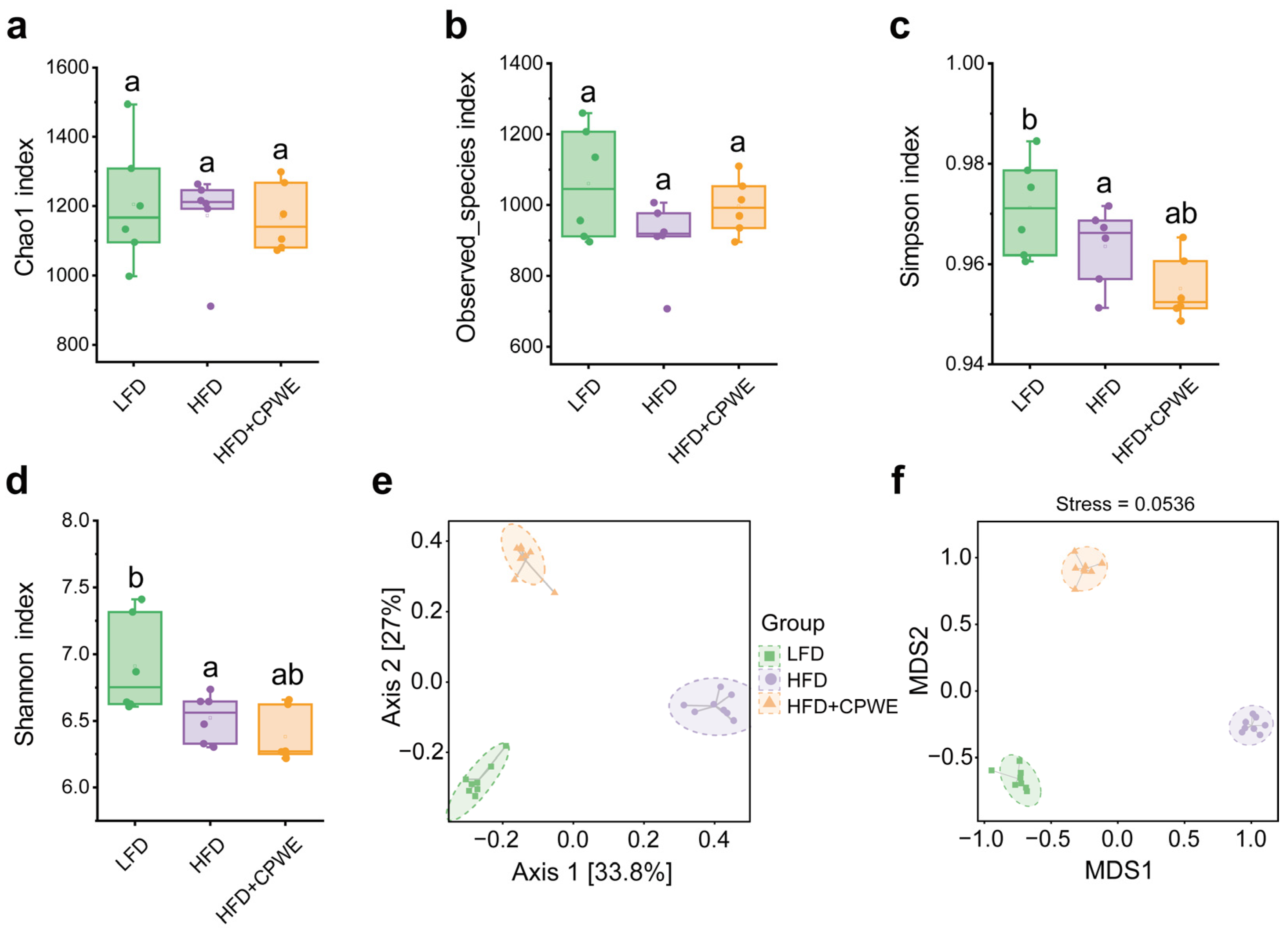
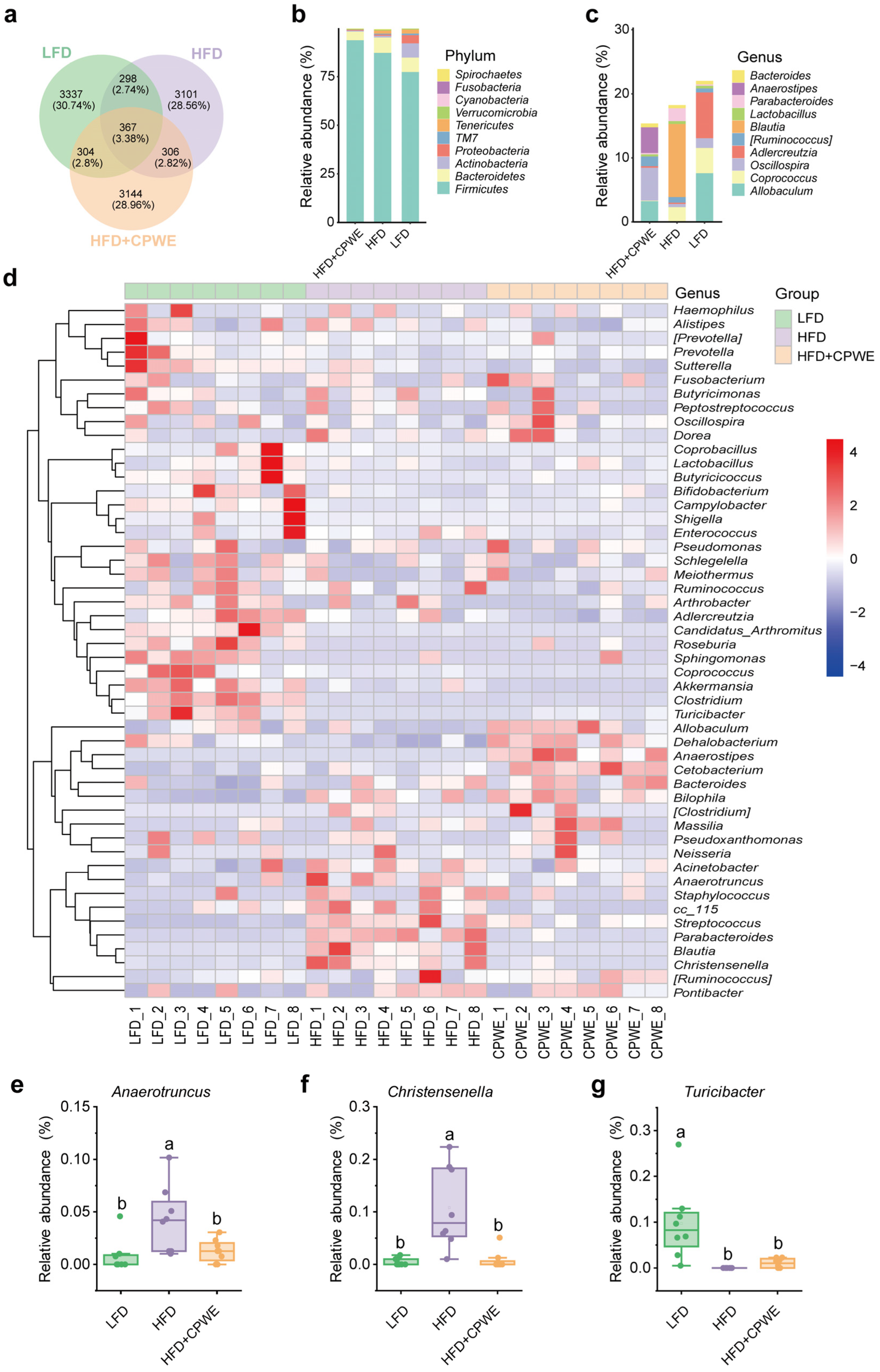
3.4. Effects of CPWE on Gut Microbiota Composition in HFD-Fed Mice
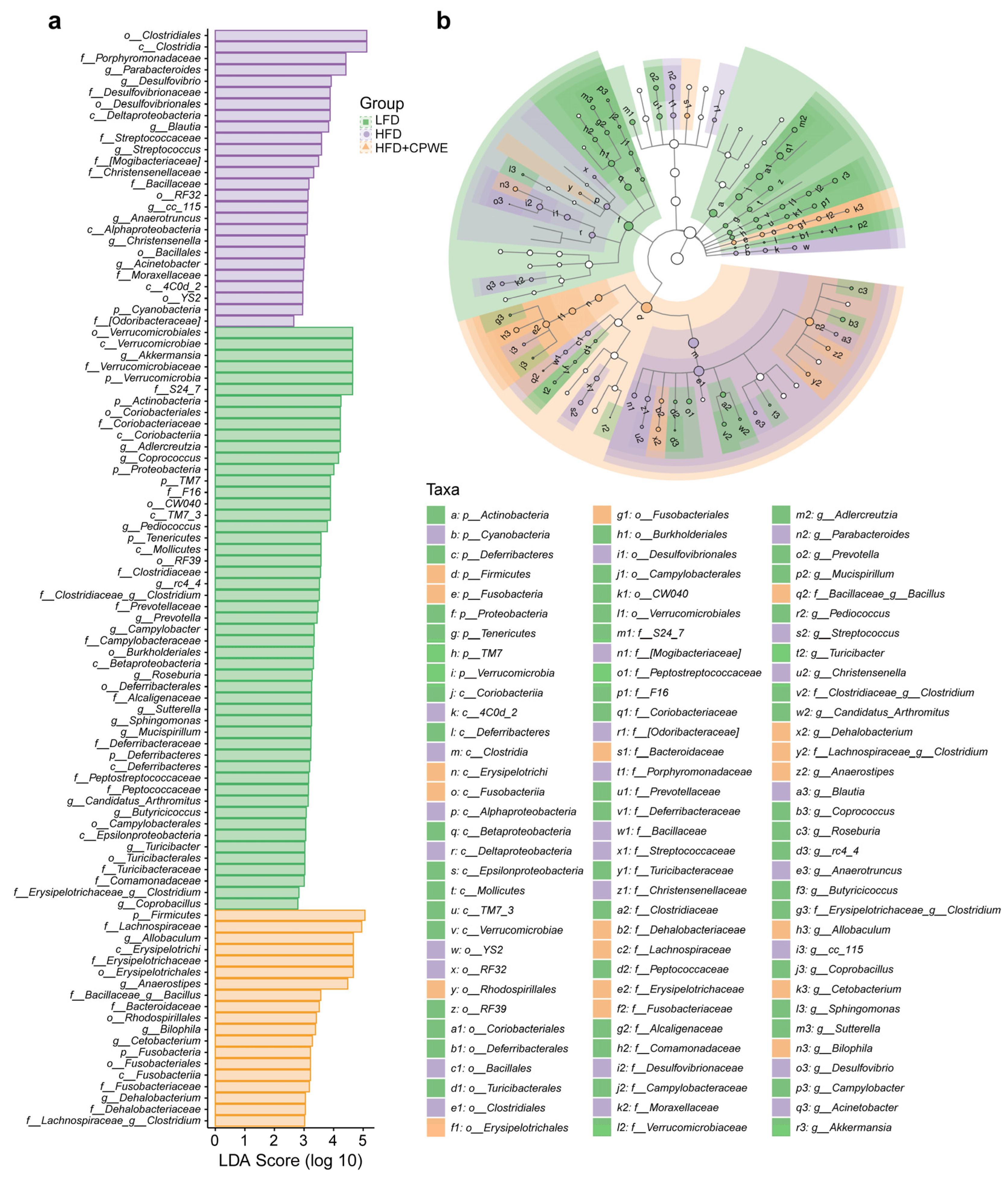
3.5. Analysis of Specific Dominant Bacterial Phenotypes
3.6. Correlation between the Obesity-Related Parameters and the Predominant Bacterial Genera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; Di Marchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.N.; Bisanz, J.E. Roles of the gut microbiome in weight management. Nat. Rev. Microbiol. 2023, 21, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Puljiz, Z.; Kumric, M.; Vrdoljak, J.; Martinovic, D.; Ticinovic Kurir, T.; Krnic, M.O.; Urlic, H.; Puljiz, Z.; Zucko, J.; Dumanic, P.; et al. Obesity, Gut microbiota, and metabolome: From pathophysiology to nutritional interventions. Nutrients 2023, 15, 2236. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P.D. The gut microbiota in obesity and weight management: Microbes as friends or foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Daubioul, C.A.; Taper, H.S.; De Wispelaere, L.D.; Delzenne, N.M. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese zucker rats. J. Nutr. 2000, 130, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chu, Q.; Ma, S.; Ma, H.; Song, H. Dietary fiber and its potential role in obesity: A focus on modulating the gut microbiota. J. Agric. Food Chem. 2023, 71, 14853–14869. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Blanck, H.M.; Garfield, K.M.; Wassung, A.; Petersen, R. A food is medicine approach to achieve nutrition security and improve health. Nat. Med. 2022, 28, 2238–2240. [Google Scholar] [CrossRef]
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Wang, B.; Su, M.; Zhou, S.; Luo, P.; Chen, L. Prevention and control effects of edible fungi and their active ingredients on obesity: An updated review of research and mechanism. J. Funct. Foods 2023, 107, 105621. [Google Scholar] [CrossRef]
- Li, R.; Luan, F.; Zhao, Y.; Wu, M.; Lu, Y.; Tao, C.; Zhu, L.; Zhang, C.; Wan, L. Crataegus pinnatifida: A botanical, ethnopharmacological, phytochemical, and pharmacological overview. J. Ethnopharmacol. 2023, 301, 115819. [Google Scholar] [CrossRef]
