Formulation of Polymeric Microparticles Using Eco-Friendly Extracted Crude Fucoidans from Edible Brown Seaweed Undaria pinnatifida
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Raw Material
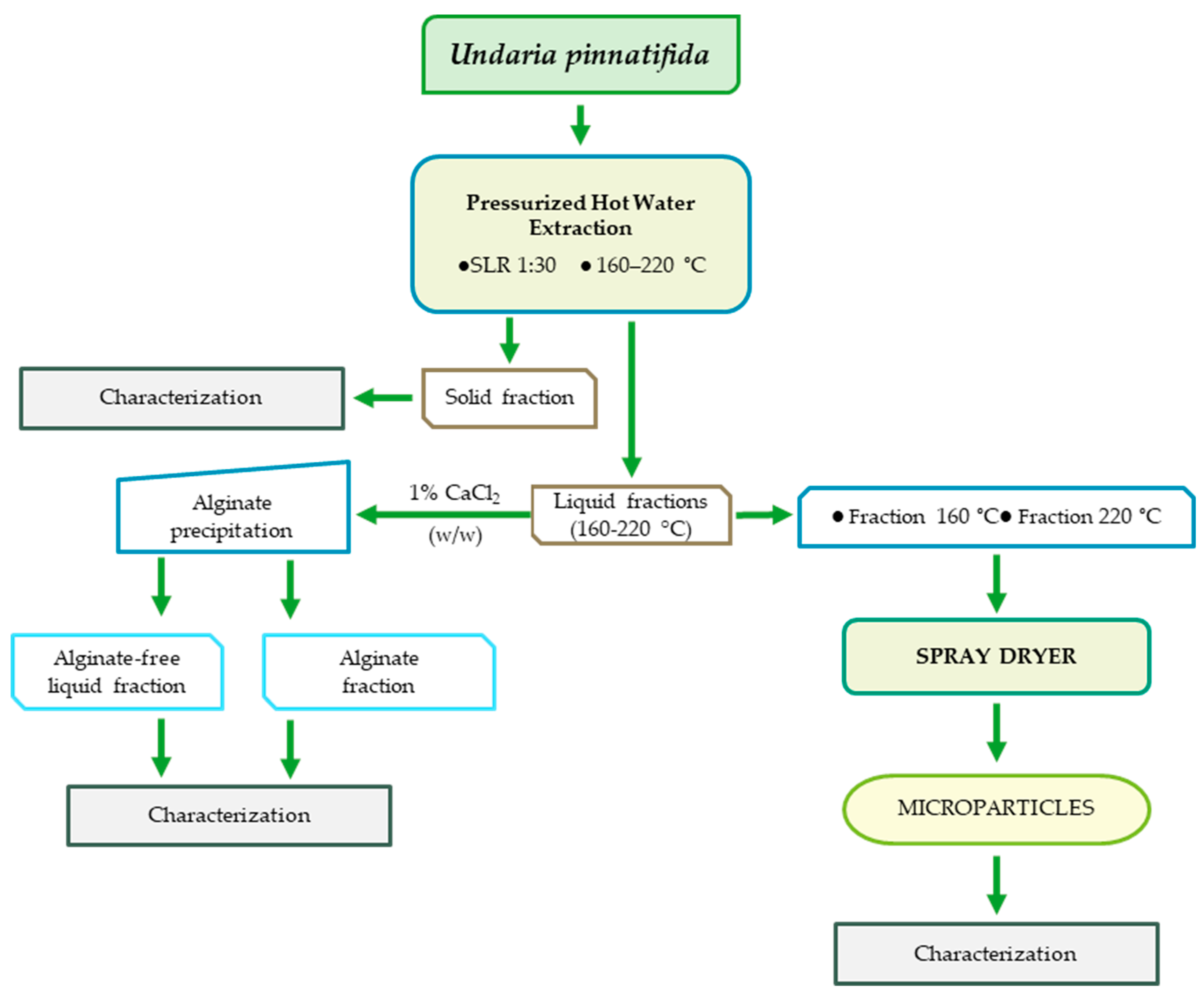
2.2. Extraction Process
Pressurized Hot Water Extraction
2.3. Solid Residues Fractions Characterization
Minerals
2.4. Liquid Fractions Characterization
2.4.1. Protein Content
2.4.2. Antioxidant Features
2.4.3. Oligosaccharide Determination
2.4.4. Sulfate Content
2.5. Structural Profiles
2.5.1. High-Performance Size Exclusion Chromatography
2.5.2. Fourier-Transform Infrared Spectroscopy
2.6. Production of Polymeric Microparticles
2.7. Characterization of Microparticles
2.7.1. Yield of Production
2.7.2. Scanning Electron Microscope
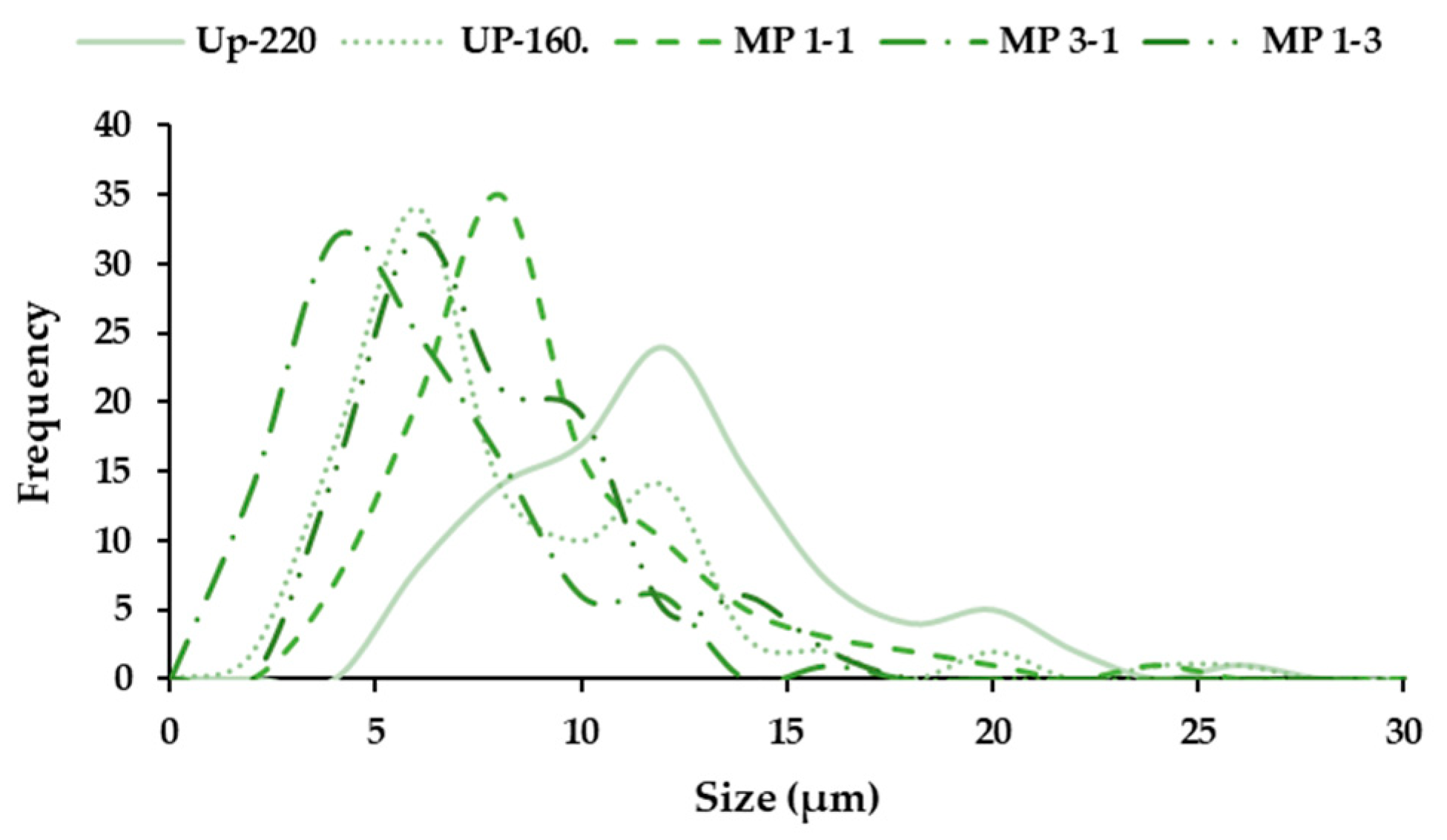
2.7.3. Size of Microparticles
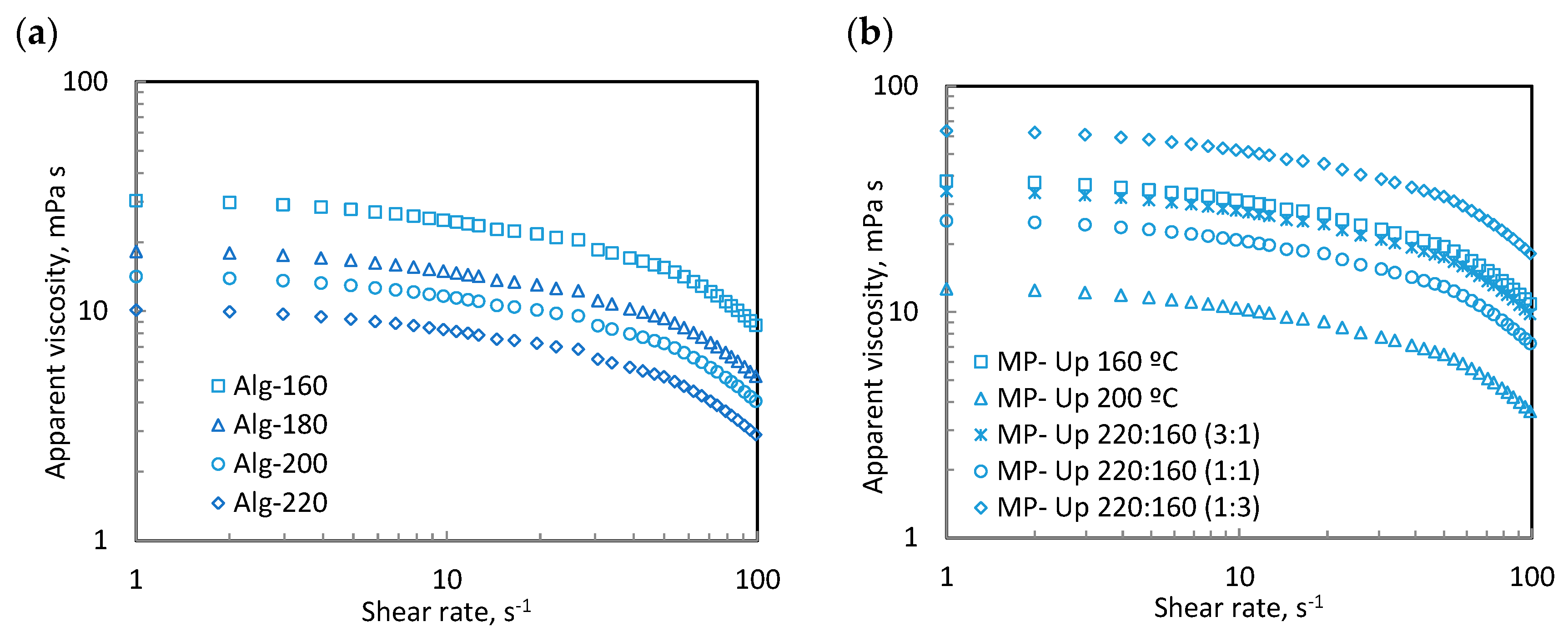
2.8. Rheological Measurements
2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction Process
3.1.1. Solid Residues Fractions Characterization
3.1.2. Liquid Fractions Properties
3.2. Structural Profiles
3.3. Production of Polymeric Microparticles
3.4. Rheological Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128027936. [Google Scholar]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.T.; Jeon, Y.J. Bioactive Potentials of Sulfated Polysaccharides Isolated from Brown Seaweed Sargassum spp in Related to Human Health Applications: A Review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a Bioactive Marine Sulphated Polysaccharide as a Key Constituent of Hybrid Biomaterials: A Review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Prasetyaningrum, A.; Praptyana, I.R.; Nurfiningsih; Ratnawati. Carrageenan: Nutraceutical and Functional Food as Future Food. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012068. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.-T.; Kim, J.-S.; Kim, W.-S.; Lee, J.S.; Jeon, Y.-J. A Review on Fucoidan Structure, Extraction Techniques, and Its Role as an Immunomodulatory Agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive Peptides and Carbohydrates from Seaweed for Food Applications: Natural Occurrence, Isolation, Purification, and Identification. Alga.l Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure Characterization and Antioxidant Activity of Fucoidan Isolated from Undaria pinnatifida Grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Song, K.