Isoflavone Content and Nutritional-Related Properties of Debittered Seeds from Two Andean Lupin (Lupinus mutabilis Sweet) Ecotypes Propagated in Two Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Phase I: L. mutabilis Propagation
2.1.1. Plant Material
2.1.2. Germination and Propagation
2.2. Phase II: Chemical Characterization of L. mutabilis Seeds Harvested from Propagated Plants
2.2.1. Raw Seed Protein Determination
2.2.2. Raw Seed Hydroalcoholic Extraction under Acidic Hydrolysis
2.2.3. Alkaloid Removal Process (Debittering)
- I.
- neutral extractant, i.e., the boiling water was replaced by fresh recirculating water (1:3 seed/water ratio), using a Daihan Maxircu-CH 12 bath circulator (DKSH Holding Ltd., Zurich, Switzerland), and the seed residue was maintained for several h at 16 °C until alkaloid depletion. The water was changed three times a day (i.e., every 8 h), using the 1:3 seed/water ratio. Plastic mesh bags (0.5 mm mesh) were used to store the seeds during extraction. The resulting alkaloid-free seeds were labeled with the subscript acronym “af” (i.e., LCaf-sl, LPaf-sl, LCaf-scl, LPaf-scl, LPaf-sl) to code the alkaloid removal.
- II.
- acidic extractant, i.e., the boiling water was replaced by 0.5 M citric acid solution. Extraction conditions were identical to those described for water (neutral extraction). This acidic process was only performed with LC-sl seeds for comparative purposes, and the resulting extract was denoted as LCaf-sl + A.
2.2.4. Alkaloid Extraction for Quantitative Purposes
2.2.5. GC-MS and GC-FID Analyses
2.2.6. Protein Determination of Alkaloid-Free Seeds
2.2.7. Hydroalcoholic Extraction from Alkaloid-Free L. mutabilis Seeds
2.2.8. Total Phenolic Content
