Characteristics of Quinoa Protein Isolate Treated by Pulsed Electric Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of a Native Quinoa Protein Isolate (N-QPI)
2.3. PEF Treatment of Quinoa Protein Isolate (PEF-QPIs)
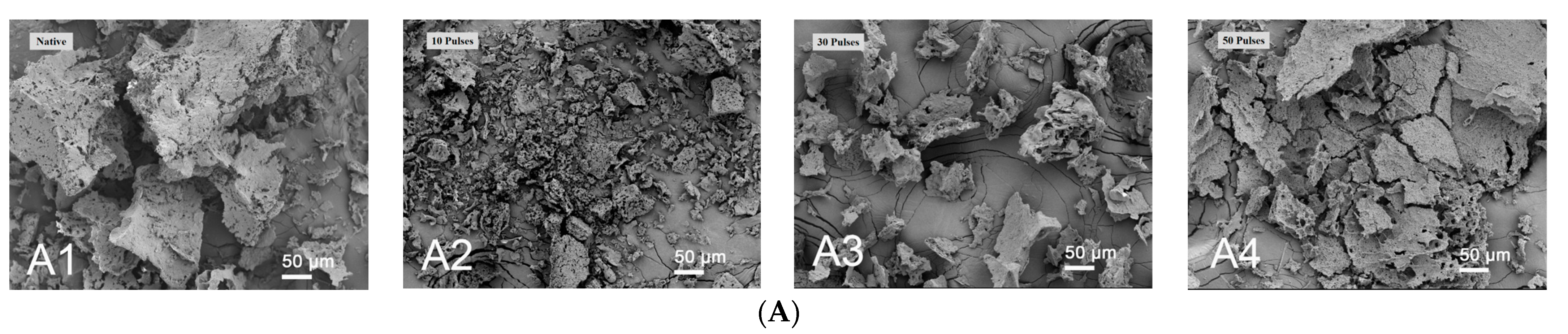
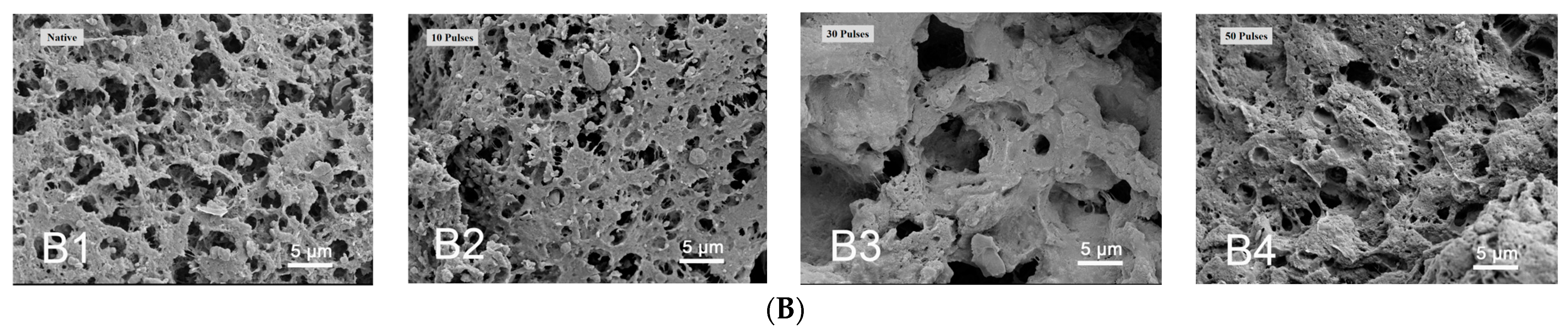
2.4. Scanning Electron Microscopy (SEM)
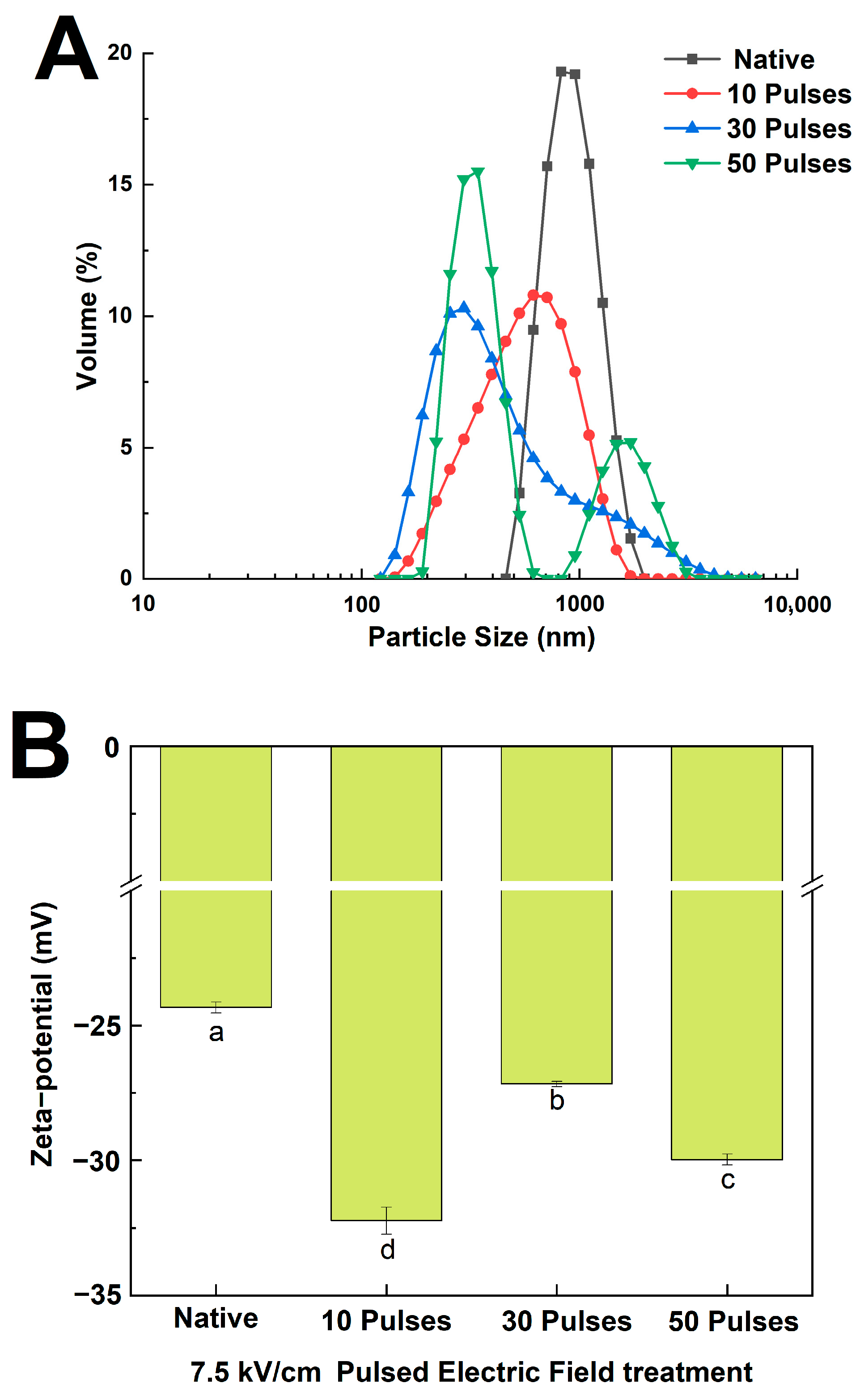
2.5. Determination of Particle Size and Zeta Potential
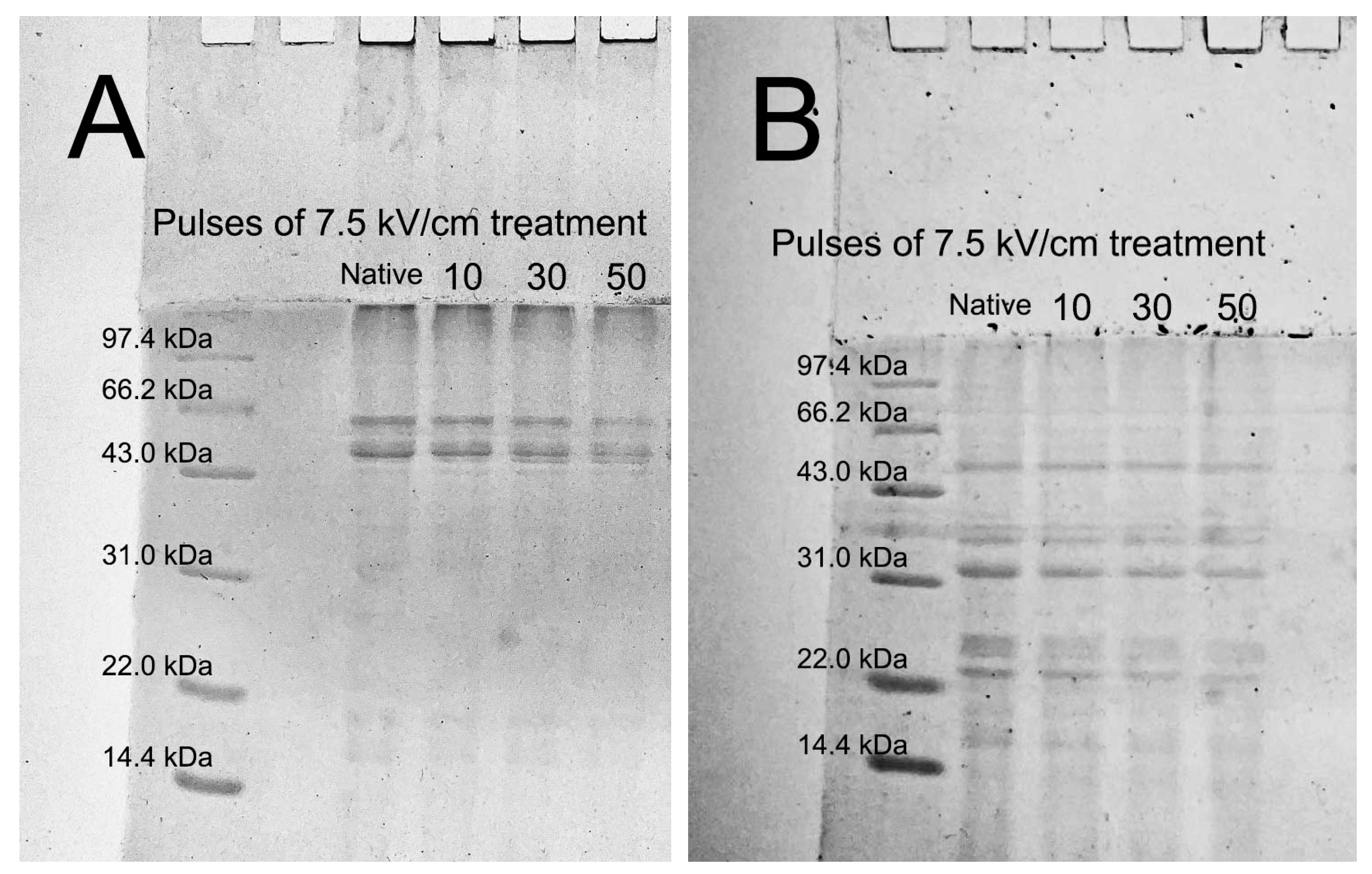
2.6. SDS-PAGE Analysis
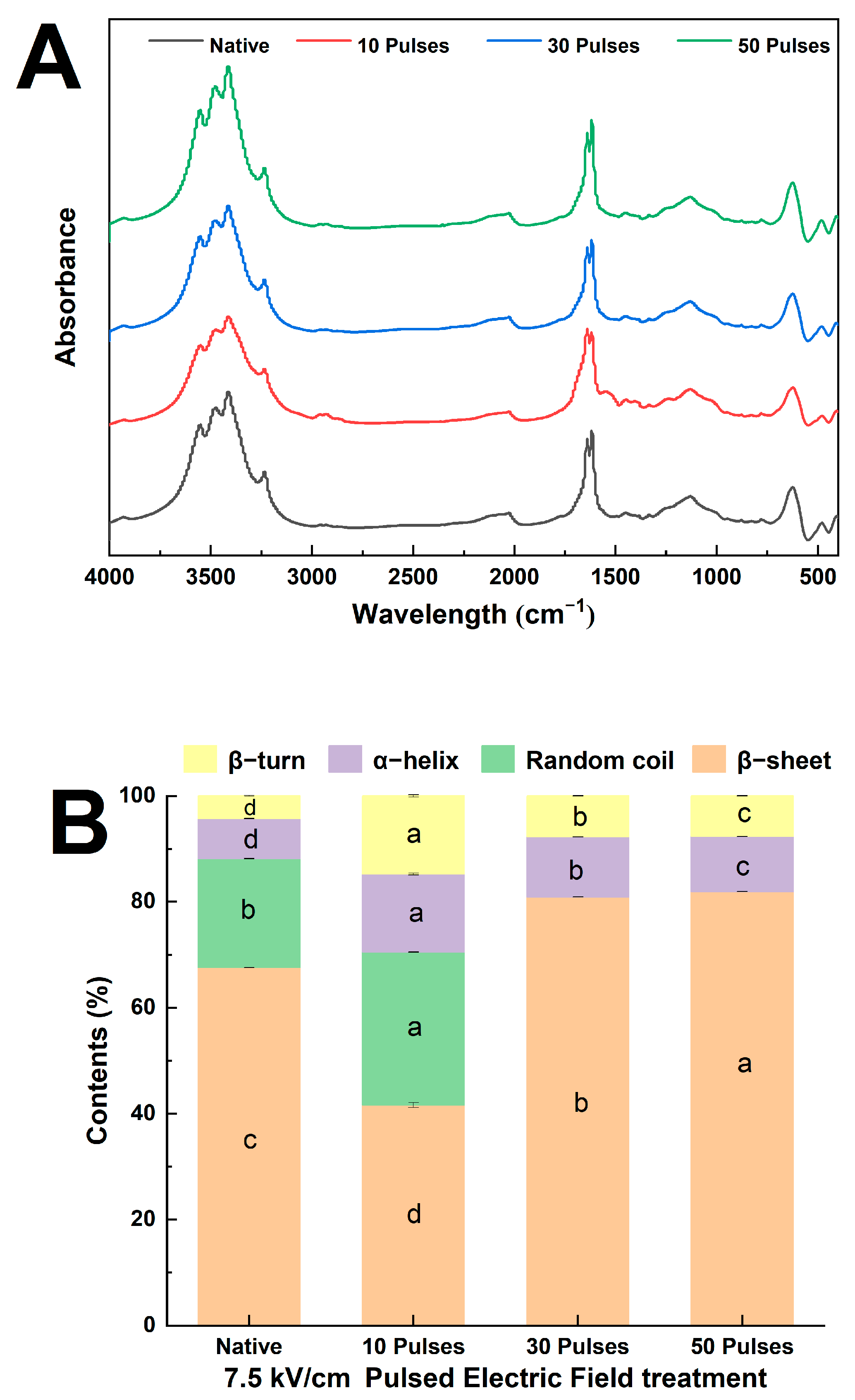
2.7. Determination of Fourier-Transform Infrared Spectroscopy
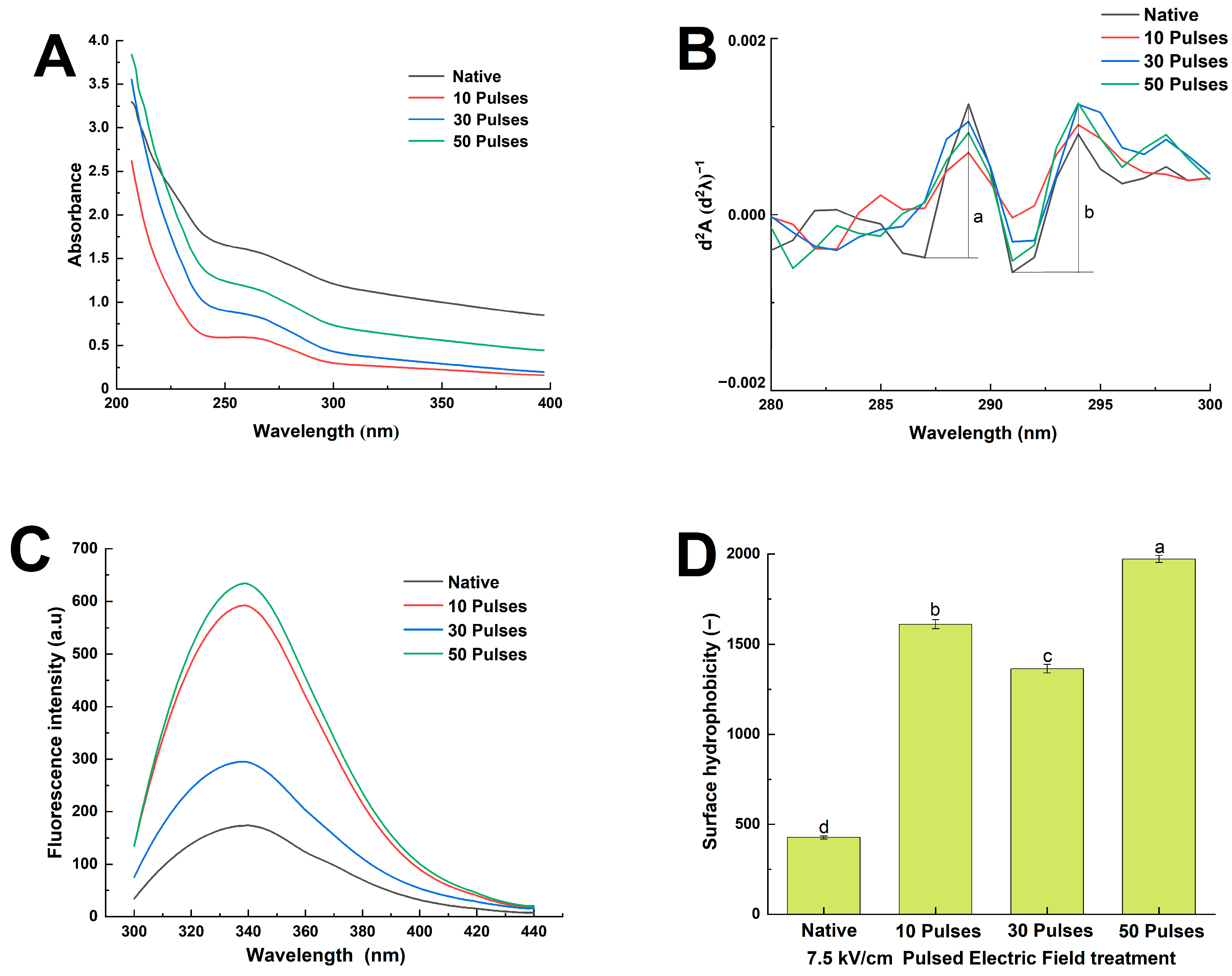
2.8. Determination of UV Spectrum
2.9. Determination of Intrinsic Fluorescence Spectrum
2.10. Determination of Surface Hydrophobicity
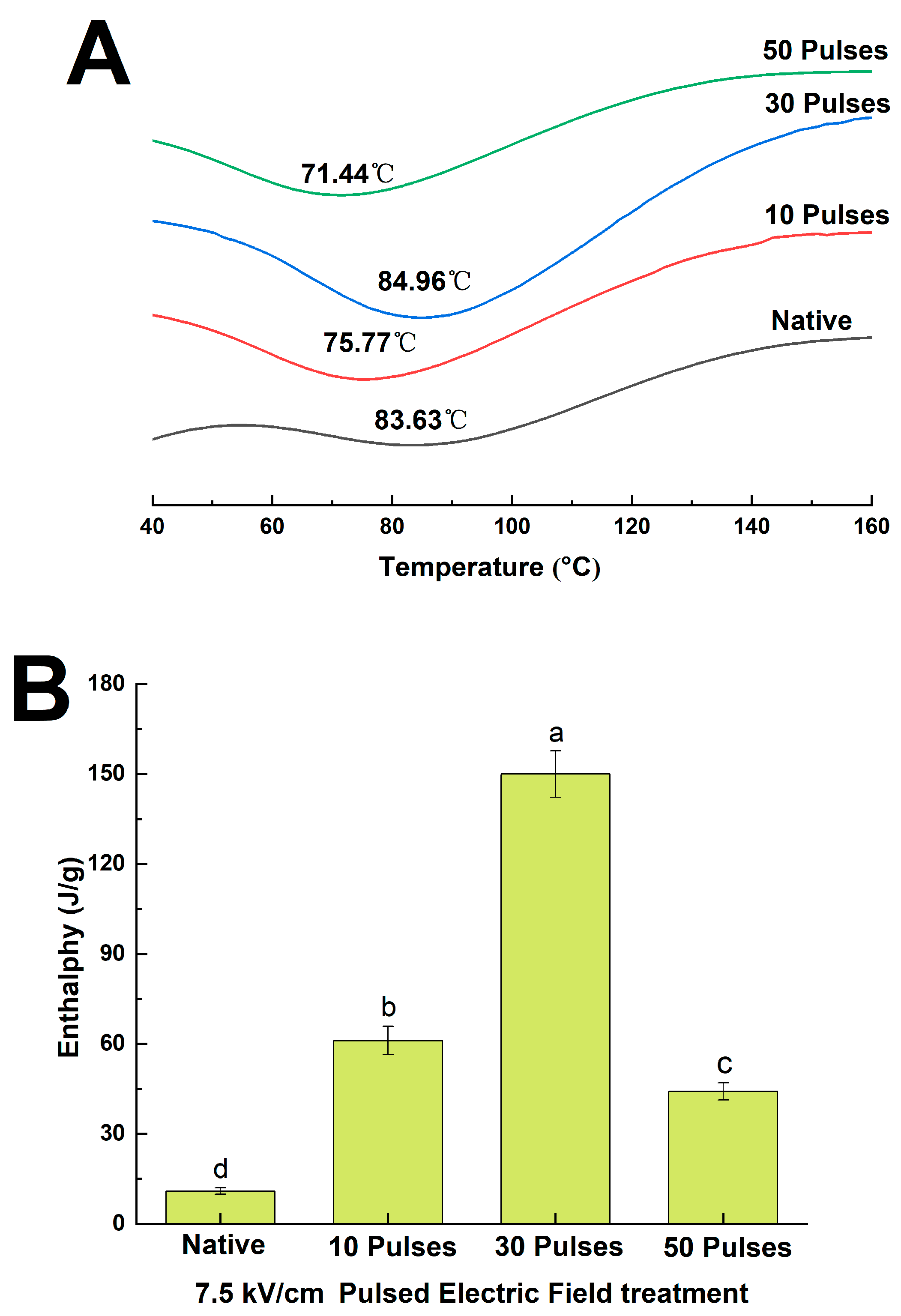
2.11. Differential Scanning Calorimetry
2.12. Determination of the Functional Properties of the Quinoa Protein Isolates
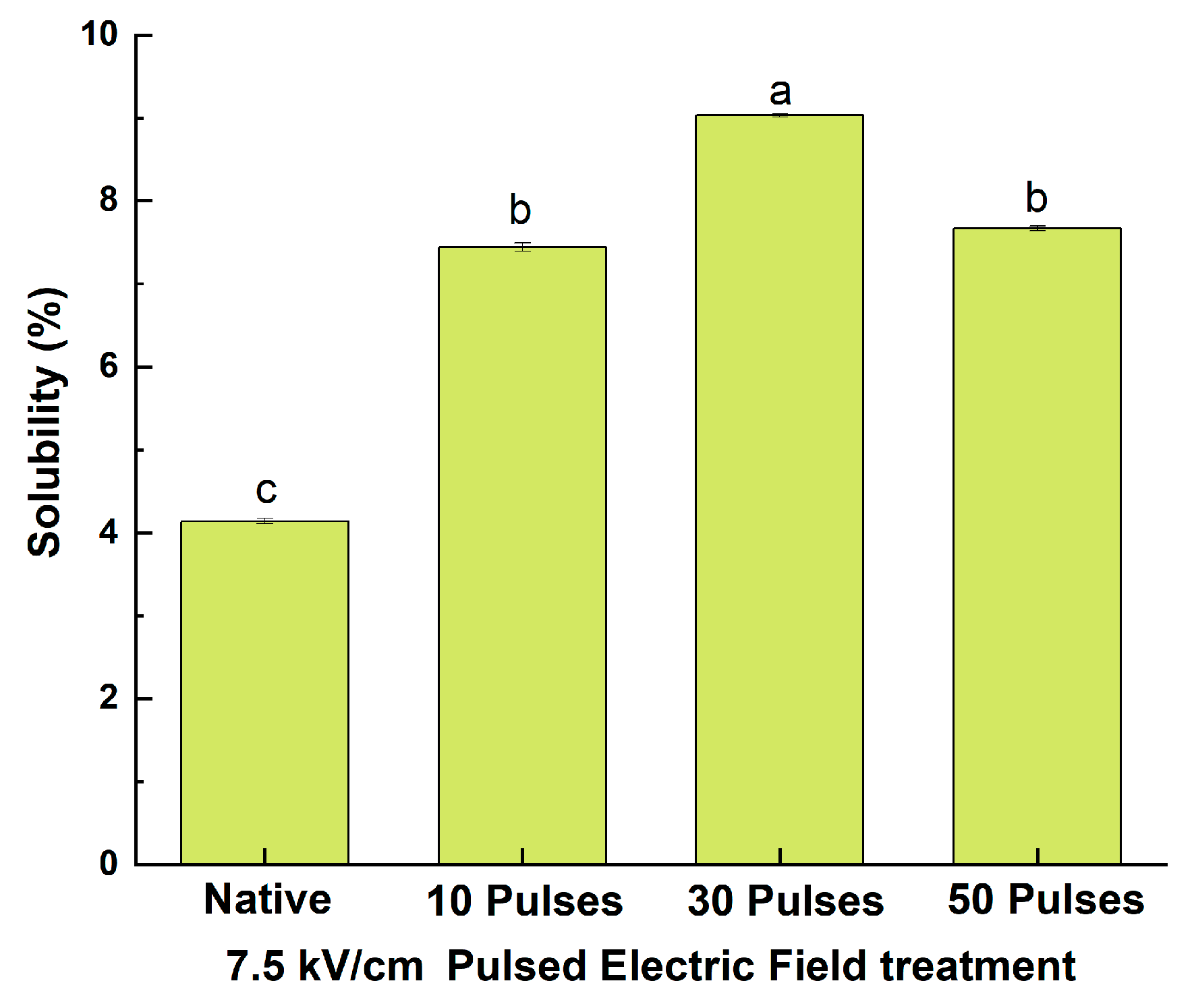