-M.; Ha, S.J.; Lee, J.-E.; Kim, S.-H.; Kim, Y.H.; Kim, Y.; Hong, S.P.; Jung, S.K.; Lee, N.H. High Yield Ultrasonication Extraction Method for Undaria pinnatifida Sporophyll and Its Anti-Inflammatory Properties Associated with AP-1 Pathway Suppression. LWT-Food Sci. Technol. 2015, 64, 1315–1322. [Google Scholar] [CrossRef]
- Martinez, M.A.; Becherucci, M.E. Study of the Potential Use of the Invasive Marine Algae Undaria pinnatifida in the Preliminary Development of a Functional Textile. J. Ind. Text. 2020, 51, 8127S–8141S. [Google Scholar] [CrossRef]
- Nadeeshani, H.; Hassouna, A.; Lu, J. Proteins Extracted from Seaweed Undaria pinnatifida and Their Potential Uses as Foods and Nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 6187–6203. [Google Scholar] [CrossRef]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef]
- Catarino, M.D.; Pires, S.M.G.; Silva, S.; Costa, F.; Braga, S.S.; Pinto, D.C.G.A.; Silva, A.M.S.; Cardoso, S.M. Overview of Phlorotannins’ Constituents in Fucales. Mar. Drugs 2022, 20, 754. [Google Scholar] [CrossRef]
- Lin, E.-T.; Lee, Y.-C.; Wang, H.-M.; Chiu, C.-Y.; Chang, Y.-K.; Huang, C.-Y.; Chang, C.-C.; Tsai, P.-C.; Chang, J.-S. Efficient Fucoidan Extraction and Purification from Sargassum cristaefolium and Preclinical Dermal Biological Activity Assessments of the Purified Fucoidans. J. Taiwan Inst. Chem. Eng. 2022, 137, 104294. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Zulham; Wilar, G.; Susilawati, Y.; Subarnas, A.; Chaerunisaa, A.Y. Microparticles of Herbal Extracts with Antioxidant Activity. Pharmacogn. J. 2021, 13, 285–295. [Google Scholar] [CrossRef]
- Guerreiro, F.; Swedrowska, M.; Patel, R.; Flórez-Fernández, N.; Dolores Torres, M.; Rosa da Costa, A.M.; Forbes, B.; Grenha, A. Engineering of Konjac Glucomannan into Respirable Microparticles for Delivery of Antitubercular Drugs. Int. J. Pharm. 2021, 604, 120731. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Torge, A.; Grützmacher, P.; Mücklich, F.; Schneider, M. The Influence of Mannitol on Morphology and Disintegration of Spray-Dried Nano-Embedded Microparticles. Eur. J. Pharm. Sci. 2017, 104, 171–179. [Google Scholar] [CrossRef]