2.2.9. LC-MS Analysis of Seed-Derived Extracts
2.2.10. Antioxidant Capacity
2.2.11. Iron Content
2.3. Phase III: Determination of the Emulsifying Capacity of a Lupin-Seed-Based Drink Formulation
2.4. Statistical Analyses
3. Results
3.1. Phase I: Propagation of Two L. mutabilis Ecotypes Using Two Soil Types
3.2. Chemical Characterization of Raw Seeds from Propagated L. mutabilis Plants
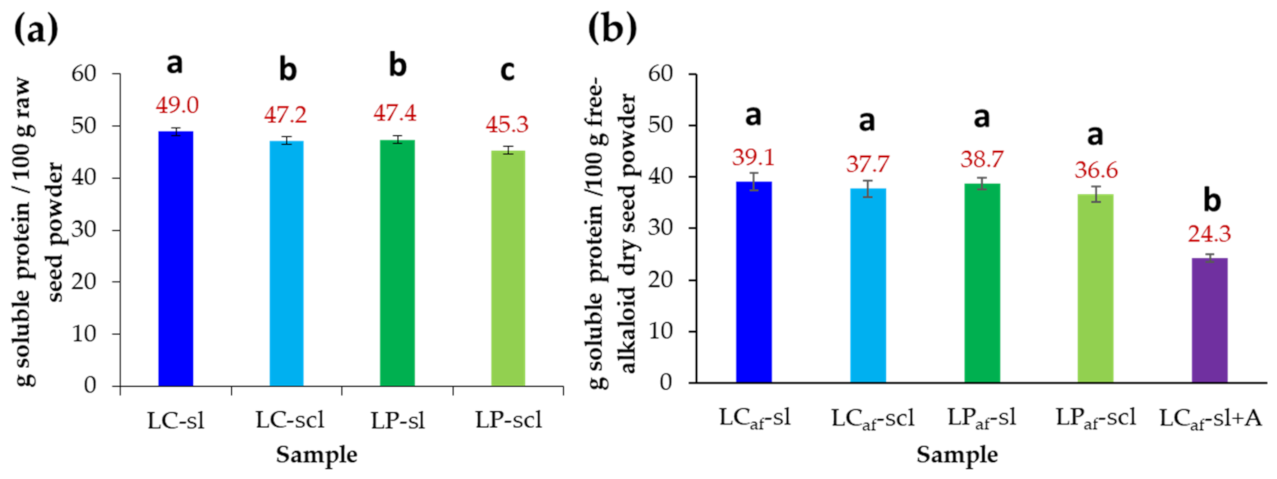
3.2.1. Raw Seed Protein Determination
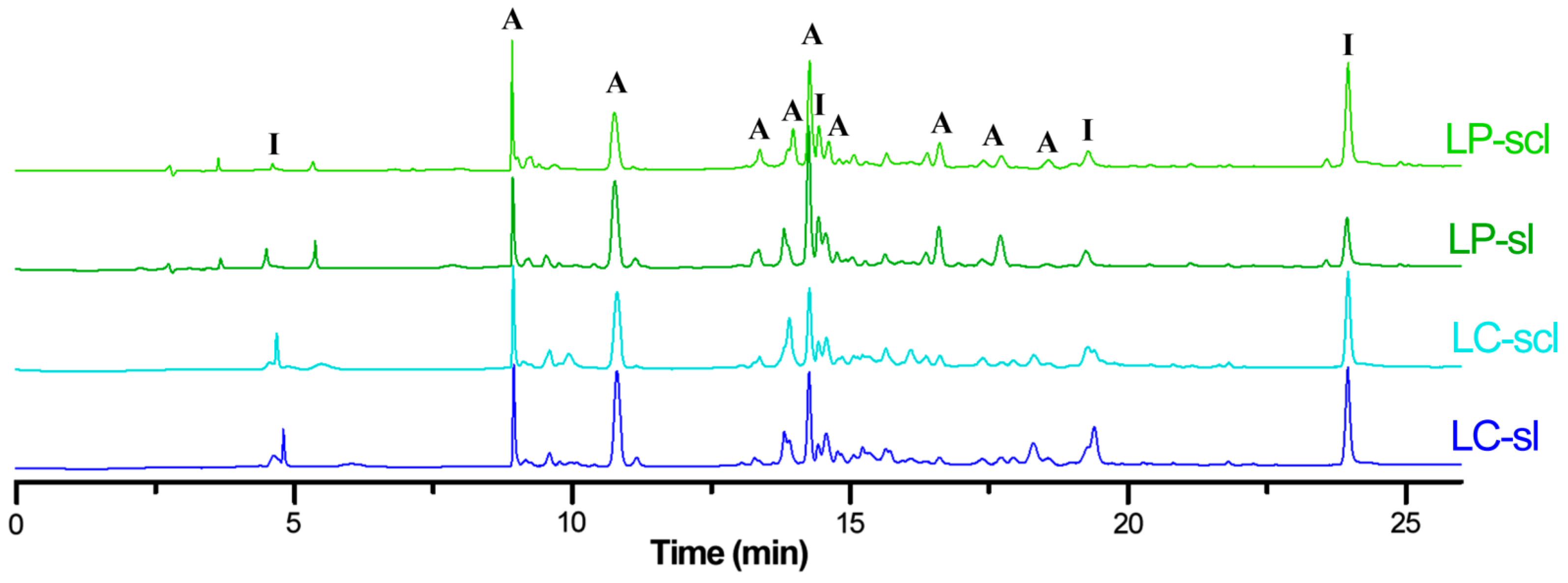
3.2.2. LC-MS Analysis of L. mutabilis Raw Seed-Derived Extracts
3.3. Alkaloid Removal Process and Protein Measurements in Alkaloid-Free L. mutabilis Seeds
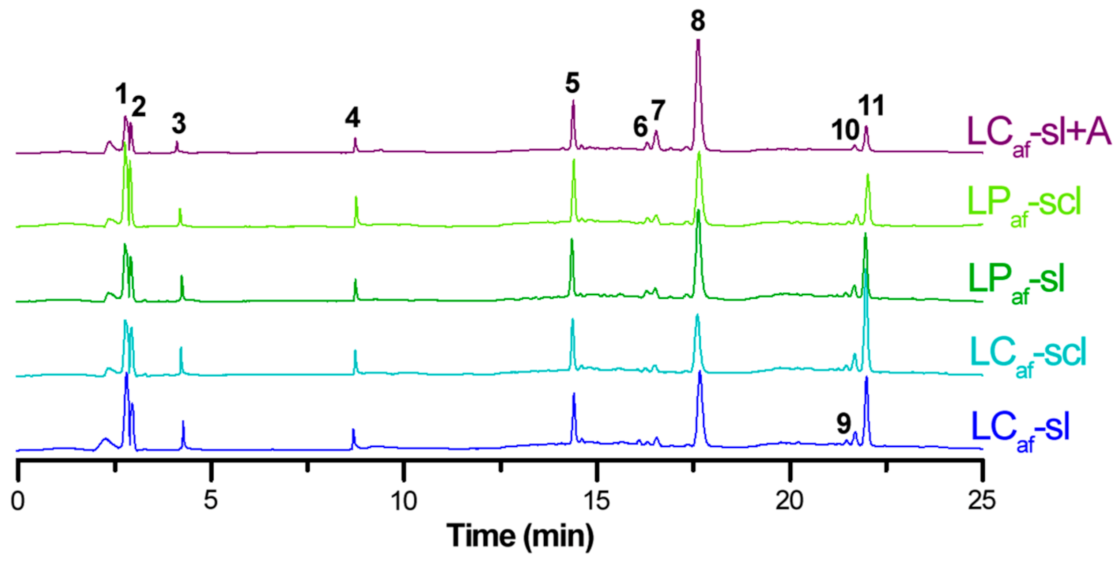
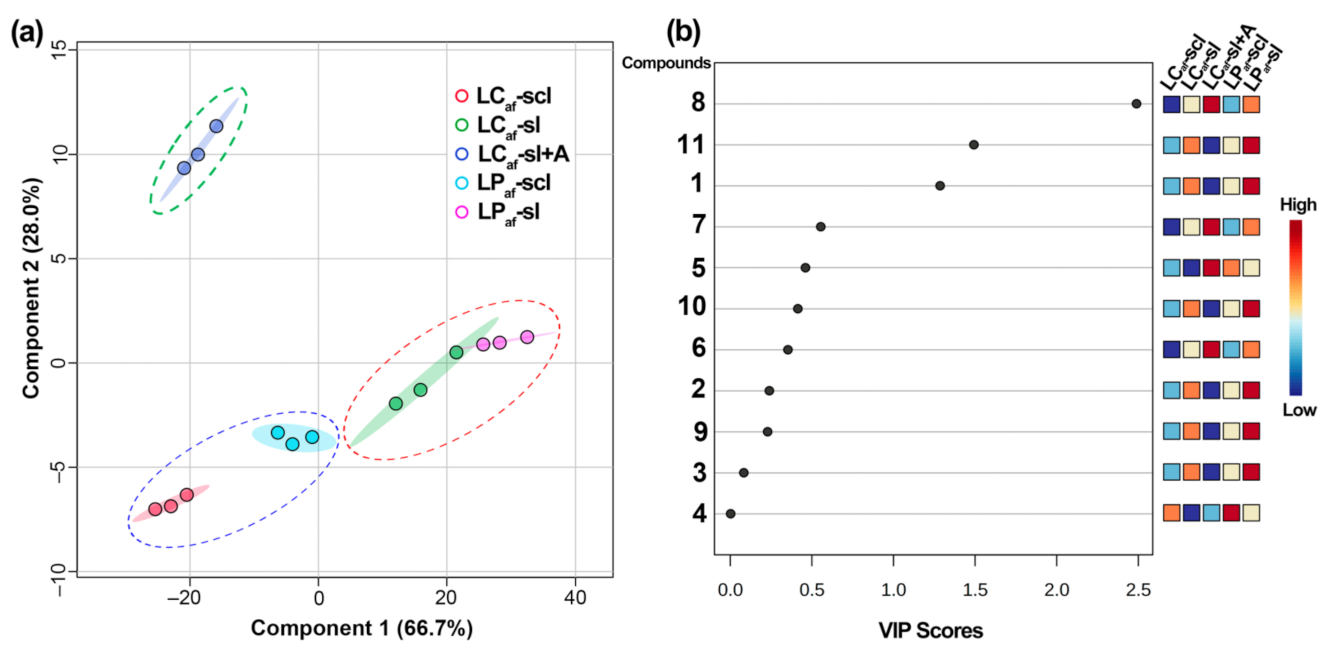
3.4. LC-MS-Based Isoflavone Analysis
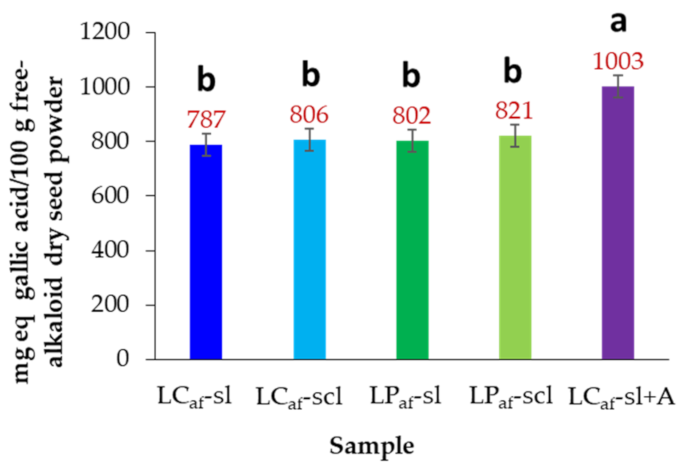
3.5. Total Phenolic Contents of Alkaloid-Free L. mutabilis Seeds
3.6. Antioxidant Capacity of Alkaloid-Free L. mutabilis Seeds
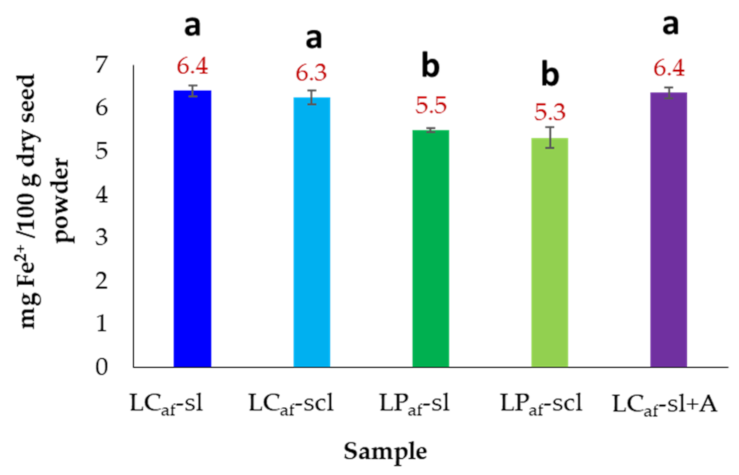
3.7. Iron Content of Alkaloid-Free L. mutabilis Seeds
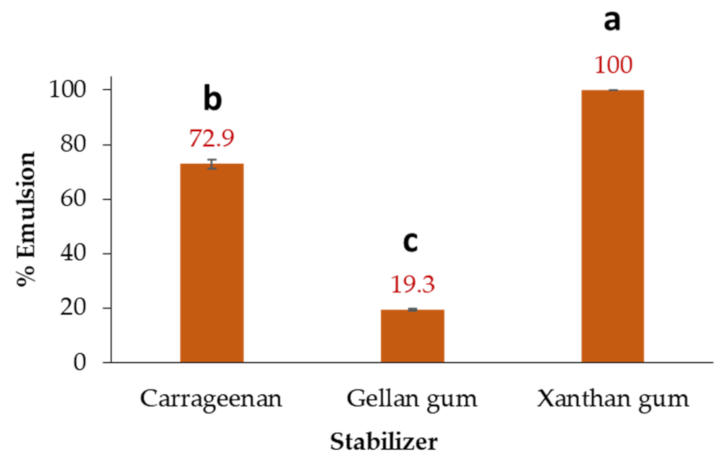
3.8. Emulsifying Capacity of Lupin Seed-Based Protein
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristic | Sandy Clay Loam (scl) Soil | Silty Loam (sl) Soil |
|---|---|---|
| pH | 4.79 | 5.76 |
| Electric conductivity (dS/m) | 0.22 | 0.29 |
| Cation exchange capacity (meq/100 g) | 11.9 | 14.4 |
| Mean humidity saturation (%) | 23.9 | 41.9 |
| Oxidable organic carbon (%) | 0.708 | 9.72 |
| Organic matter (%) | 1.22 | 16.8 |
| Total nitrogen (%) | 0.059 | 0.810 |
| Apparent density (g/cm3) | 0.956 | 0.616 |
| Clay texture (%) | 32.0 | 18.0 |
| Sand texture (%) | 50.0 | 14.0 |
| Silt texture (%) | 18.0 | 68.0 |
References
