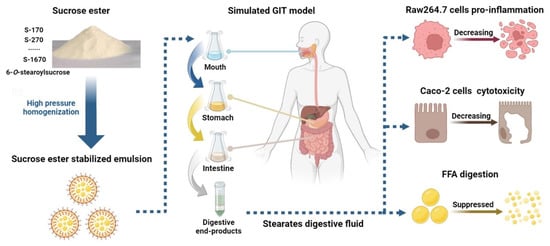
Sucrose Stearates Stabilized Oil-in-Water Emulsions: Gastrointestinal Fate, Cell Cytotoxicity and Proinflammatory Effects after Simulated Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Emulsion Preparation
2.3. Droplet Size and Charge Measurements
2.4. Study on the Lipid Digestive Profiles of Emulsions Stabilized by Sucrose Stearate In Vitro
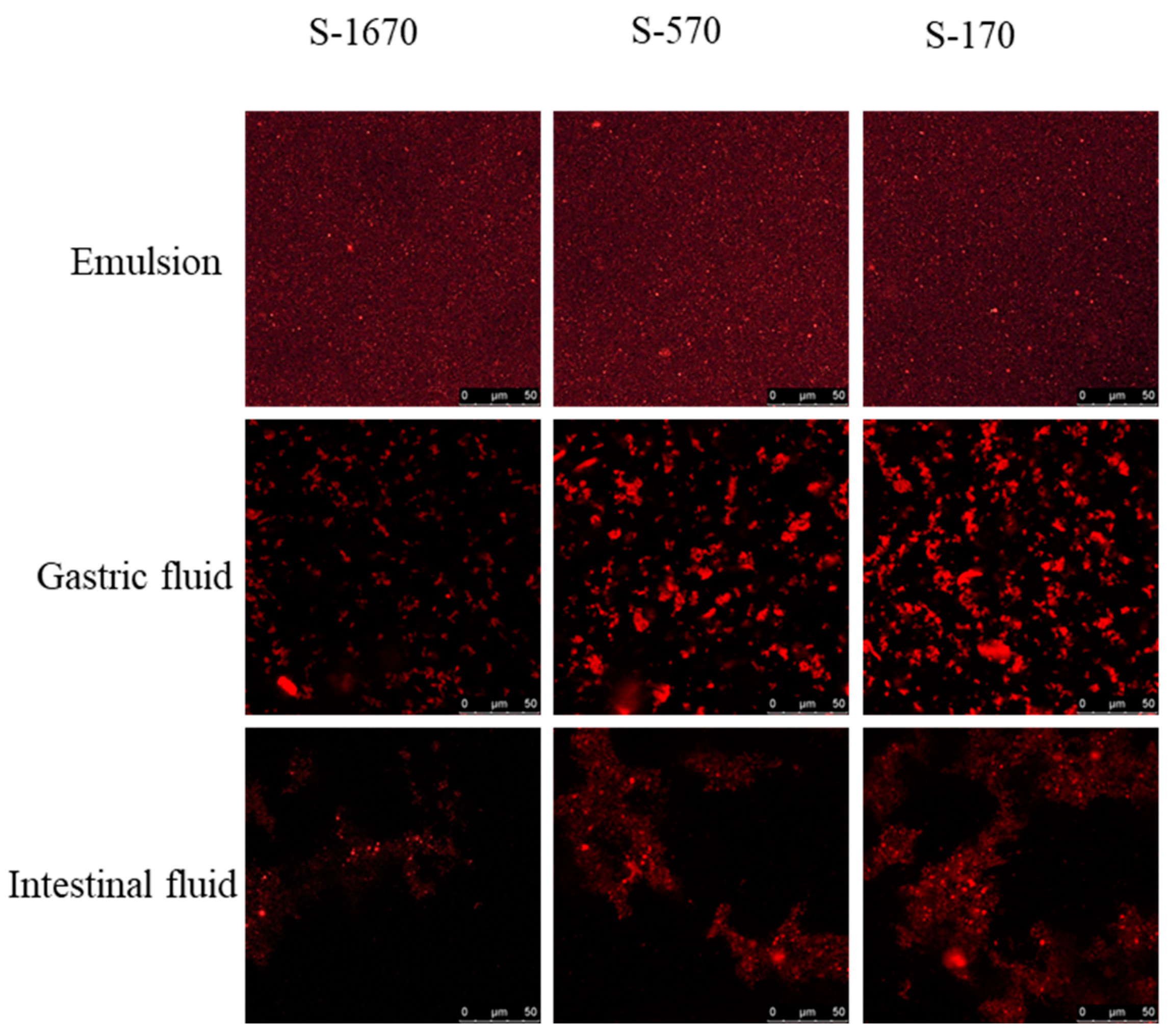
2.5. Confocal Microscopy
2.6. Study on the Digestive Behavior of S-170 to S-1670 in a Simulated GIT
2.7. High-Performance Liquid Chromatography (HPLC) Analysis
2.8. Cell Viability
2.9. Nitric Oxide (NO) Assay
2.10. Statistical Analysis
3. Results and Discussion
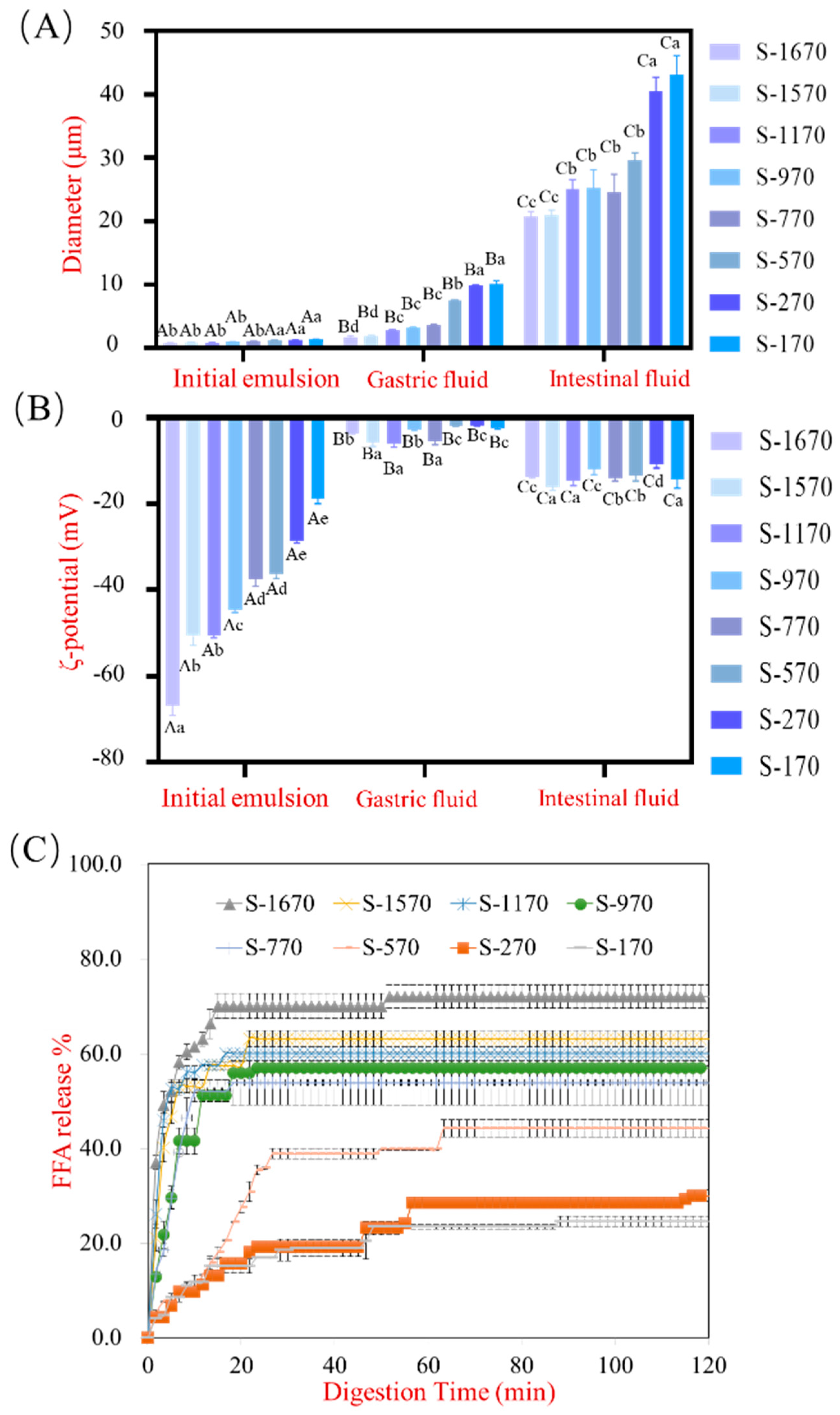
3.1. The Effect of Sucrose Stearate with Different HLB Values on Lipid Digestion in Emulsion under GIT
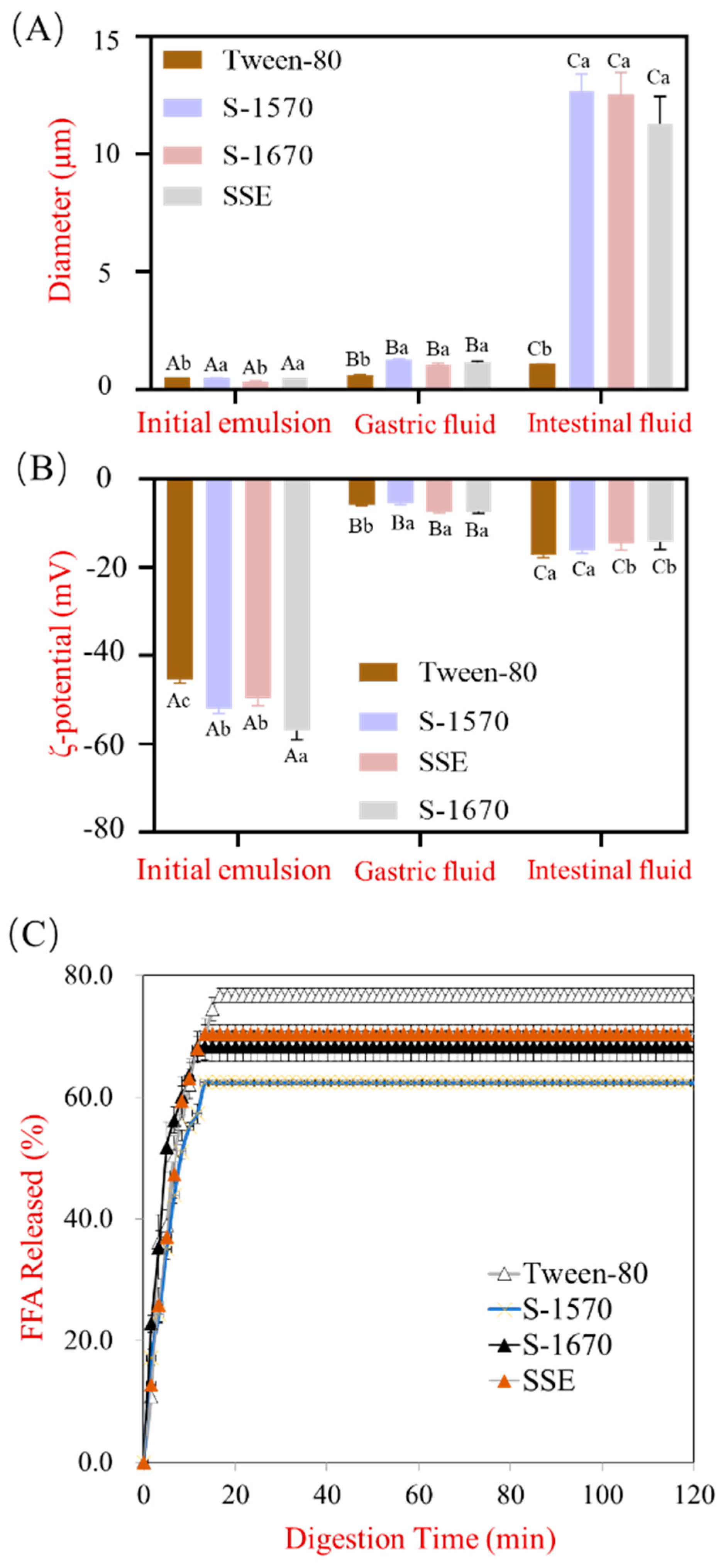
3.2. The Effect of Different Types of Emulsifiers with the Same HLB Value on Lipolysis Behavior
3.3. The Digestive Behavior of a Series of Sucrose Stearate under Gastrointestinal Conditions
3.4. The Quantification of Sucrose Stearates after In Vitro Digestion
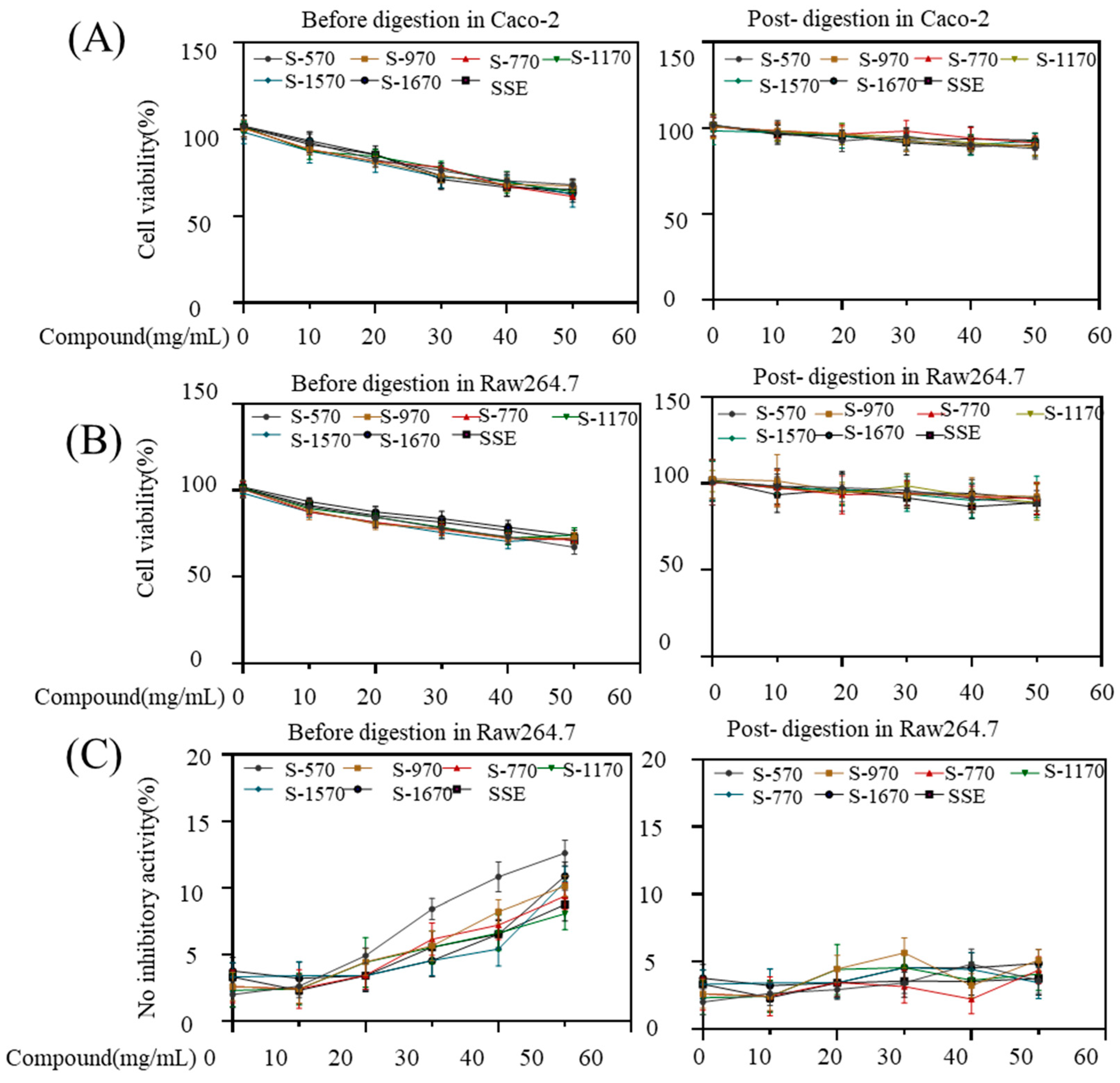
3.5. Bioactivity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Name | Mono Ester (%) | Poly Esters (%) | HLB |
|---|---|---|---|
| S-170 | 1 | 99 | 1 |
| S-270 | 10 | 90 | 2 |
| S-570 | 30 | 70 | 5 |
| S-770 | 40 | 60 | 7 |
| S-970 | 50 | 50 | 9 |
| S-1170 | 55 | 45 | 11 |
| S-1570 | 70 | 30 | 15 |
| S-1670 | 75 | 25 | 16 |
| SSE | 100 | 0 | 16 |
References
- Infantes-Garcia, M.R.; Verkempinck, S.H.; Hendrickx, M.E.; Grauwet, T. Kinetic modeling of in vitro small intestinal lipid digestion as affected by the emulsion interfacial composition and gastric prelipolysis. J. Agric. Food Chem. 2021, 69, 4708–4719. [Google Scholar] [CrossRef] [PubMed]
