Abstract
Enteromorpha prolifera (EP) is a green alga that causes green bloom worldwide. This study aimed to isolate and identify n-3 polyunsaturated fatty acids (PUFAs) from EP oil obtained via supercritical fluid extraction (SFE) and to explore its preventive effects against dextran sodium sulfate (DSS)-induced ulcerative colitis in C57BL/6J mice. In EP oil, we found the novel n-3 polyunsaturated fatty acid C16:4n-3 and two unusual fatty acids C18:4n-3 and C16:3n-3, using GC-MS. The administration of EP oil reduced histopathological of symptoms colitis and the shortening of the colon length. Pro-inflammatory cytokines of IL-6 and TNF-α in serum of EP oil treatment were lower than DSS treatment (by 37.63% and 83.52%), and IL-6 gene expression in the colon was lower in than DSS group by 48.28%, and IL-10 in serum was higher than DSS group by 2.88-fold. Furthermore, the protein expression of p-STAT3 by the EP oil treatment was significantly reduced compared with DSS treatment group by 73.61%. Lipidomics study suggested that phosphatidylcholine and phosphatidylethanolamine were positively associated with the anti-inflammatory cytokine IL-10, while cholesteryl ester and sphingomyelin were negatively related to inflammation cytokines in the EP oil group. The present results indicated that EP oil rich in n-3 PUFA contains a novel fatty acid C16:4n-3, as well as two uncommon fatty acids C18:4n-3 and C16:3n-3. EP oil could prevent DSS-induced ulcerative colitis by regulating the JAK/STAT pathway and lipid metabolism.
1. Introduction
Inflammatory bowel disease (IBD) is a gastrointestinal inflammatory disease including Crohn’s disease and ulcerative colitis (UC), with an estimated prevalence of 5 million cases of UC in 2023 [1]. UC is characterized by ulcers in the rectum and colon, and the typical symptoms are rectal bleeding, tenesmus, increased stool frequency or episodes of diarrhea, urgent bowel movements, and cramp-like abdominal pain [2]. The cause of UC is complex and not completely clear, and might be related to many factors like genetic, microbiota, immune system, stress, and diet [3]. Risk factors, including a Westernized diet characterized by a high intake of saturated fats and refined sugar, smoking, stress, and antibiotics can increase the incidence of UC [4]. Medications such as 5-aminosalicylic acids (sulfasalazine, mesalamine, balsalazide and olsalazine), corticosteroids (prednisone and budesonide), thiopurines, immunomodulators, and other biologics are typically used for the maintenance of UC remission [2]. However, these treatments are not suitable for long-term use due to their adverse effects such as bone marrow and liver toxicity, pancreatitis, lymphoma, osteoporosis, depression, type 2 diabetes mellitus, and herpes zoster [2]. Recently, researchers have looked at specific dietary interventions like the Mediterranean diet and anti-inflammatory diets to protect intestinal immune homeostasis [5].
Marine-derived n-3 polyunsaturated fatty acids (PUFAs) like eicosapentaenoic acid (EPA, C20:5n-3), docosapentaenoic acid (DPA, C22:5n-3), and docosahexaenoic acid (DHA, C22:6n-3) and their precursors like α-linolenic acid (ALA, C18:3n-3) and stearidonic acid (SDA, C18:4n-3) may influence the inflammatory state of endothelial cells [6]. Especially, EPA and DHA may protect against TNBS- or DSS- induced UC in vivo, but ALA might not reduce the development of UC [6]. Besides, n-3 PUFAs like EPA, DPA, and DHA could be converted to specialized pro-resolving mediators (SPMs) such as resolvins, protectins, and maresins, which may play important roles in the inflammatory response [7]. However, it is still needed to investigate the potential SPMs of C18:4n-3 and the beneficial effects on IBD. Moreover, fish oil is not recommended for vegetarians and people with fish allergies those that dislike its strong odor and flavor. In this case, algae oil may be a potential alternative PUFA.
Enteromorpha prolifera (EP) is a green alga distributed globally that is used as food and traditional medicine in China and Japan [8]. In recent decades, EP bloom has caused large-scale green tides, which have led to serious problems for marine aquaculture and the environment [9]. EP is rich in proteins, sulfated polysaccharides, unsaturated fatty acids, and mineral elements like ferrum and calcium. Many studies focus on polysaccharides, which have biological effects such as against oxidative stress, insulin resistance, inflammation, and cancer [8,10]. In addition, the ethanol extract of EP was active against lipid accumulation, inflammatory and oxidative stress on high-fat diet-fed mice [11]. However, the composition of the fatty acids in EP oil and the functional properties are less well-studied. Although the lipids account for less than 5% of green algae, algae oil is usually rich in PUFAs, which may be effective against inflammation, cardiovascular disease, and metabolic disorders [12].
Consequently, the aim of the study was to investigate the fatty acids of EP oil and the anti-inflammatory effect. EP oil was isolated by supercritical fluid extraction and the fatty acid composition of GC-MS was analyzed. We evaluated the mechanisms of anti-inflammatory activity and potential metabolite biomarkers in DSS-induced colitis in C57BL/6J mice.
2. Materials and Methods
2.1. Plant Material and Reagents
Enteromorpha prolifera was collected from the offshore of Qingdao, Shandong, China (36°05′ N, 120°30′ E), dried at 60 °C, and then ground into powder. The molecular mass of dextran sodium sulfate (DSS) was 36~50 kDa (Yeasen Biotechnology (Shanghai) Co., Ltd., Shanghai, China). Acetonitrile, methanol, formic acid, and ammonium acetate were provided by Merck (Darmstadt, Germany) and the other reagents were of analytical grade.