- Jing, Y.; Yan, M.; Liu, D.; Tao, C.; Hu, B.; Sun, S.; Zheng, Y.; Wu, L. Research progress on the structural characterization, biological activity and product application of polysaccharides from Crataegus pinnatifida. Int. J. Biol. Macromol. 2023, 244, 125408. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Yan, M.; Zhang, R.; Hu, B.; Sun, S.; Zhang, D.; Zheng, Y.; Wu, L. Structural characterization of a heteropolysaccharide from the fruit of Crataegus pinnatifida and its bioactivity on the gut microbiota of immunocompromised mice. Food Chem. 2023, 413, 135658. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Chen, M.; Li, D.; Liu, R.; Sun, T. Isolation, structural properties and bioactivities of polysaccharides from Crataegus pinnatifida. J. Ethnopharmacol. 2024, 323, 117688. [Google Scholar] [CrossRef]
- Ye, T.; Zheng, Y.; Guan, Y.; Sun, Y.; Chen, C. Rapid determination of chemical components and antioxidant activity of the fruit of Crataegus pinnatifida Bunge by NIRS and chemometrics. Spectrochim. Acta A 2023, 289, 122215. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Z.-T.; Wang, Y.-Z. The traditional usages, chemical components and pharmacological activities of Wolfiporia cocos: A review. Am. J. Chin. Med. 2022, 50, 389–440. [Google Scholar] [CrossRef]
- Tan, Y.-y.; Yue, S.-r.; Lu, A.-p.; Zhang, L.; Ji, G.; Liu, B.-c.; Wang, R.-r. The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-κB/CCL3/CCR1 axis. Phytomedicine 2022, 103, 154208. [Google Scholar] [CrossRef]
- Jiang, Y.; Ren, A.; Sun, X.; Yang, B.; Peng, H.; Huang, L. Fuling production areas in China: Climate and distribution changes (A.D. 618–2100). Front. Plant Sci. 2024, 15, 1289485. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.J.; Lai, N.P.Y.; Ng, W.M.; Siah, K.T.H.; Gan, R.-Y.; Zhong, L.L.D. Chemical structures, extraction and analysis technologies, and bioactivities of edible fungal polysaccharides from Poria cocos: An updated review. Int. J. Biol. Macromol. 