- Infantes-Garcia, M.R.; Verkempinck, S.; Gonzalez-Fuentes, P.; Hendrickx, M.; Grauwet, T. Lipolysis products formation during in vitro gastric digestion is affected by the emulsion interfacial composition. Food Hydrocoll. 2021, 110, 106163. [Google Scholar] [CrossRef]
- Jain, S.; Winuprasith, T.; Suphantharika, M. Encapsulation of lycopene in emulsions and hydrogel beads using dual modified rice starch: Characterization, stability analysis and release behaviour during in-vitro digestion. Food Hydrocoll. 2020, 104, 105730. [Google Scholar] [CrossRef]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.; Jakob, S.; Mosenthin, R. Principles of physiology of lipid digestion. Asian-Australas. J. Anim. Sci. 2005, 18, 282–295. [Google Scholar] [CrossRef]
- Szűts, A.; Szabó-Révész, P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Degner, B.; Olson, K.; Rose, D.; Schlegel, V.; Hutkins, R.; McClements, D. Influence of freezing rate variation on the microstructure and physicochemical properties of food emulsions. J. Food Eng. 2013, 119, 244–253. [Google Scholar] [CrossRef]
- Jadhav, J.; Pratap, A.P. Enzymatic synthesis and characterization of sucrose erucate. Tenside Surfactants Deterg. 2017, 54, 539–545. [Google Scholar] [CrossRef]
- Shao, S.Y.; Shi, Y.G.; Wu, Y.; Bian, L.Q.; Zhu, Y.J.; Huang, X.Y.; Pan, Y.; Zeng, L.Y.; Zhang, R.R. Lipase-catalyzed synthesis of sucrose monolaurate and its antibacterial property and mode of action against four pathogenic bacteria. Molecules 2018, 23, 1118. [Google Scholar] [CrossRef]
- Verkempinck, S.; Salvia-Trujillo, L.; Moens, L.; Charleer, L.; Van Loey, A.; Hendrickx, M.; Grauwet, T. Emulsion stability during gastrointestinal conditions effects lipid digestion kinetics. Food Chem. 2018, 246, 179–191. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, D.; Gan, L.; Lu, X.; Wang, Y. Octenyl succinate esterified gum arabic stabilized emulsions: Preparation, stability and in vitro gastrointestinal digestion. LWT 2021, 149, 112022. [Google Scholar] [CrossRef]