- Queffelec, J.; Flórez-Fernández, N.; Dominguez, H.; Torres, M.D. Microwave Hydrothermal Processing of Undaria pinnatifida for Bioactive Peptides. Bioresour. Technol. 2021, 342, 125882. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Recovery of Bioactive and Gelling Extracts from Edible Brown Seaweed Laminaria ochroleuca by Non-Isothermal Autohydrolysis. Food Chem. 2019, 277, 353–361. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of Lignocellulosics by Steam-Aqueous Pretreatments. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; da S. Pereira, L.O.; Marquez, U.M.L. Amino Acid Composition, Protein Content and Calculation of Nitrogen-to-Protein Conversion Factors for 19 Tropical Seaweeds. Phycological. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the Protein Fraction with High Antioxidant Activity from Red Seaweed Industrial Solid Residue after Agar Extraction by Subcritical Water Treatment. J. Appl. Phycol. 2021, 33, 1181–1194. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of Solubre, Cell-Wall-Bound and Exuded Phlorotannins in the Brown Alga Fucus vesiculosus, with Implications on Their Ecological Functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- González-López, N.; Moure, A.; Domínguez, H. Hydrothermal Fractionation of Sargassum muticum Biomass. J. Appl. Phycol. 2012, 24, 1569–1578. [Google Scholar] [CrossRef]
- Balboa, M.E.; Rivas, S.; Moure, A.; Domínguez, H.; Parajó, J.C. Simultaneous Extraction and Depolymerization of Fucoidan from Sargassum muticum in Aqueous Media. Mar. Drugs 2013, 11, 4612–4627. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; González-Muñoz, M.J.; Domínguez, H. Influence of Molecular Weight on the Properties of Sargassum muticum Fucoidan. Algal. Res. 2019, 38, 101393. [Google Scholar] [CrossRef]
- Dodgson, K.S. Determination of Inorganic Sulphate in Studies on the Enzymic and Non-Enzymic Hydrolysis of Carbohydrate and Other Sulphate Esters. Biochem. J. 1961, 78, 312–319. [Google Scholar] [CrossRef]
- Silva, C.; Torres, M.D.; Chenlo, F.; Moreira, R. Rheology of Aqueous Mixtures of Tragacanth and Guar Gums: Effects of Temperature and Polymer Ratio. Food Hydrocoll. 2017, 69, 293–300. [Google Scholar] [CrossRef]