2.12.1. Determination of Solubility
2.12.2. Determination of Emulsifying Properties
2.12.3. Determination of Foaming Properties
2.13. Statistical Analyses
3. Results and Discussion
3.1. Effect of PEF Treatment on Scanning Electron Microscopy (SEM) of QPIs
3.2. Effect of PEF Treatment on the Particle Size and Zeta Potential of QPIs
3.3. SDS-PAGE Analysis of N-QPIs and PEF-QPIs
3.4. Effect of PEF Treatment on the Secondary Structure of QPIs
3.5. Effects of PEF Treatment on the UV Spectrum, Intrinsic Fluorescence Spectrum, and Surface Hydrophobicity of QPIs
3.6. Effects of PEF Treatment on the Thermal Stability of QPIs
3.7. Effect of PEF Treatment on the Functional Properties of QPIs
3.7.1. Effect of PEF Treatment on the Solubility of QPIs
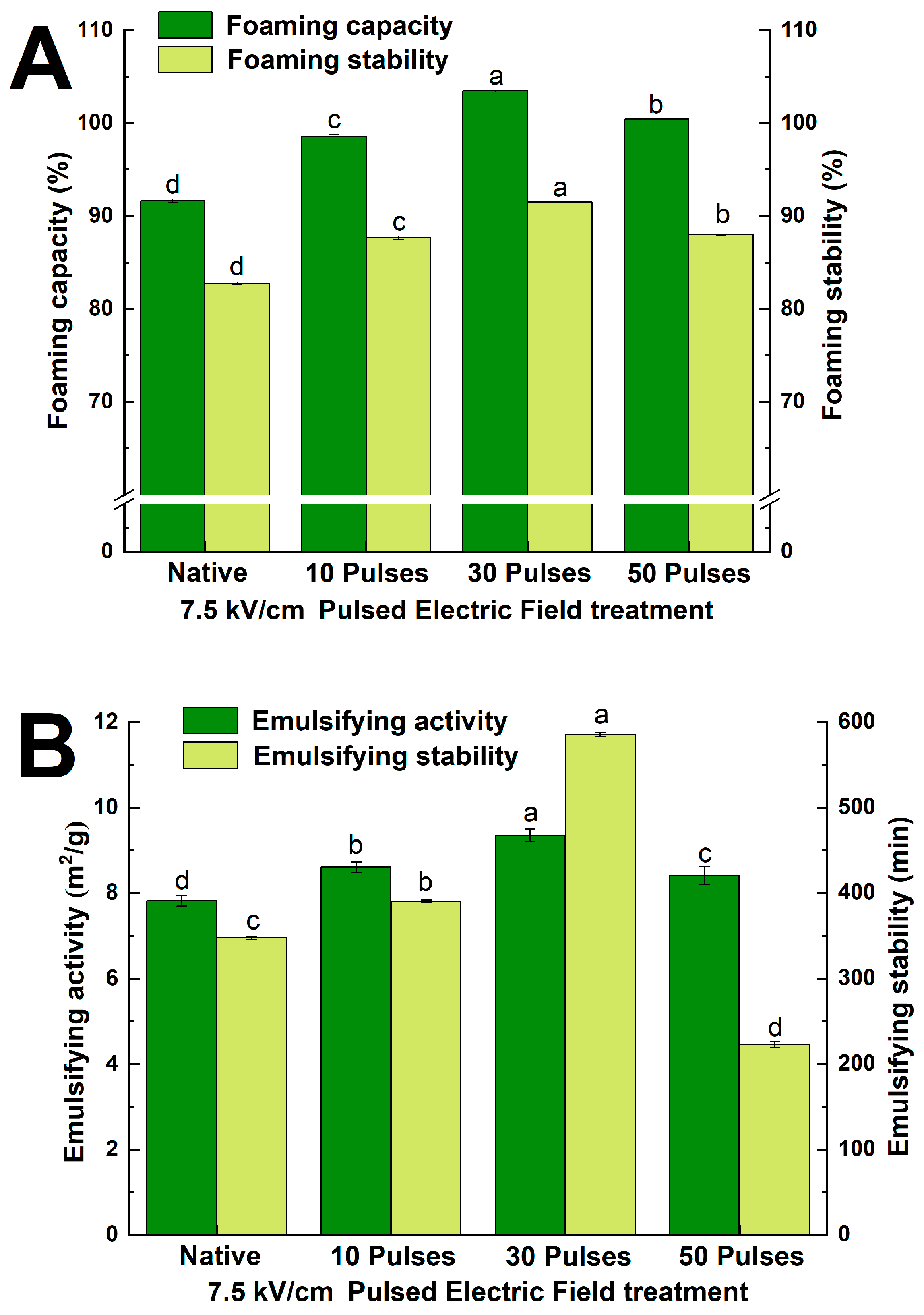
3.7.2. Effect of PEF Treatment on Foaming and Emulsifying Properties of QPIs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Zhang, Y.; Cao, B.; Wei, X.; Shen, Z.; Su, N. Assessment