- Raku, T.; Kitagawa, M.; Shimakawa, H.; Tokiwa, Y. Enzymatic synthesis of hydrophilic undecylenic acid sugar esters and their biodegradability. Biotechnol. Lett. 2003, 25, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hai, Y.-W.; Ma, D.; Chen, J.; Banwell, M.G.; Lan, P. Fatty acid ester surfactants derived from raffinose: Synthesis, characterization and structure-property profiles. J. Colloid Interface Sci. 2019, 556, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-P.; Ma, Y.-R.; Teng, Y.; Chen, J.; Banwell, M.G.; Lan, P. Emulsifying properties of an homologous series of medium-and long-chain D-maltotriose esters and their impacts on the viabilities of selected cell lines. J. Agric. Food Chem. 2020, 68, 9004–9013. [Google Scholar] [CrossRef] [PubMed]
- Baker, I.J.; Willing, R.I.; Furlong, D.N.; Grieser, F.; Drummond, C.J. Sugar fatty acid ester surfactants: Biodegradation pathways. J. Surfactants Deterg. 2000, 3, 13–27. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lizundia, E. A review on the thermomechanical properties and biodegradation behaviour of polyesters. Eur. Polym. J. 2019, 121, 109296. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Hao, T.; Li, S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015, 187, 370–377. [Google Scholar] [CrossRef]
- Petrova, K.T.; Barros, M.T.; Calhelha, R.C.; Soković, M.; Ferreira, I.C. Antimicrobial and cytotoxic activities of short carbon chain unsaturated sucrose esters. Med. Chem. Res. 2018, 27, 980–988. [Google Scholar] [CrossRef]