2024, 261, 129555. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Hu, C.; Cheng, J.; Qiu, W.; Wang, Q.; Chen, X.; Chang, C.; Hu, J.; Qiu, Z.; Zheng, G. The extraction, structure characterization and hydrogel construction of a water-insoluble β-glucan from Poria cocos. Carbohyd Res. 2023, 534, 108960. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Zhang, F.; Li, Y.; Li, X.; Pu, X.; He, X.; Zhang, M.; Yang, X.; Yu, Q.; et al. Effects of Poria cocos extract on metabolic dysfunction-associated fatty liver disease via the FXR/PPARα-SREBPs pathway. Front. Pharmacol. 2022, 13, 1007274. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hong, B.; Shan, X.; Cheng, Y.; Peng, D.; Hu, R.; Wang, L.; Chen, W. The effect of Poria cocos polysaccharide PCP-1C on M1 macrophage polarization via the Notch signaling pathway. Molecules 2023, 28, 2140. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, Y.; Chen, Y.; Wang, D.; Lei, Y.; Pan, M.; Wang, Z.; Xiao, W.; Dai, Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Poria cocos. Int. J. Biol. Macromol. 2024, 261, 129878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, J.; Zheng, D.; Zhao, P.; Wang, Y.; Zhao, J.; Li, P. Immunomodulatory activity and its mechanisms of two polysaccharides from Poria cocos. Molecules 2023, 29, 50. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Yang, H.; Zheng, J.; Hu, H.; Zhu, T.; Zou, X.; Hu, B.; Liu, H. Poria cocos oligosaccharides ameliorate dextran sodium sulfate-induced colitis mice by regulating gut microbiota dysbiosis. Food Funct. 2023, 14, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Ni, X.; Yao, Y.; Sun, Y.; Yu, X.; Xia, D.; Yang, Z.; Hu, M.G.; Hou, X. Hyperoside prevents high-fat diet-induced obesity by increasing white fat browning and lipophagy via CDK6-TFEB pathway. J. Ethnopharmacol. 2023, 307, 116259. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cui, Z.; Gao, X.; Liu, H.; Wang, L.; Gong, J.; Wang, A.; Zhang, J.; Ma, Q.; Huang, Y.; et al. Corosolic acid ameliorates non-alcoholic steatohepatitis induced by high-fat diet and carbon tetrachloride by regulating TGF-β1/Smad2, NF-κB, and AMPK signaling pathways. Phytother. Res. 2021, 35, 5214–5226. [Google Scholar] [CrossRef]