2.2. Isolation of Enteromorpha prolifera Oil by SFE
Lipids were isolated from EP following the previous procedure detailed in Yang et al. [13]. Supercritical fluid extraction (SFE) was carried out using a supercritical fluid extraction system (HA220-40-11) (Jiangsu Huaan Scientific Research Devices Co., Ltd., Nantong, China). EP powder was mixed with 95% ethanol (4:1 w/w) and fed into a 10 L extraction vessel. The temperatures of separator I and II were 55 °C and 37 °C whereas the pressures were 8 and 5 MPa, respectively. The temperature and pressure of the extractor were 45 °C and 28 MPa. EP oil was collected from the two separators every 45 min and then combined.
2.3. Fatty Acid Methyl Esters and GC-MS Analysis
Lipids from samples were extracted by chloroform and methanol (2:1, v/v). Then, lipids were transesterified to fatty acid methyl esters (FAMEs) by the previous procedure [14]. Briefly, 3 mL 0.9 mol/L H2SO4/methanol and 1 mL methylbenzene were added to 1 mL of the sample. The tubes were capped, placed in a 70 °C water bath for 2 h, and shaken every 30 min. Then, 2 mL hexane and 2 mL water were added to the sample and centrifuged at 2000 rpm for 10 min. The oil suspension was filtered using a Supelclean LC-Si SPE silica column (505048, Supelo, Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China) and the lipid extract was dried using gaseous nitrogen.
GC-MS analysis was performed with an Agilent 7000D GC and 5973 (EI) MS detector (Agilent Technologies, Palo Alto, CA, USA). Agilent MassHunter Qualitative Analysis 10.0 software was used for data processing [15]. The flow rate was 1 mL/min and an HP-INNOWax column (30 m × 250 μm × 0.25 μm, Agilent 19091N-133I) was used. Program was set for 5 min from 160 °C at 20 °C/min; then 12 min from 180 °C at 20 °C/min; 8 min from 200 °C at 20 °C; 11 min from 205 °C at 20 °C; 5 min from 280 °C at 6 °C. The GC was operated in split injection mode and split ratio was 10:1 at a split flow rate of 10 mL/min with helium as the carrier gas (15 mL/min). Injection volume was 1 μL and inlet temperature was 230 °C. Detector temperature was set at 320 °C and scanning range was from 300~800 m/z. Peaks were identified by retention time and characteristic ions by comparison with a standard 37-component FAME mix (Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China) and the NIST 11 library.
2.4. Animals and Treatment
Male C57BL/6J mice (20~24 g) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Ethical approval was obtained from the Qingdao University Laboratory Animal Welfare Ethics Committee (No. 20211231C576020220111070). All mice were housed at 22 ± 2 °C with 50% ± 5% relative humidity and a 12 h light/dark cycle light. After one week of acclimation, the mice were randomly allocated (6 mice per group) to the four groups. All mice had free access to a standard diet (AIN-93M) and were given oil by intragastric administration, as follows: (1) control (olive oil, 0.1 mL/20 g), (2) DSS (olive oil, 0.1 mL/20 g), (3) EP oil (EP oil, 0.1 mL/20 g), (4) SASP (salicylazosulphapyridine, 100 mg/kg and olive oil, 0.1 mL/20 g) [16]. The control group was given sterile tap water, whereas the other three groups were provided 2.0% DSS in the drinking water on days 1~4, days 14~18, and days 29~33 to induce chronic colitis [17,18]. Body weight was recorded daily, and mice were fasted overnight at the end of experiment and sacrificed after anesthesia. Blood was obtained from the orbital venous plexus bleeding, and after allowed to stand for 30 min, serum was collected by centrifuging at 2000× g for 15 min in 4 °C.
2.5. Histopathological Examination
Colon tissues were formalin-fixed and paraffin embedded, and sections were sliced into 5 μm slices stained with either hematoxylin and eosin (H&E) or Alcian blue/periodic acid–Schiff (AB-PAS). The sections were visualized under a Nikon light microscope (Tokyo, Japan). Histomorphological scores of 0~4 were given to the slides based on the degree of mucosal damage and inflammatory cell infiltration in the colon. The following point system was used as a guide when assessing the slides: 0, intact crypt with no inflammatory cell infiltration; 1, minimal loss of goblet cells and inflammatory cell immersion; 2, extensive loss of goblet cells and inflammatory infiltration in the mucosa; 3, extensive loss of goblet cells, minimal loss of crypts, and inflammation continuously distributed in the mucosa; 4, extensive loss of crypts and full-thickness inflammatory cells seriously infiltrated [19].
2.6. Serum Inflammatory Cytokines
Cytokines in serum were quantified using the enzyme-linked immunosorbent assay (ELISA) kits for mouse IL-6, IL-10, and TNF-α (Thermo Fisher Scientific Co., Ltd., Shanghai, China). Protein concentrations were measured by BCA protein assay kit (Dalian Meilun Biotechnology Co., Ltd., Dalian, China).
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
Total RNA of colon tissue was extracted using Trizol, and reverse transcription was performed by cDNA synthesis kit (Yeasen Biotechnology (Shanghai) Co., Ltd., Shanghai, China). The amplification and detection were performed on a QuantStudio 1 Real-Time PCR System, and data were analyzed using 2−ΔΔCT method by Quantstudio™ Design and Analysis Software v1.5.1 (Applied Biosystems, Foster City, CA, USA). Primer sequences are shown in Table 1.

Table 1.
Primer sequences for qRT-PCR analysis.