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public. Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, J.A.A.S. Current Trends and Technologies in Nutraceutical Industry. In Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods; Amalraj, A., Kuttappan, S., Varma, A.C.K., Matharu, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 347–360. ISBN 978-0-323-90794-1. [Google Scholar]
- Arnoldi, G. Legumes Are Valuable Sources of Tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef]
- Ishaq, A.R.; El-Nashar, H.A.S.; Younis, T.; Mangat, M.A.; Shahzadi, M.; Ul Haq, A.S.; El-Shazly, M. Genus Lupinus (Fabaceae): A Review of Ethnobotanical, Phytochemical and Biological Studies. J. Pharm. Pharmacol. 2022, 74, 1700–1717. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.R.; Koziol, M.; van Boekel, M.A.J.S. Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Crit. Rev. Food Sci. Nutr. 2016, 56, 1454–1487. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Isoflavones and Antioxidant Capacity of Peruvian and Brazilian Lupin Cultivars. J. Food Compos. Anal. 2009, 22, 397–404. [Google Scholar] [CrossRef]
- Ortega-David, E.; Rodríguez, A.; David, A.; Rodríguez, A. Characterization Properties of Lupin (Lupinus mutabilis) Seeds Grown in the Colombian Andean Region. Acta Agron. 2010, 59, 111–118. [Google Scholar]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant. Sci. 2021, 12, 670103. [Google Scholar] [CrossRef]
- Rípodas, C.; Via, V.D.; Aguilar, O.M.; Zanetti, M.E.; Blanco, F.A. Knock-down of a Member of the Isoflavone Reductase Gene Family Impairs Plant Growth and Nodulation in Phaseolus vulgaris. Plant Physiol. Biochem. 2013, 68, 81–89. [Google Scholar] [CrossRef]