- Shimazaki, A.; Sakamoto, J.J.; Furuta, M.; Tsuchido, T. Antifungal activity of diglycerin ester of fatty acids against yeasts and its comparison with those of sucrose monopalmitate and sodium benzoate. Biocontrol Sci. 2016, 21, 123–130. [Google Scholar] [CrossRef][Green Version]
- Bernal, C.-A.; Castellanos, L.; Aragón, D.M.; Martínez-Matamoros, D.; Jiménez, C.; Baena, Y.; Ramos, F.A. Peruvioses A to F, sucrose esters from the exudate of Physalis peruviana fruit as α-amylase inhibitors. Carbohydr. Res. 2018, 461, 4–10. [Google Scholar] [CrossRef]
- Valdés, K.; Morilla, M.J.; Romero, E.; Chávez, J. Physicochemical characterization and cytotoxic studies of nonionic surfactant vesicles using sucrose esters as oral delivery systems. Colloids Surf. B Biointerfaces 2014, 117, 1–6. [Google Scholar] [CrossRef]
- Andrade, J.; Wright, A.J.; Corredig, M. In vitro digestion behavior of water-in-oil-in-water emulsions with gelled oil-water inner phases. Food Res. Int. 2018, 105, 41–51. [Google Scholar] [CrossRef]
- Xie, M.-F.; White, L.V.; Banwell, M.G.; Wang, Y.; Lan, P. Solvent-Free Synthesis of High-Purity Sucrose Fatty Acid Monoesters and a Comparison of Their Properties with Those of Their Commercial Counterparts. ACS Food Sci. Technol. 2021, 1, 1550–1560. [Google Scholar] [CrossRef]
- Bao, C.; Kramata, P.; Lee, H.J.; Suh, N. Regulation of hedgehog signaling in cancer by natural and dietary compounds. Mol. Nutr. Food Res. 2018, 62, 1700621. [Google Scholar] [CrossRef]
- Hubert, F.; Loiseau, C.; Ergan, F.; Pencréaćh, G.; Poisson, L. Fast fatty acid analysis by core-shell reversed-phase liquid chromatography coupled to evaporative light-scattering detector. Food Nutr. Sci. 2017, 8, 1051–1062. [Google Scholar] [CrossRef][Green Version]