and comparison of nutritional qualities of thirty quinoa (Chenopodium quinoa Willd.) seed varieties. Food Chem. X 2023, 19, 100808. [Google Scholar] [CrossRef] [PubMed]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.-E.; Shabala, S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Walia, S.; Kumar, R. Functional composition, physiological effect and agronomy of future food quinoa (Chenopodium quinoa Willd.): A review. J. Food Compos. Anal. 2023, 118, 105192. [Google Scholar] [CrossRef]
- Rodriguez Gomez, M.J.; Matias Prieto, J.; Cruz Sobrado, V.; Calvo Magro, P. Nutritional characterization of six quinoa (Chenopodium quinoa Willd.) varieties cultivated in Southern Europe. J. Food Compos. Anal. 2021, 99, 103876. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring. Food Chem. 2019, 272, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Huang, K.; Shi, J.; Li, M.; Sun, R.; Guan, W.; Cao, H.; Guan, X.; Zhang, Y. Intervention of microwave irradiation on structure and quality characteristics of quinoa protein aggregates. Food Hydrocoll. 2022, 130, 107677. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.-Q.; Jiang, W.; Wu, M.; Rao, S.-Q.; Qian, J.-Y. Pulsed Electric Field as a Means to Elevate Activity and Expression of -Amylase in Barley (Hordeum vulgare L.) Malting. Food Bioprocess Technol. 2019, 12, 1010–1020. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Singha, P.; Singh, S.K. A comprehensive review on impact of non-thermal processing on the structural changes of food components. Food Res. Int. 2021, 149, 110647. [Google Scholar] [CrossRef]
- Blahovec, J.; Vorobiev, E.; Lebovka, N. Pulsed Electric Fields Pretreatments for the Cooking of Foods. Food Eng. Rev. 2017, 9, 71–81. [Google Scholar] [CrossRef]
- Lyu, C.; Huang, K.; Yang, N.; Wang, H.; Wang, J. Combination of Thermosonication and Pulsed Electric Fields Treatments for Controlling Saccharomyces cerevisiae in Chinese Rice Wine. Food Bioprocess Technol. 2016, 9, 1854–1864. [Google Scholar] [CrossRef]