2.8. Western Blotting
Proteins from colon tissue were separated by SDS-PAGE, and transferred to 0.45 μm PVDF membranes that were blocked in 5% BSA. Primary antibodies were incubated overnight at 4 °C: anti-STAT3 (signal transducer and activator of transcription 3, STAT3) (1:1000, ab68153; Abcam, Cambridge, MA, USA), anti-p-STAT3 (phosphor Y705) (1:2000, ab76315; Abcam, USA), and anti-β-actin (1:2000, ab8227; Abcam, USA). The membranes were washed and incubated with horseradish peroxidase-labeled secondary antibody at room temperature for 1 h. Images were visualized with using ECL reagent on a Tanon-5200 Multi automatic image analyzer (Tanon, Shanghai, China) and the intensity was calculated with ImageJ 1.45s software.
2.9. Hepatic Lipidomics Analysis
Hepatic lipid extraction was processed by the Folch method with minor modifications [20]. Livers (liver:deionized water 1:10, v/v) were homogenized using tissue homogenizer, and liver homogenate was added to chloroform/methanol (2:1, v/v) in a 1:9 ratio, and then centrifuged at 10,000 rpm for 10 min. The organic phase was collected and dried using nitrogen. Isopropanol (200 μL) was added to redissolve, and the supernatant obtained after centrifuging at 10,000 rpm for 10 min was filtered for lipidomics analysis. Quality control (QC) samples of 10 μL were collected for each sample.
Hepatic lipidomics was performed using Agilent Technologies 6530C Q-TOF LC/MS in positive ESI detection mode. The ACQUITY UPLC BEHC18 column (2.1 mm × 100 mm, 1.7 μm) was used and the flow rate was 0.3 mL/min. Mobile phase A was acetonitrile: water (60:40, v/v) containing 5 mM ammonium acetate, and mobile phase B was isopropanol alcohol:acetonitrile (90:10, v/v). The following elution gradient was applied: initial gradient of 10% mobile phase B for 2 min, followed by a linear increase to 60% B in 6 min, which was held for 3 min, increased to 75% at 13 min, 78% at 17 min, and 99% at 19 min, which was then held for 6 min, followed by a decrease to 10% at 26 min to equilibrate the system [21]. Injection volume was 2 μL, and the other parameters set as follows: column temperature: 40 °C, scan range: 50~1000 m/z, capillary voltage: 3 kV, cone hole voltage: 40 V, capillary temperature: 320 °C, auxiliary gas heater temperature: 350 °C. All samples were randomly injected and QC samples were injected every 10 samples.
Hepatic lipidomic data were converted by Abf converter v1.8 software; lipid metabolites were integrated by MS-FLO (https://msflo.fiehnlab.ucdavis.edu, accessed on 21 November 2023) and identified based on MS-DIAL [22]. The MS data were matched by lipidBlast database by comparing with retention times, m/z value, peak area, and ion intensities. The metabolites were analyzed using MetaboAnalyst 4.0 software (Wishart Research Group, University of Alberta, Edmonton, AB, Canada) and human Metabolome Database (HMDB) [23].
2.10. Statistical Analysis
Data in histograms were shown as the mean ± standard deviation. The results were statistically analyzed by one-way analysis of variance (ANOVA) for Duncan’s test using IBM SPSS Statistics 20 and GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA). Lipidomic data were selected by significant features (p < 0.01), fold-change > 2, p-value < 0.05, and VIP scores > 2.0.
3. Results and Discussion
3.1. Identification of Fatty Acids of Enteromorpha prolifera Oil Extracted by SFE
EP oil was extracted by SFE, and the fatty acid composition was analyzed by GC-MS (Table 2). Linoleic acid and α-linolenic acid, which are essential fatty acids for human health, were found to be the predominant fatty acids in EP oil, accounting for 13.14% and 16.41%, respectively.

Table 2.
The fatty acid composition of Enteromorpha prolifera oil.
The SFA:MUFA:PUFA ratio was nearly 3:2:5, whereby 32.17% of the total fatty acids were n-3 PUFAs. C16:3n-3, C16:4n-3, and C18:4n-3 of n-3 PUFAs were identified from EP oil, and C16:4n-3 and C18:4n-3 were 5.96% and 6.23% of the total fatty acids. Results showed that EP oil was rich in C16 and C18 fatty acids, especially two unusual fatty acids of C16:4n-3 and C18:4n-3 (Figure 1).
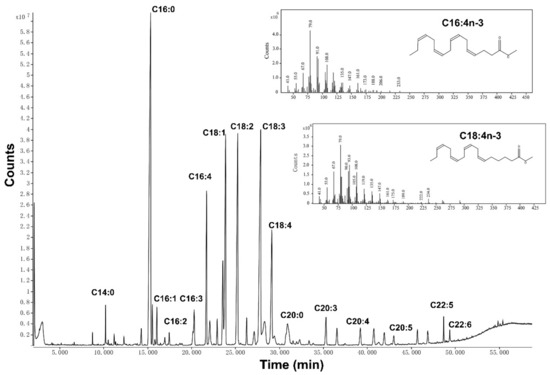
Figure 1.
Representative GC-MS chromatogram of EP oil with the major peak and the abundance of m/z for C16:4n-3 and C18:4n-3.
C16 and C18 PUFAs were the main fatty acids in green algae, and these can be used to synthesize EPA and DHA in both algae and mammals [24]. ALA needs the Δ6-desaturase to transform to C18:4n-3, which is a rate-limiting step, then converted to EPA via elongase and Δ5-desaturase. On the other hand, C16:3n-3 converts to C16:4n-3 via Δ15-desaturase, and then transforms to C18:4n-3 via elongase, which is more easily converted to EPA and DHA. The fatty acid composition of EP oil was similar to the green seaweeds such as Ulva species [25]. In addition, C16 n-3 PUFAs can be found in Karenia mikimotoi and Euphausia pacifica, which play important roles in the marine food chain [26,27].