- Cernadas, H.; Flórez-Fernández, N.; González-Muñoz, M.J.; Domínguez, H.; Torres, M.D. Retrieving of High-Value Biomolecules from Edible Himanthalia elongata Brown Seaweed Using Hydrothermal Processing. Food Bioprod. Process. 2019, 7, 275–286. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pacheco, D.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L.; Figueirinha, A.; Dias, J.M. Valorisation of Marine Macroalgae Waste Using a Cascade Biorefinery Approach: Exploratory Study. J. Clean. Prod. 2023, 385, 135672. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Pedersen, T.H.; Rosendahl, L.A. Biocrude Production via Non-Catalytic Supercritical Hydrothermal Liquefaction of Fucus vesiculosus Seaweed Processing Residues. Recycling 2021, 6, 45. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of Heating Values of Biomass Fuel from Elemental Composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Gan, A.; Baroutian, S. Subcritical Water Extraction for Recovery of Phenolics and Fucoidan from New Zealand Wakame (Undaria pinnatifida) Seaweed. J. Supercrit. Fluids 2022, 190, 105732. [Google Scholar] [CrossRef]
- Neri, T.A.N.; Rohmah, Z.; Ticar, B.F.; Palmos, G.N.; Choi, B.D. Evaluation of Sea Mustard (Undaria pinnatifida) Sporophylls from South Korea as Fucoidan Source and Its Corresponding Antioxidant Activities. Fish Aquatic. Sci. 2019, 22, 24. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Tuvikene, R.; Somasiri, M.N.R.; De Silva, M.; Adhikari, R.; Ranahewa, T.H.; Wijesundara, R.; Wijesekera, S.K.; Dissanayake, I.; Wangchuk, P.; et al. A Novel Therapeutic Effect of Mannitol-Rich Extract from the Brown Seaweed Sargassum ilicifolium Using in Vitro and in Vivo Models. BMC Complement. Med. Ther. 2023, 23, 26. [Google Scholar] [CrossRef]
- Puhari, S.S.M.; Yuvaraj, S.; Vasudevan, V.; Ramprasath, T.; Rajkumar, P.; Arunkumar, K.; Amutha, C.; Selvam, G.S. Isolation and Characterization of Fucoidan from Four Brown Algae and Study of the Cardioprotective Effect of Fucoidan from Sargassum wightii against High Glucose-Induced Oxidative Stress in H9c2 Cardiomyoblast Cells. J. Food Biochem. 2022, 46, e14412. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR Spectroscopy as a Tool for Polysaccharide Identification in Edible Brown and Red Seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Belattmania, Z.; Kaidi, S.; Atouani, S.E.