- Qiu, S.; Zhou, Y.; Kim, J.T.; Bao, C.; Lee, H.J.; Chen, J. Amentoflavone inhibits tumor necrosis factor-α-induced migration and invasion through AKT/mTOR/S6k1/hedgehog signaling in human breast cancer. Food Funct. 2021, 12, 10196–10209. [Google Scholar] [CrossRef]
- Zheng, B.; Zheng, B.; Carr, A.J.; Yu, X.; McClements, D.J.; Bhatia, S.R. Emulsions stabilized by inorganic nanoclays and surfactants: Stability, viscosity, and implications for applications. Inorganica Chim. Acta 2020, 508, 119566. [Google Scholar] [CrossRef]
- Cabezas, D.M.; Diehl, B.W.; Tomás, M.C. Emulsifying properties of hydrolysed and low HLB sunflower lecithin mixtures. Eur. J. Lipid Sci. Technol. 2016, 118, 975–983. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, A.; Du, Y.; Cao, Y.; Zhang, E.; Zhou, Q.; Hai, H.; Zhen, Y.; Zhang, S. Effects of sucrose ester structures on liposome-mediated gene delivery. Acta Biomater. 2018, 72, 278–286. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Designing excipient emulsions to increase nutraceutical bioavailability: Emulsifier type influences curcumin stability and bioaccessibility by altering gastrointestinal fate. Food Funct. 2015, 6, 2475–2486. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.; Sun, L.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: Influence of emulsion droplet size. Food Chem. 2017, 229, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Ménard, O.; Etienne, J.; Ossemond, J.; Durand, A.; Buffin, R.; Loizon, E.; Meugnier, E.; Deglaire, A.; Dupont, D.; et al. Human milk pasteurisation reduces pre-lipolysis but not digestive lipolysis and moderately decreases intestinal lipid uptake in a combination of preterm infant in vitro models. Food Chem. 2020, 329, 126927. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, S.; Jolibois, É.; Britten, M. Effect of emulsifiers on linseed oil emulsion structure, lipolysis and oxidation during in vitro digestion. Food Funct. 