Interestingly, owing to the high n-6/n-3 fatty acid ratio (20~50:1) of modern diets related to chronic disease, the ratio of EP oil was 0.53:1, which indicates that EP oil may balance the high n-6/n-3 ratio diet [28]. Results showed that EP oil rich in n-3 PUFA contained a novel fatty acid, C16:4n-3, as well as two uncommon fatty acids, C18:4n-3 and C16:3n-3.
3.2. Impact of Enteromorpha prolifera Oil on DSS-Induced Mice
The protective effect of EP oil against UC was investigated in C57BL/6J mice exposed to 2% DSS for three times to induce chronic UC in a mouse model. At the end, the body weight of mice in all experimental groups exposed to DSS was significantly lower in the than control group (Figure 2A). The colonic length in the DSS-treated groups was shortened compared with the control group and clinical manifestations of DSS-induced UC including diarrhea and blood in the feces and anus was observed (Figure 2B). EP oil alleviated edema in the colon relative to the DSS group (Figure 2C).
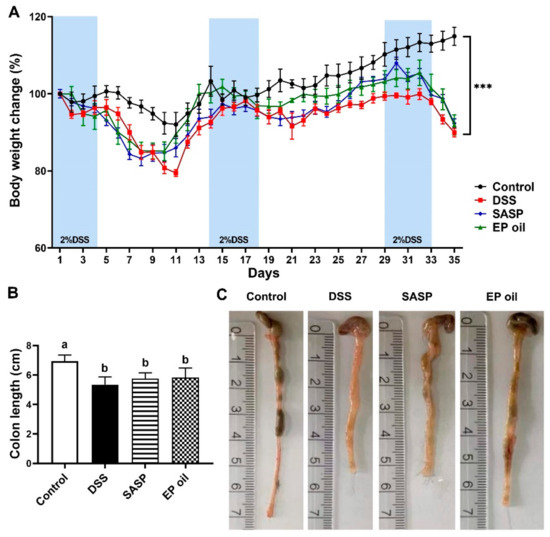
Figure 2.
Effect of EP oil on DSS-treated mice on (A) body weight change; (B) colon length; and (C) representative images of the colon length. *** p < 0.001 compared with the DSS group. Lowercase letters in histograms indicate p < 0.05 according to Duncan’s test.
The results showed that EP oil alleviated DSS damage to the colon, whereas the DSS group fed with olive oil rich in C18:1n-9 experienced no amelioration of UC. Another study also reported that olive oil and flaxseed oil rich in C18:3n-3 had no preventive effect on DSS-induced acute UC [29]. These results suggested that EP oil rich in C16:4n-3, C18:4n-3, and EPA could potentially attenuate DSS-induced colitis in mice.
3.3. Effect of Enteromorpha prolifera Oil on Colon Histomorphology
H&E staining of colon segments in the DSS group showed severe colon damage, characterized by edema, cell infiltration, distorted crypt, and destructed epithelial barrier as the white arrow pointed (Figure 3A). Histomorphological observations showed that 2% DSS induced a severe chronic UC mice model. EP oil-fed mice showed little basal plasmacytosis and goblet cell depletion compared with DSS-induced mice. Moreover, AB-PAS staining results showed neatly arranged goblet cells and less edema in the mucus membrane in EP oil and SASP groups compared to DSS mice (Figure 3C). EP oil and SASP treatment also resulted in lower histomorphological scores than the DSS group (Figure 3B,D). These results showed that EP oil could alleviate the symptoms of DSS-induced colitis in colon.
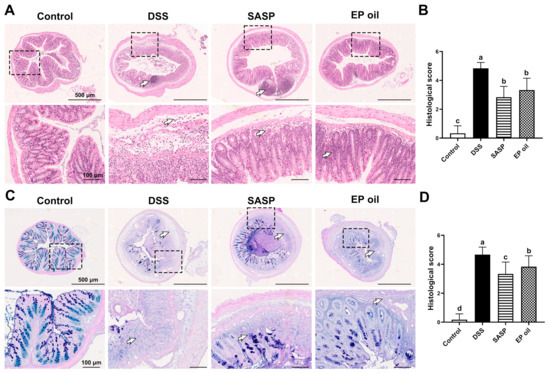
Figure 3.
Effect of EP oil on DSS-induced mice colon by H&E and AB-PAS. (A) Histological staining of H&E (50× and 200×). (B) Histological scores of H&E. (C) Histological staining of AB-PAS (50× and 200×). (D) Histological scores of AB-PAS. White arrow pointed edema, cell infiltration, distorted crypt, or destructed epithelial barrier. Lowercase letters in histograms represent p < 0.05 according to Duncan’s test.
3.4. Anti-Inflammatory Effects of Enteromorpha prolifera Oil on DSS-Induced Mice
The anti-inflammatory activities of EP oil were investigated by measuring serum cytokines using ELISA, and the expression of these cytokines genes in the colon tissue was tested by qRT-PCR. IL-6 and TNF-α levels in the DSS group were significantly higher than in the other groups, and those of IL-10 were lower (p < 0.05). Mice treated with EP oil had significantly lower serum levels of IL-6 and TNF-α, by 37.63% and 83.52% respectively, when compared to the DSS group mice. Mice in EP and SASP groups had significantly higher expression of IL-10 than the DSS group, by 2.88-fold (p < 0.05) (Figure 4A–C). These results showed that inflammatory cytokines (IL-6 and TNF-α) increased after DSS treatment, whereas EP oil and SASP suppressed the secretion of pro-inflammatory cytokines and improved the secretion of anti-inflammatory cytokines.