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR Characterization of Alginates from the Main Alginophyte Species of the Atlantic Coast of Morocco. Molecules 2020, 25, 4335. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A Green Approach for Alginate Extraction from Sargassum muticum Brown Seaweed Using Ultrasound-Assisted Technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Bittkau, K.S.; Alban, S. Size Distribution and Chain Conformation of Six Different Fucoidans Using Size-Exclusion Chromatography with Multiple Detection. J. Chromatogr. A 2020, 1612, 460658. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Design and Characterization of Controlled-Release Vitamin A Microparticles Prepared by a Spray-Drying Process. Powder. Technol. 2017, 305, 411–417. [Google Scholar] [CrossRef]
- Grenha, A.; Guerreiro, F.; Lourenço, J.P.; Lopes, J.A.; Cámara-Martos, F. Microencapsulation of Selenium by Spray-Drying as a Tool to Improve Bioaccessibility in Food Matrix. Food Chem. 2023, 402, 134463. [Google Scholar] [CrossRef]
- Baltrusch, K.L.; Torres, M.D.; Domínguez, H.; Flórez-Fernández, N. Spray-Drying Microencapsulation of Tea Extracts Using Green Starch, Alginate or Carrageenan as Carrier Materials. Int. J. Biol. Macromol. 2022, 203, 417–429. [Google Scholar] [CrossRef]
- Bäther, S.; Hundschell, C.S.; Kieserling, H.; Wagemans, A.M. Impact of the Solvent Properties on Molecular Interactions and Phase Behaviour of Alginate-Gelatin Systems. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130455. [Google Scholar] [CrossRef]
- Pérez-Larrán, P.; Balboa, E.M.; Torres, M.D.; Domínguez, H. Antioxidant and Antitumoral Properties of Aqueous Fractions from Frozen Sargassum muticum. Waste Biomass Valorization 2020, 11, 1261–1269. [Google Scholar] [CrossRef]
- Mazza, K.E.L.; Costa, A.M.M.; da Silva, J.P.L.; Alviano, D.S.; Bizzo, H.R.; Tonon, R.V. Microencapsulation of Marjoram Essential Oil as a Food Additive Using Sodium Alginate and Whey Protein Isolate. Int. J. Biol. Macromol. 2023, 233, 123478. [Google Scholar] [CrossRef]






| Relation (w/w) | Extract 160 °C | Extract 220 °C |
|---|---|---|
| 3:1 | 3 | 1 |
| 1:1 | 1 | 1 |
| 1:3 | 1 | 3 |