- Liu, Y.; Hassan, S.; Kidd, B.N.; Garg, G.; Mathesius, U.; Singh, K.B.; Anderson, J.P. Ethylene Signaling Is Important for Isoflavonoid-Mediated Resistance to Rhizoctonia solani in Roots of Medicago truncatula. Mol. Plant Microbe Interact. 2017, 30, 691–700. [Google Scholar] [CrossRef]
- Rizzo, G.; Feraco, A.; Storz, M.A.; Lombardo, M. The Role of Soy and Soy Isoflavones on Women’s Fertility and Related Outcomes: An Update. J. Nutr. Sci. 2022, 11, e17. [Google Scholar] [CrossRef]
- Cherdshewasart, W.; Sutjit, W. Correlation of Antioxidant Activity and Major Isoflavonoid Contents of the Phytoestrogen-Rich Pueraria Mirifica and Pueraria Lobata Tubers. Phytomedicine 2008, 15, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Fujita, M.; Kamei, Y. Health Promotion Effects of Soy Isoflavones. J. Nutr. Sci. Vitaminol. 2020, 66, 502–507. [Google Scholar] [CrossRef]
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones Derived from Plant Raw Materials: Bioavailability, Anti-Cancer, Anti-Aging Potentials, and Microbiome Modulation. Crit. Rev. Food Sci. Nutr. 2023, 63, 261–287. [Google Scholar] [CrossRef]
- Mir, R.H.; Sabreen, S.; Mohi-Ud-Din, R.; Wani, T.U.; Jaleel, A.; Jan, R.; Banday, N.; Maqbool, M.; Mohi-Ud-Din, I.; Mir, B.I.; et al. Isoflavones of Soy: Chemistry and Health Benefits. In Edible Plants in Health and Diseases; Volume 1: Cultural, Practical and Economic Value; Masoodi, M.H., Rehman, M.U., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 303–324. ISBN 978-981-16-4880-9. [Google Scholar]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and Their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef]