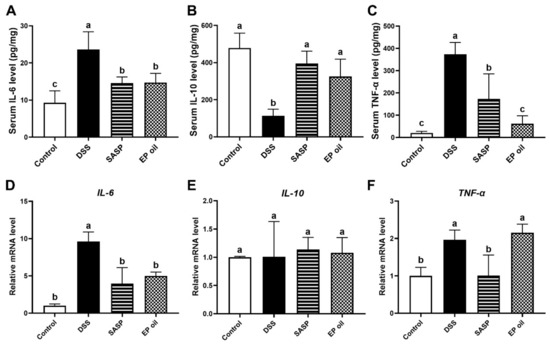
Figure 4.
Anti-inflammatory activity of EP oil on cytokines by ELISA. (A) IL-6, (B) IL-10, and (C) TNF-α. The mRNA expression of cytokines (D) IL-6, (E) IL-10, and (F) TNF-α. Lowercase letters in histograms represent p < 0.05 according to Duncan’s test.
Meanwhile, EP oil and SASP significantly blocked IL-6 expression compared with the DSS group by 48.28% and 58.83% (p < 0.05) (Figure 4D). This was especially evident in SASP-treated mice, although there was no differences in mRNA levels of TNF-α between the EP oil group and DSS group (p > 0.05) (Figure 4F). There were no differences in mRNA expression of IL-10 in colon between all groups (Figure 4E). This observation is consistent with previous studies that n-3 PUFA alleviated inflammatory and decreased serum TNF-α through the formation of resolvins and protectins in fat-1 transgenic mice [30]. These showed that EP oil may play a role in cytokine modulation and exhibit anti-inflammatory activity by inhibiting IL-6.
3.5. Enteromorpha prolifera Oil Inhibited Inflammation by JAK/STAT Signaling Pathway
The JAK/STAT signaling pathway is involved in the pathology of IBD [28]. To investigate the mechanism of EP oil on DSS-induced mice, we tested the expression of key mRNAs of JAK/STAT signaling pathway (Figure 5). JAK1, JAK2, STAT2, SOCS3, and TYK2 mRNA levels in the DSS treatment group were lower than in the control group (p < 0.05). JAK1, JAK2, STAT2, STAT3, and SOCS3 expression in mice fed SASP were lower than in other groups (p < 0.05). The expression of JAK1, STAT2, and SOCS3 in the EP oil group was significantly higher than in the DSS group (p < 0.05). Moreover, the protein expression levels of p-STAT3 in the colon were significantly decreased following EP oil treatment, by 73.61% compared to the DSS group (Figure 5G,H). This suggests that EP oil may modulate the JAK/STAT pathway.
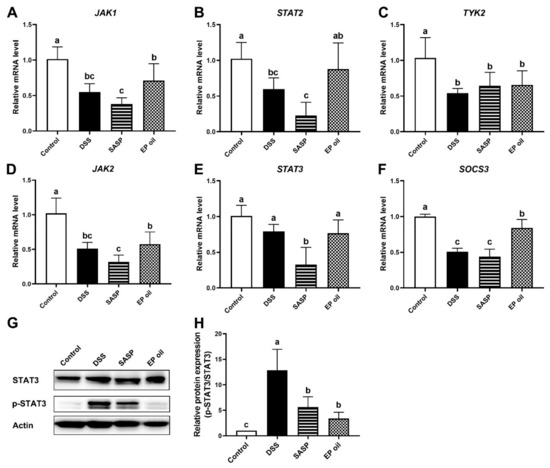
Figure 5.
EP oil inhibited inflammation by JAK/STAT signaling pathway. (A) JAK1, (B) STAT2, (C) SOCS3, (D) JAK2, (E) STAT3, (F) TYK2 mRNA expression of EP oil, fish oil, and flaxseed oil. (G) Representative Western blotting images depicting STAT3 and p-STAT3, and (H) STAT3 and p-STAT3 protein level in colon. Lowercase letters in histograms represent p < 0.05 according to Duncan’s test.
Very few studies have investigated the bioactivity of C18:4n-3 and C16:4n-3 in EP oil. Some studies reported that C18:4n-3 and C16:4n-3 from Undaria pinnatifida and Ulva pertusa may convert to EPA, DHA, and pro-resolving lipid mediators, which could exert anti-inflammatory activity [31,32]. Algal oil and marine-derived bioactive compounds alleviated DSS-induced inflammation and colitis by altering the gut microbiota [12,33]. Moreover, specialized pro-resolving mediators such as resolvins, protectins, and mavrins were synthesized from EPA and DHA, and were shown to improve colon histology and reduce pro-inflammatory cytokines in the JAK/STAT signaling pathway [34,35]. This study indicated that EP oil may protect against UC via the modulation of the JAK/STAT signaling pathway.
3.6. Effect of Enteromorpha prolifera Oil on Hepatic Lipid Metabolism
To investigate effect of EP oil on hepatic lipid metabolism in DSS-treated mice, lipidomics were investigated using an untargeted metabolomics approach. Potential functional biomarkers of lipid metabolites were analyzed in the control, DSS, and EP oil groups. In the liver, 122 lipids were identified and a PCA-based clustering approach revealed a strong segregation with PC1 (53.4%) and PC2 (18.9%) (Figure 6A).