- Hao, P.; Yang, X.; Yin, W.; Wang, X.; Ling, Y.; Zhu, M.; Yu, Y.; Chen, S.; Yuan, Y.; Quan, X.; et al. A study on the treatment effects of Crataegus pinnatifida polysaccharide on non-alcoholic fatty liver in mice by modulating gut microbiota. Front. Vet. Sci. 2024, 11, 1383801. [Google Scholar] [CrossRef]
- Liou, C.J.; Dai, Y.W.; Wang, C.L.; Fang, L.W.; Huang, W.C. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019, 33, 11791–11803. [Google Scholar] [CrossRef]
- Sun, S.-S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.-W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ye, C.; Hu, B.; Xia, H.; Bian, Q.; Liu, Y.; Kong, M.; Zhou, S.; Liu, H. Regulation of gut microbiota and intestinal metabolites by Poria cocos oligosaccharides improves glycolipid metabolism disturbance in high-fat diet-fed mice. J. Nutr. Biochem. 2022, 107, 109019. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shi, Q.; Chen, J.; Lu, J.; Wang, L.; Qiu, M.; Liu, J. Effects of 23-epi-26-deoxyactein on adipogenesis in 3T3-L1 preadipocytes and diet-induced obesity in C57BL/6 mice. Phytomedicine 2020, 76, 153264. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chu, J.; Wang, M.; Chen, L.; Zhang, L.; Xie, Z.; Zhang, J.; Ho, C.-T.; Li, D.; Wan, X. Large yellow tea attenuates macrophage-related chronic inflammation and metabolic syndrome in high-fat diet treated mice. J. Agric. Food Chem. 2018, 66, 3823–3832. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, H.; Shui, S.; Lin, Y.; Wang, F.; Wang, L.; Chen, J.; Liu, J. Chrysin stimulates subcutaneous fat thermogenesis in mice by regulating PDGFRα and MicroRNA expressions. J. Agric. Food Chem. 2021, 69, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumerova, B.; Rosolova, H. Obesity and dyslipidemia. Curr. Atheroscler. Rep. 2023, 25, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zou, Y.; Tang, H.; Zhuang, J.; Ye, Z.; Wei, T.; Lin, J.; Zheng, Q. Cordyceps militaris polysaccharides modulate gut microbiota and improve metabolic disorders in mice with diet-induced obesity. J. Sci. Food Agric. 