- Hu, C.; Wong, W.-T.; Wu, R.; Lai, W.-F. Biochemistry and Use of Soybean Isoflavones in Functional Food Development. Crit. Rev. Food Sci. Nutr. 2020, 60, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Sayyar Khan, M. Nutritional Quality of Important Food Legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Ben Hassine, A.; Rocchetti, G.; Zhang, L.; Senizza, B.; Zengin, G.; Mahomoodally, M.F.; Ben-Attia, M.; Rouphael, Y.; Lucini, L.; El-Bok, S. Untargeted Phytochemical Profile, Antioxidant Capacity and Enzyme Inhibitory Activity of Cultivated and Wild Lupin Seeds from Tunisia. Molecules 2021, 26, 3452. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.; Jürgens, H.-U.; Schliephake, E.; Ordon, F. Effect of the Soil PH on the Alkaloid Content of Lupinus angustifolius. Int. J. Agron. 2012, 2012, 269878. [Google Scholar] [CrossRef]
- Cazzato, E.; Laudadio, V.; Stellacci, A.M.; Ceci, E.; Tufarelli, V. Influence of Sulphur Application on Protein Quality, Fatty Acid Composition and Nitrogen Fixation of White Lupin (Lupinus albus L.). Eur. Food Res. Technol. 2012, 235, 963–969. [Google Scholar] [CrossRef]
- Cowling, W.A.; Tarr, A. Effect of Genotype and Environment on Seed Quality in Sweet Narrow-Leafed Lupin (Lupinus angustifolius L.). Aust. J. Agric. Res. 2004, 55, 745–751. [Google Scholar] [CrossRef]
- Gulisano, A.; Alves, S.; Rodriguez, D.; Murillo, A.; van Dinter, B.-J.; Torres, A.F.; Gordillo-Romero, M.; de Torres, M.L.; Neves-Martins, J.; Paulo, M.-J.; et al. Diversity and Agronomic Performance of Lupinus mutabilis Germplasm in European and Andean Environments. Front. Plant. Sci. 2022, 13, 903661. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and Breeding of Lupinus mutabilis: An Emerging Protein Crop. Front. Plant. Sci. 2019, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Silvério, S.C.; Moreira, S.; Milagres, A.M.F.; Macedo, E.A.; Teixeira, J.A.; Mussatto, S.I. Interference of Some Aqueous Two-Phase System Phase-Forming Components in Protein Determination by the Bradford Method. Anal. Biochem. 