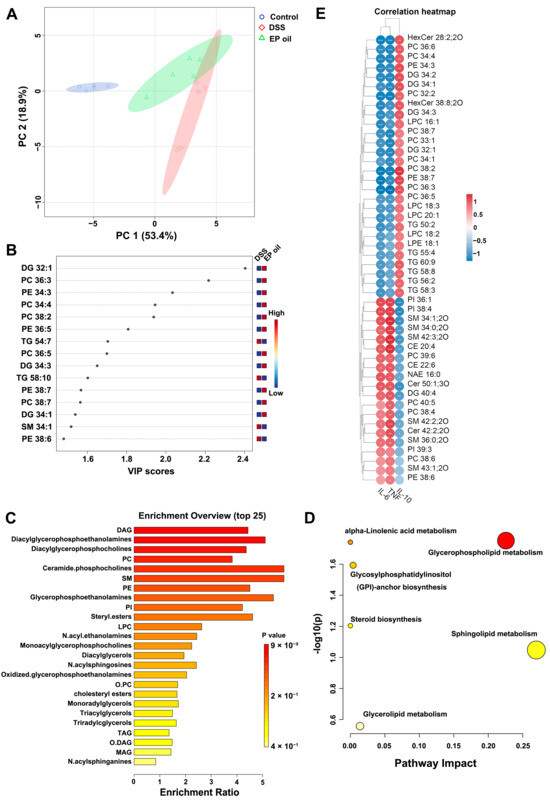
Figure 6.
EP oil influenced lipid metabolism in liver: (A) PCA score plots (n = 5); (B) VIP map of PLS-DA between DSS and EP oil group; (C) enrichment of lipid metabolites; (D) pathway impact of lipid metabolites; (E) heatmap of association between cytokines and significantly changed metabolites.
PLS-DA analysis assessed the changes in lipid metabolites after DSS-induced colitis and EP oil treatment (Figure S1). VIP (variable importance in projection) for the top 15 significant lipid metabolites were selected in the EP oil and DSS groups (Figure 6B). The lipid metabolites including diacylglycerol (DG), phosphatidylcholine (PC), and phosphatidylethanolamine (PE) with polyunsaturated fatty acids were higher in the EP oil group than in the DSS group. The DSS and control groups were separated by PLS-DA, and the VIP scores of the DSS group were clustered with DG, ceramide (Cer), glycerophospholipids (PI), and sphingomyelin (SM) (Figure S1C).
The heatmap showed that mice in the DSS group had significantly lower triacylglycerol expression (TG) and significantly higher expression of PI, SM, cholesteryl ester (CE), PC, and PE compared to the control group (Figure S2). Significantly changed lipid metabolites were selected for by the volcano plot based on fold-change and t tests (Figure S3). There were clear differences in the expression of lipid metabolites including DG 32:1, DG 34:3, PC 36:3, PE 34:3, PC34:4, PC36:5, and PC 38:2 between the DSS group and the EP oil group.
Enrichment analysis showed that PI, ceramide phosphocholines, SM, DG, and PC were associated with DSS-induced colitis (Figure S4). EP oil had an effect on the expression of metabolites including phospholipids, SM, ceramide phosphocholines, PC, PE, PI, and steryl esters (Figure 6C). Furthermore, pathway analysis showed that glycerophospholipid metabolism, sphingolipid metabolism, glycerolipid metabolism, steroid biosynthesis, glycosylphosphatidylinositol (GPI) biosynthesis were associated with the EP oil treatment group and the DSS-induced colitis group (Figure 6D and Figure S5).
The correlations of lipid metabolites and cytokines were analyzed by Pearson correlation test (Figure 6E). In total, 48 lipid metabolites were significantly correlated with inflammatory cytokines. HexCer, PC, PE, LPC, and TG were related to IL-10, particularly DG 32:1, PC 36:3, PE 34:3, PC34:4, and PC 38:2. Moreover, PI, SM, CE, and Cer were found to be significantly associated with IL-6 and TNF-α. The above results suggested that lipid metabolites containing long-chain and unsaturated fatty acids exerted stronger anti-inflammatory effects.
Abnormal lipid metabolism have associated with the risk of intestinal inflammatory; notably, PC, Cer, and SM were most significantly changed [36,37]. Lipidomics analysis showed that DSS induced changes in hepatic lipid metabolite composition. TGs in the DSS group were also significantly lower than in the control group. LPC, PC (16:0/20:4), TG (16:0/18:0/18:1), SM (18:2/24:0), TG (14:0/16:0/18:2), TG (18:1/18:2/20:4), CE (14:1), PE (O-16:0/20:4), SM (d18:1/21:0) and sitosterol sulfate are biomarkers of UC and Crohn’s disease [38,39]. Mice in the DSS group had increased PI, SM, CE, LPC, and PC, which was correlated with IL-6 and TNF-α [38,40,41]. Sphingolipids, including hexosylceramide (HexCer 38:8), PC36:6, PC36:3, PC38:2, PE38:7, PC38:7, and TG58:8, which contain long-chain polyunsaturated fatty acids had a positive association with IL-10. These results indicate that EP oil has the potential ability to regulate lipid metabolism and alleviate inflammation owing to its content of C16 and C18 n-3 PUFAs.
4. Conclusions
In summary, our findings that highlight EP oil is rich in C16 and C18 n-3 PUFAs, especially C16:3n-3, C16:4n-3, and C18:4n-3, which could alleviate DSS-induced colitis and regulate lipid metabolism. As the monomer compounds of C16:3n-3, C16:4n-3, and C18:4n-3 were difficult to prepare, further study is needed to clarify the anti-inflammatory mechanisms of C16:3n-3, C16:4n-3, and C18:4n-3. These findings suggest that Enteromorpha prolifera may be a promising new method of dietary prevention for UC treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13010046/s1, Figure S1: PLS-DA scores plot of (A) control and DSS groups and (B) EP oil and DSS groups. (C) VIP of PLS-DA between control and DSS groups; Figure S2: Heat map of (A) control and DSS groups and (B) EP oil and DSS groups; Figure S3: Volcano plot of (A) control and DSS groups and (B) EP oil and DSS groups; Figure S4: Lipid metabolite enrichment analysis between control and DSS groups; Figure S5: Metabolism pathway analysis of lipid metabolites between control and DSS groups.