2020, 11, 10126–10136. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, X.; Wang, X.; Jin, Q.; McClements, D.J. Influence of dairy emulsifier type and lipid droplet size on gastrointestinal fate of model emulsions: In vitro digestion study. J. Agric. Food Chem. 2018, 66, 9761–9769. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Zhang, S.; Li, J.; Zheng, H.; Jin, H.; Xu, J. Comparison of different protein emulsifiers on physicochemical properties of β-carotene-loaded nanoemulsion: Effect on formation, stability, and in vitro digestion. Nanomaterials 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Verkempinck, S.; Salvia-Trujillo, L.; Moens, L.; Carrillo, C.; Van Loey, A.; Hendrickx, M.; Grauwet, T. Kinetic approach to study the relation between in vitro lipid digestion and carotenoid bioaccessibility in emulsions with different oil unsaturation degree. J. Funct. Foods 2018, 41, 135–147. [Google Scholar] [CrossRef]
- Abedi, S.; Suteria, N.S.; Chen, C.-C.; Vanapalli, S.A. Microfluidic production of size-tunable hexadecane-in-water emulsions: Effect of droplet size on destabilization of two-dimensional emulsions due to partial coalescence. J. Colloid Interface Sci. 2019, 533, 59–70. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef]
- Li, C.; Shen, Y.; Sun, C.; Cheraga, N.; Tu, J. Immunosafety and chronic toxicity evaluation of monomethoxypoly (ethylene glycol)-b-poly (lactic acid) polymer micelles for paclitaxel delivery. Drug Deliv. 2016, 23, 878–885. [Google Scholar] [CrossRef]

| Sample | Initial | Intestinal | ||||||
|---|---|---|---|---|---|---|---|---|
| Monoester (mg/mL) | Monoester (%) | Polyester (mg/mL) | Polyester (%) | Monoester (mg/mL) | Monoester (%) | Polyester (mg/mL) | Polyester (%) | |