- Han, S.-F.; Jin, W.; Yang, Q.; Abomohra, A.E.-F.; Zhou, X.; Tu, R.; Chen, C.; Xie, G.-J.; Wang, Q. Application of pulse electric field pretreatment for enhancing lipid extraction from Chlorella pyrenoidosa grown in wastewater. Renew. Energy 2019, 133, 233–239. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, S.; Yan, W.; Tang, Y.; Yang, R.; Zhao, W. Inactivation of apple (Malus domestica Borkh) polyphenol oxidases by radio frequency combined with pulsed electric field treatment. Int. J. Food Sci. Technol. 2018, 53, 2054–2063. [Google Scholar] [CrossRef]
- Lin, Z.-R.; Zeng, X.-A.; Yu, S.-J.; Sun, D.-W. Enhancement of Ethanol-Acetic Acid Esterification Under Room Temperature and Non-catalytic Condition via Pulsed Electric Field Application. Food Bioprocess Technol. 2012, 5, 2637–2645. [Google Scholar] [CrossRef]
- Huang, K.; Jiang, T.; Wang, W.; Gai, L.; Wang, J. A Comparison of Pulsed Electric Field Resistance for Three Microorganisms with Different Biological Factors in Grape Juice via Numerical Simulation. Food Bioprocess Technol. 2014, 7, 1981–1995. [Google Scholar] [CrossRef]
- Polachini, T.C.; Norwood, E.-A.; Le-Bail, P.; Le-Bail, A.; Carcel, J.A. Pulsed electric field (PEF) application on wheat malting process: Effect on hydration kinetics, germination and amylase expression. Innov. Food Sci. Emerg. 2023, 86, 103375. [Google Scholar] [CrossRef]
- Karki, R.; Oey, I.; Bremer, P.; Leong, S.Y.; Silcock, P. Effect of chilled storage on the quality, in-vitro protein digestibility, and microbial growth of sous vide processed beef short ribs pre-treated with pulsed electric field (PEF). Innov. Food Sci. Emerg. Technol. 2023, 89, 103485. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Zhang, Y.; Zeng, X.-A.; El-Mashad, H.; Pan, Z.-L.; Wang, Q.-J. Effect of Pulsed Electric Field on Microstructure of Some Amino Acid Group of Soy Protein Isolates. Int. J. Food Eng. 2014, 10, 113–120. [Google Scholar] [CrossRef]
- Thongkong, S.; Yawootti, A.; Klangpetch, W.; Fashakin, O.O.; Tangjaidee, P.; Rawdkuen, S.; Phongthai, S. A novel application of pulsed electric field as a key process for quick-cooking rice production. Innov. Food Sci. Emerg. Technol. 2023, 90, 103494. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.-C.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H.; Aleid, G.M.; Alhamad, M.N.; Maghaydah, S. Evaluation of quality and protein structure of natural water kefir-fermented quinoa protein concentrates. Food Chem. 2023, 404, 134614. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Available online: http://www.aoac.org/official (accessed on 26 May 2022).
- Pulsed Electric Fields Extraction Systems. Guangzhou Xin’an Food Technol. 2020. Available online: http://www.pefxa.com/ (accessed on 15 June 2021).