2023, 103, 1885–1894. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrients 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Peng, W.; He, C.-X.; Li, R.-L.; Qian, D.; Wang, L.-Y.; Chen, W.-W.; Zhang, Q.; Wu, C.-J. Zanthoxylum bungeanum amides ameliorates nonalcoholic fatty liver via regulating gut microbiota and activating AMPK/Nrf2 signaling. J. Ethnopharmacol. 2024, 318, 1166848. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.; Hou, C.; Chen, L.; Zhang, Y.; Li, J. Effects of pomegranate peel polyphenols combined with inulin on gut microbiota and serum metabolites of high-fat-induced obesity rats. J. Agric. Food Chem. 2023, 71, 5733–5744. [Google Scholar] [CrossRef]
- Ma, K.L.; Kei, N.; Yang, F.; Lauw, S.; Chan, P.L.; Chen, L.; Cheung, P.C.K. In vitro fermentation characteristics of fungal polysaccharides derived from Wolfiporia cocos and their effect on human fecal microbiota. Foods 2023, 12, 4014. [Google Scholar] [CrossRef]
- Lai, Y.; Deng, H.; Fang, Q.; Ma, L.; Lei, H.; Guo, X.; Chen, Y.; Song, C. Water-insoluble polysaccharide extracted from Poria cocos alleviates antibiotic-associated diarrhea based on regulating the gut microbiota in mice. Foods 2023, 12, 3080. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, Q.; Gao, Z.; Bianca Xu, G.; Chen, H.; Chitrakar, B.; Sun, Y.; Zhao, W.; Lin, X.; Zhou, K.; et al. Characterization of procyanidin extracts from hawthorn (Crataegus pinnatifida) in human colorectal adenocarcinoma cell line Caco-2, simulated digestion, and fermentation identified unique and novel prebiotic properties. Food Res. Int. 2023, 165, 112393. [Google Scholar] [CrossRef]
- Zou, Y.-T.; Zhou, J.; Wu, C.-Y.; Zhang, W.; Shen, H.; Xu, J.-D.; Zhang, Y.-Q.; Long, F.; Li, S.-L. Protective effects of Poria cocos and its components against cisplatin-induced intestinal injury. J. Ethnopharmacol. 2021, 269, 113722. [Google Scholar] [CrossRef] [PubMed]





| Ingredient (g) | LFD | HFD | HFD+CP | HFD+WE | HFD+CPWE |