Author Contributions
Conceptualization, H.W.; methodology, H.W. and X.W.; software, X.W.; validation, H.W.; formal analysis, H.W.; investigation, H.W. and X.W.; resources, P.M.L.; data curation, H.W.; writing—original draft preparation, H.W.; writing—review and editing, P.M.L.; visualization, H.W.; supervision, D.L.; project administration, D.L.; Funding acquisition, H.W. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Postdoctoral Science Foundation of Qingdao, grant number RZ2100005372 and Postdoctoral Innovation Project of Shandong Province, grant number SDCX-ZG-202203012.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Qingdao University Laboratory Animal Welfare Ethics Committee (protocol code No. 20211231C576020220111070 and 31 December 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Panah, F.M.; Nielsen, K.D.; Simpson, G.L.; Schönherz, A.; Schramm, A.; Lauridsen, C.; Nielsen, T.S.; Højberg, O.; Fredborg, M.; Purup, S.; et al. A westernized diet changed the colonic bacterial composition and metabolite concentration in a dextran sulfate sodium pig model for ulcerative colitis. Front. Microbiol. 2023, 14, 1018242. [Google Scholar] [CrossRef]
- Saadh, M.J.; Pal, R.S.; Arias-Gonzáles, J.L.; Orosco Gavilán, J.C.; Jc, D.; Mohany, M.; Al-Rejaie, S.S.; Bahrami, A.; Kadham, M.J.; Amin, A.H.; et al. A Mendelian Randomization Analysis Investigates Causal Associations between Inflammatory Bowel Diseases and Variable Risk Factors. Nutrients 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Durkin, L.A.; Childs, C.E.; Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and the Intestinal Epithelium—A Review. Foods 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Nusrat, A. Saving Problematic Mucosae: SPMs in Intestinal Mucosal Inflammation and Repair. Trends Mol. Med. 2019, 25, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Dong, L.; Yan, Q.; Dong, Y.; Wang, L.; Wang, F. Preparation and Characterization of an Anticancer Peptide from Oriental Tonic Food Enteromorpha prolifera. Foods 2022, 11, 3507. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; He, P.; Wang, Z.; Shi, Y.; Wang, Y. The world’s largest macroalgal bloom in the Yellow Sea, China: Formation and implications. Estuar. Coast. Shelf Sci. 2013, 129, 2–10. [Google Scholar] [CrossRef]
- Zhao, S.; He, Y.; Wang, C.; Assani, I.; Hou, P.; Feng, Y.; Yang, J.; Wang, Y.; Liao, Z.; Shen, S. Isolation, Characterization and Bioactive Properties of Alkali-Extracted Polysaccharides from Enteromorpha prolifera. Mar. Drugs 2020, 18, 552. [Google Scholar] [CrossRef]
- Song, W.; Wang, Z.; Zhang, X.; Li, Y. Ethanol Extract from Ulva prolifera Prevents High-Fat Diet-Induced Insulin Resistance, Oxidative Stress, and Inflammation Response in Mice. Biomed Res. Int. 2018, 2018, 1374565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Chen, W.; Huang, F.; Chen, S.; Wang, X.; Yang, C. Algal oil alleviates antibiotic-induced intestinal inflammation by regulating gut microbiota and repairing intestinal barrier. Front. Nutr. 2022, 9, 1081717. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ye, D.; Yuan, L.; Sun, Y.; Han, D.; Hu, Q. Solid Matrix-Supported Supercritical CO₂ Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Mar. Drugs 2019, 17, 203. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Siriamornpun, S.; Li, D. Polyunsaturated Fatty Acid Content of Edible Insects in Thailand. J. Food Lipids 2006, 13, 277–285. [Google Scholar] [CrossRef]
- Xu, X.; Lu, S.; Li, X.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Zeng, M.; Zhao, Y. Effects of microbial diversity and phospholipids on flavor profile of caviar from hybrid sturgeon (Huso dauricus × Acipenser schrencki). Food Chem. 2022, 377, 131969. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Fan, Z.-K.; Gao, X.; Zhou, F.; Guo, X.-F.; Sinclair, A.J.; Li, D. n-3 polyunsaturated fatty acids in phospholipid or triacylglycerol form attenuate nonalcoholic fatty liver disease via mediating cannabinoid receptor 1/adiponectin/ceramide pathway. J. Nutr. Biochem. 2024, 123, 109484. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, H.; Zhao, C.; Zhong, F.; Li, D. Oyster polysaccharides relieve DSS-induced colitis via anti-inflammatory and maintaining the physiological hypoxia. Int. J. Biol. Macromol. 2023, 238, 124150. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, A.G.; Jaja-Chimedza, A.; Graf, B.L.; Waterman, C.; Verzi, M.P.; Raskin, I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE 2017, 12, e0184709. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Wang, X.; Lan, H.; Sun, T.; Cong, P.; Xue, C.; Xu, J. Serum metabolomics analysis reveals amelioration effects of sea cucumber ether phospholipids on oxidative stress and inflammation in high-fat diet-fed mice. Food Funct. 2022, 13, 10134–10146. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-K.; Ma, W.-J.; Zhang, W.; Li, H.; Zhai, J.; Zhao, T.; Guo, X.