| 160 °C | 180 °C | 200 °C | 220 °C | |
| Ash * | 18.50 | 13.43 | 8.05 | 6.48 |
| Sulphate (%) | 0.78 ± 0.01 | 0.65 ± 0.01 | 0.37 ± 0.01 | 0.25 ± 0.01 |
| C (%) | 40.45 ± 0.24 | 43.50 ± 0.19 | 50.64 ± 1.49 | 54.20 ± 0.15 |
| Protein (%) | 24.83 ± 0.34 | 27.49 ± 0.84 | 31.88 ± 0.95 | 29.86 ± 1.14 |
| H (%) | 6.06 ± 0.09 | 5.95 ± 0.01 | 6.66 ± 0.01 | 6.65 ± 0.06 |
| N (%) | 4.62 ± 0.06 | 5.11 ± 0.16 | 5.93 ± 0.18 | 5.55 ± 0.21 |
| HHV (kJ/kg) | 16,665.72 ± 110.75 | 17,876.38 ± 52.42 | 21,146.91 ± 719.21 | 22,803.15 ± 70.22 |
| Mineral ** | (mg/kg) | |||
| Na | 101,812 | 98,203 | 104,423 | 102,408 |
| K | 99,979 | 97,246 | 102,996 | 99,294 |
| Mg | 13,103 | 13,376 | 15,094 | 15,032 |
| Ca | 5365 | 6111 | 8822 | 10,014 |
| P | 3331 | 3393 | 3933 | 4016 |
| I | 383 | 369 | 396 | 418 |
| Fe | 124.7 | 17.00 | 31.14 | 17.52 |
| Zn | 7.01 | 3.23 | 7.77 | 5.21 |
| Cu | 2.94 | 1.54 | 3.75 | 2.27 |
| Cd | 0 | 0 | 0.16 | 0.16 |
| Production Yield (%) | |
|---|---|
| MP-160 | 57.88 ± 1.57 |
| MP-220 | 74.20 ± 3.55 |
| MP-1:3 (220:160) | 67.37 ± 0.57 |
| MP-3:1 (220:160) | 71.05 ± 0.45 |
| MP-1:1 (220:160) | 74.77 ± 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Anta, T.; Torres, M.D.; Dominguez, H.; Flórez-Fernández, N. Formulation of Polymeric Microparticles Using Eco-Friendly Extracted Crude Fucoidans from Edible Brown Seaweed Undaria pinnatifida. Foods 2023, 12, 1859. https://doi.org/10.3390/foods12091859
Ferreira-Anta T, Torres MD, Dominguez H, Flórez-Fernández N. Formulation of Polymeric Microparticles Using Eco-Friendly Extracted Crude Fucoidans from Edible Brown Seaweed Undaria pinnatifida. Foods. 2023; 12(9):1859. https://doi.org/10.3390/foods12091859
Chicago/Turabian StyleFerreira-Anta, Tania, Maria Dolores Torres, Herminia Dominguez, and Noelia Flórez-Fernández. 2023. "Formulation of Polymeric Microparticles Using Eco-Friendly Extracted Crude Fucoidans from Edible Brown Seaweed Undaria pinnatifida" Foods 12, no. 9: 1859. https://doi.org/10.3390/foods12091859
APA StyleFerreira-Anta, T., Torres, M. D., Dominguez, H., & Flórez-Fernández, N. (2023). Formulation of Polymeric Microparticles Using Eco-Friendly Extracted Crude Fucoidans from Edible Brown Seaweed Undaria pinnatifida. Foods, 12(9), 1859. https://doi.org/10.3390/foods12091859