|---|---|---|---|---|---|
| Casein | 200 | 200 | 200 | 200 | 200 |
| Maltodextrin 10 | 35 | 100 | 100 | 100 | 100 |
| Lard | 20 | 177.5 | 177.5 | 177.5 | 177.5 |
| Calcium carbonate | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| Cellulose | 50 | 50 | 47.3 | 7.1 | 4.4 |
| Corn starch | 315 | 72.8 | 54 | 72.8 | 54 |
| Dicalcium phosphate | 13 | 13 | 13 | 13 | 13 |
| Soybean oil | 25 | 25 | 25 | 25 | 25 |
| Vitamin Mix V10001 | 10 | 10 | 10 | 10 | 10 |
| L-Cystine | 3 | 3 | 3 | 3 | 3 |
| Potassium citrate | 16.5 | 16.5 | 16.5 | 16.5 | 16.5 |
| Mineral Mix 10026 | 10 | 10 | 10 | 10 | 10 |
| Sucrose | 350 | 172.8 | 172.8 | 172.8 | 172.8 |
| Choline bitartrate | 2 | 2 | 2 | 2 | 2 |
| CP | 21.5 | 21.5 | |||
| WE | 42.9 | 42.9 | |||
| Total weight (g) | 1055 | 858.1 | 858.1 | 858.1 | 858.1 |
| Total energy (kcal) | 4057 | 4057 | 4057 | 4057 | 4057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Hu, Y.; Yang, D.; Zhou, A.; Luo, S.; Xu, N.; Dong, J.; He, Q.; Zhang, C.; Zhang, X.; et al. The Effects of Crataegus pinnatifida and Wolfiporia extensa Combination on Diet-Induced Obesity and Gut Microbiota. Foods 2024, 13, 1633. https://doi.org/10.3390/foods13111633
Yuan J, Hu Y, Yang D, Zhou A, Luo S, Xu N, Dong J, He Q, Zhang C, Zhang X, et al. The Effects of Crataegus pinnatifida and Wolfiporia extensa Combination on Diet-Induced Obesity and Gut Microbiota. Foods. 2024; 13(11):1633. https://doi.org/10.3390/foods13111633
Chicago/Turabian StyleYuan, Jingjing, Yueyun Hu, Dongmei Yang, An Zhou, Shengyong Luo, Na Xu, Jiaxing Dong, Qing He, Chenxu Zhang, Xinyu Zhang, and et al. 2024. "The Effects of Crataegus pinnatifida and Wolfiporia extensa Combination on Diet-Induced Obesity and Gut Microbiota" Foods 13, no. 11: 1633. https://doi.org/10.3390/foods13111633
APA StyleYuan, J., Hu, Y., Yang, D., Zhou, A., Luo, S., Xu, N., Dong, J., He, Q., Zhang, C., Zhang, X., Ji, Z., Li, Q., & Chu, J. (2024). The Effects of Crataegus pinnatifida and Wolfiporia extensa Combination on Diet-Induced Obesity and Gut Microbiota. Foods, 13(11), 1633. https://doi.org/10.3390/foods13111633