| S-170 | 0.024 | 1.21% | 1.9699 | 98.49% | 0.0066 | 0.33% | 0.1638 | 8.19% |
| S-270 | 0.276 | 13.82% | 1.679 | 83.95% | 0 | 0 | 0.1278 | 6.39% |
| S-570 | 0.5144 | 25.72% | 1.478 | 73.90% | 0 | 0 | 0.129 | 6.45% |
| S-770 | 0.689 | 34.45% | 1.2956 | 64.78% | 0 | 0 | 0.195 | 9.75% |
| S-970 | 0.8634 | 43.17% | 1.1226 | 56.13% | 0 | 0 | 0.148 | 7.40% |
| S-1170 | 1.1622 | 58.11% | 0.8322 | 41.61% | 0 | 0 | 0.145 | 7.25% |
| S-1570 | 1.3984 | 69.92% | 0.5698 | 28.49% | 0 | 0 | 0.1652 | 8.26% |
| S-1670 | 1.558 | 77.90% | 0.393 | 19.65% | 0 | 0 | 0.1476 | 7.38% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Guan, W.; Chen, J.; Zeng, C.; Tan, S.; Chen, J.; Ma, D. Sucrose Stearates Stabilized Oil-in-Water Emulsions: Gastrointestinal Fate, Cell Cytotoxicity and Proinflammatory Effects after Simulated Gastrointestinal Digestion. Foods 2024, 13, 175. https://doi.org/10.3390/foods13010175
Zheng D, Guan W, Chen J, Zeng C, Tan S, Chen J, Ma D. Sucrose Stearates Stabilized Oil-in-Water Emulsions: Gastrointestinal Fate, Cell Cytotoxicity and Proinflammatory Effects after Simulated Gastrointestinal Digestion. Foods. 2024; 13(1):175. https://doi.org/10.3390/foods13010175
Chicago/Turabian StyleZheng, Danhong, Weiyan Guan, Jiaqing Chen, Cuicui Zeng, Shen Tan, Jing Chen, and Da Ma. 2024. "Sucrose Stearates Stabilized Oil-in-Water Emulsions: Gastrointestinal Fate, Cell Cytotoxicity and Proinflammatory Effects after Simulated Gastrointestinal Digestion" Foods 13, no. 1: 175. https://doi.org/10.3390/foods13010175
APA StyleZheng, D., Guan, W., Chen, J., Zeng, C., Tan, S., Chen, J., & Ma, D. (2024). Sucrose Stearates Stabilized Oil-in-Water Emulsions: Gastrointestinal Fate, Cell Cytotoxicity and Proinflammatory Effects after Simulated Gastrointestinal Digestion. Foods, 13(1), 175. https://doi.org/10.3390/foods13010175