- Zhang, L.; Chen, X.; Wang, Y.; Guo, F.; Hu, S.; Hu, J.; Xiong, H.; Zhao, Q. Characteristics of rice dreg protein isolate treated by high-pressure microfluidization with and without proteolysis. Food Chem. 2021, 358, 129861. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Chen, M.; Wang, Y.; Zhang, D.; Zhao, S.; Liu, J.; Pan, F.; Zhang, T. Foaming properties of egg white proteins improved by enzymatic hydrolysis: The changes in structure and physicochemical properties. Food Hydrocoll. 2023, 141, 108681. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification in album (Chenopodium album) protein isolates due to controlled thermal modification and its relationship with protein digestibility and functionality. Food Hydrocoll. 2020, 103, 105708. [Google Scholar] [CrossRef]
- Ling, B.; Ouyang, S.; Wang, S. Effect of radio frequency treatment on functional, structural and thermal behaviors of protein isolates in rice bran. Food Chem. 2019, 289, 537–544. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Huang, H.; Xiong, M.; Yang, Y.; Lin, C.; Yang, F.; Xie, Y.; Yuan, Y. Improvement of the emulsifying properties of Zanthoxylum seed protein by ultrasonic modification. Ultrason. Sonochem. 2023, 100, 106638. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.-J.; Jiang, W.; Qian, J.-Y. Effect of pulsed electric field on functional and structural properties of canola protein by pretreating seeds to elevate oil yield. LWT-Food Sci. Technol. 2017, 84, 73–81. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.-B. Structural and functional properties of self-assembled peanut protein nanoparticles prepared by ultrasonic treatment: Effects of ultrasound intensity and protein concentration. Food Chem. 2023, 413, 135626. [Google Scholar] [CrossRef]
- Maekinen, O.E.; Hager, A.-S.; Arendt, E.K. Localisation and development of proteolytic activities in quinoa (Chenopodium quinoa) seeds during germination and early seedling growth. J. Cereal Sci. 2014, 60, 484–489. [Google Scholar] [CrossRef]
- Maekinen, O.E.; Zannini, E.; Koehler, P.; Arendt, E.K. Heat-denaturation and aggregation of quinoa (Chenopodium quinoa) globulins as affected by the pH value. Food Chem. 2016, 196, 17–24. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Romero, N.; Tapia, C.A.; Silva, J.; Rivera, M. Study of some physicochemical and functional properties of quinoa (chenopodium quinoa willd.) protein isolates. J. Agric. Food Chem. 2008, 56, 4745–4750. [Google Scholar] [CrossRef] [PubMed]
- Alavi, F.; Emam-Djomeh, Z.; Momen, S.; Hosseini, E.; Moosavi-Movahedi, A.A. Fabrication and characterization of acid-induced gels from thermally-aggregated egg white protein formed at alkaline condition. Food Hydrocoll. 2020, 99, 105337. [Google Scholar] [CrossRef]
- Lange, R.; Balny, C. UV-visible derivative spectroscopy under high pressure. Biochim. Biophys. Acta 2002, 1595, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Geng, Z.; Zhang, X.; Ma, T.; Liu, H.; Wang, L. Ultrasonic treatment influences the compactness of quinoa protein microstructure and improves the structural integrity of quinoa protein at the interfaces of high internal phase emulsion. Food Res. Int. 2023, 168, 108681. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sun, R.; Shi, J.; Li, M.; Guan, X.; Liu, J.; Huang, K.; Zhang, Y. Effect of ultrasonic on the structure and quality characteristics of quinoa protein oxidation aggregates. Ultrason. Sonochem. 2021, 77, 105685. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Sun, X. A comparative study of physicochemical and conformational properties in three vicilins from Phaseolus legumes: Implications for the structure-function relationship. Food Hydrocoll. 2011, 25, 315–324. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Yuan, X.; Zhao, S.; Kang, Z.; Zhu, M.; He, H.; Ma, H. Physicochemical, conformational and functional changes of quinoa protein affected by high-pressure homogenization. LWT-Food Sci. Technol. 2023, 173, 114343. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, W.; Yan, D.; Ma, L.; Ma, H. Solubility and aggregation of soy protein isolate induced by different ionic liquids with the assistance of ultrasound. Int. J. Biol. Macromol. 2020, 164, 2277–2283. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Q.; Waterink, B.; Venema, P.; de Vries, R.; Sagis, L.M.C. Physical, interfacial and foaming properties of different mung bean protein fractions. Food Hydrocoll. 2023, 143, 108885. [Google Scholar] [CrossRef]
- Wang, W.N.; Wang, X.; Zhang, H.R.; Wang, H.; Wang, L.Q.; Zhang, N.; Yu, D.Y. Effects of electric field intensity regulation on protein aggregation behaviour and foaming property of soybean 7S globulin. Int. J. Biol. Macromol. 2023, 248, 125784. [Google Scholar] [CrossRef]
- Igartua, D.E.; Dichano, M.C.; Ferrari, S.B.; Palazolo, G.G.; Cabezas, D.M. Combination of pH-shifting, ultrasound, and heat treatments to enhance solubility and emulsifying stability of rice protein isolate. Food Chem. 2024, 433, 137319. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Z.; Zheng, X.; Wen, W.; Wang, X. Characteristics of Quinoa Protein Isolate Treated by Pulsed Electric Field. Foods 2024, 13, 148. https://doi.org/10.3390/foods13010148
Zhang X, Li Z, Zheng X, Wen W, Wang X. Characteristics of Quinoa Protein Isolate Treated by Pulsed Electric Field. Foods. 2024; 13(1):148. https://doi.org/10.3390/foods13010148
Chicago/Turabian StyleZhang, Xinyue, Zhanrong Li, Xiaojiao Zheng, Wenjun Wen, and Xiaowen Wang. 2024. "Characteristics of Quinoa Protein Isolate Treated by Pulsed Electric Field" Foods 13, no. 1: 148. https://doi.org/10.3390/foods13010148
APA StyleZhang, X., Li, Z., Zheng, X., Wen, W., & Wang, X. (2024). Characteristics of Quinoa Protein Isolate Treated by Pulsed Electric Field. Foods, 13(1), 148. https://doi.org/10.3390/foods13010148