-F.; Sinclair, A.J.; Li, D. Elevated serum phosphatidylcholine (16:1/22:6) levels promoted by fish oil and vitamin D(3) are highly correlated with biomarkers of non-alcoholic fatty liver disease in Chinese subjects. Food Funct. 2022, 13, 11705–11714. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Tian, H.; Liu, C.; Zhang, X.; Peng, Y.; Yang, X.; Chen, F.; Li, J. Metformin and cyanidin 3-O-galactoside from Aronia melanocarpa synergistically alleviate cognitive impairment in SAMP8 mice. Food Funct. 2021, 12, 10994–11008. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Ripol, A.; Afonso, C.; Freire, M.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Sci. Nutr. 2017, 5, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yamazaki, Y.; Koike, S.; Hakozaki, M.; Nagahora, N.; Yuki, S.; Yano, A.; Tsurumi, K.; Okumura, T. Lipids, fatty acids and hydroxy-fatty acids of Euphausia pacifica. Sci. Rep. 2017, 7, 9944. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.G.; Lasiter, A.D.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. III. Four cold-adapted, peridinin-containing taxa and the presence of trigalactosyldiacylglycerol as an additional glycolipid. Eur. J. Phycol. 2009, 44, 439–445. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2021, 1, 30–40. [Google Scholar] [CrossRef]
- De Paula do Nascimento, R.; Lima, A.V.; Oyama, L.M.; Paiotti, A.P.R.; Cardili, L.; Martinez, C.A.R.; Pereira, J.A.; Silva, M.F.; Garofolo, I.C.; Silveira, V.L.F.; et al. Extra virgin olive oil and flaxseed oil have no preventive effects on DSS-induced acute ulcerative colitis. Nutrition 2020, 74, 110731. [Google Scholar] [CrossRef]
- Hudert, C.A.; Weylandt, K.H.; Lu, Y.; Wang, J.; Hong, S.; Dignass, A.; Serhan, C.N.; Kang, J.X. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11276–11281. [Google Scholar] [CrossRef]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Murata, M.; Kaneniwa, M.; Saito, H.; Komatsu, W.; Shinohara, K. Purification of stearidonic acid (18:4(n-3)) and hexadecatetraenoic acid (16:4(n-3)) from algal fatty acid with lipase and medium pressure liquid chromatography. Biosci. Biotechnol. Biochem. 2000, 64, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Nunes, L.V.; Duarte, M.T.S.; Ferreira, L.F.R.; Soriano, R.N.; Iqbal, H.M.N. Exploitation of Marine-Derived Robust Biological Molecules to Manage Inflammatory Bowel Disease. Mar. Drugs 2021, 19, 196. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-N.; Choi, Y.-S.; Kim, S.H.; Zhong, X.; Kim, W.; Park, J.S.; Saeidi, S.; Han, B.W.; Kim, N.; Lee, H.S.; et al. Resolvin D1 suppresses inflammation-associated tumorigenesis in the colon by inhibiting IL-6-induced mitotic spindle abnormality. FASEB J. 2021, 35, e21432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xue, Z.; Yang, H.; Zhao, F.; Liu, C.; Chen, J.; Lu, S.; Zou, Z.; Zhou, Y.; Zhang, X. Differential effects of EPA and DHA on DSS-induced colitis in mice and possible mechanisms involved. Food Funct. 2021, 12, 1803–1817. [Google Scholar] [CrossRef] [PubMed]
- Diab, J.; Hansen, T.; Goll, R.; Stenlund, H.; Ahnlund, M.; Jensen, E.; Moritz, T.; Florholmen, J.; Forsdahl, G. Lipidomics in Ulcerative Colitis Reveal Alteration in Mucosal Lipid Composition Associated With the Disease State. Inflamm. Bowel Dis. 2019, 25, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Scoville, E.A.; Allaman, M.M.; Brown, C.T.; Motley, A.K.; Horst, S.N.; Williams, C.S.; Koyama, T.; Zhao, Z.; Adams, D.W.; Beaulieu, D.B.; et al. Alterations in Lipid, Amino Acid, and Energy Metabolism Distinguish Crohn’s Disease from Ulcerative Colitis and Control Subjects by Serum Metabolomic Profiling. Metabolomics 2018, 14, 17. [Google Scholar] [CrossRef]
- Ferru-Clément, R.; Boucher, G.; Forest, A.; Bouchard, B.; Bitton, A.; Lesage, S.; Schumm, P.; Lazarev, M.; Brant, S.; Duerr, R.H.; et al. Serum Lipidomic Screen Identifies Key Metabolites, Pathways, and Disease Classifiers in Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 1024–1037. [Google Scholar] [CrossRef]
- Murgia, A.; Hinz, C.; Liggi, S.; Denes, J.; Hall, Z.; West, J.; Santoru, M.L.; Piras, C.; Manis, C.; Usai, P.; et al. Italian cohort of patients affected by inflammatory bowel disease is characterised by variation in glycerophospholipid, free fatty acids and amino acid levels. Metabolomics 2018, 14, 140. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Zhou, E.; Tao, Y.; Wang, M.; Qi, S.; Zhao, L.; Tan, Y.; Wu, L. Integrated microbiomic and metabolomic analyses reveal the mechanisms by which bee pollen and royal jelly lipid extracts ameliorate colitis in mice. Food Res. Int. 2023, 171, 113069. [Google Scholar] [CrossRef]
- Fan, F.; Mundra, P.A.; Fang, L.; Galvin, A.; Moore, X.L.; Weir, J.M.; Wong, G.; White, D.A.; Chin-Dusting, J.; Sparrow, M.P.; et al. Lipidomic Profiling in Inflammatory Bowel Disease: Comparison Between Ulcerative Colitis and Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).