2012, 421, 719–724. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Nurmi, T.; Adlercreutz, H. A Simplified HPLC Method for Total Isoflavones in Soy Products. Food Chem. 2004, 87, 297–305. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Nout, M.J.R.; van Boekel, M.A.J.S.; Koziol, M.; Linnemann, A.R. Modelling of the Aqueous Debittering Process of Lupinus mutabilis Sweet. LWT Food Sci. Technol. 2013, 53, 507–516. [Google Scholar] [CrossRef]
- Wink, M. Quinolizidin Alkaloids. In Methods in Plant Biochemistry; Waterman, P., Ed.; Academic Press: London, UK, 1993; Volume 8, pp. 197–239. [Google Scholar]
- Cely-Veloza, W.; Quiroga, D.; Coy-Barrera, E. Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules 2022, 27, 305. [Google Scholar] [CrossRef]
- Buitrago, D.; Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Comparative Examination of Antioxidant Capacity and Fingerprinting of Unfractionated Extracts from Different Plant Parts of Quinoa (Chenopodium quinoa) Grown under Greenhouse Conditions. Antioxidants 2019, 8, 238. [Google Scholar] [CrossRef]
- KNApSAcK: A Comprehensive Species-Metabolite Relationship Database. Available online: http://www.knapsackfamily.com/knapsack_core/top.php (accessed on 17 March 2023).
- Dictionary of Natural Products. Available online: https://dnp.chemnetbase.com/ (accessed on 16 March 2023).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 16 March 2023).
- Tripathi, A.D.; Gupta, K.A.; Malik, S. Iron Determination by Colorimetric Method Using O-Phenanthroline. Bull. Pure Appl. Sci. Chem. 2019, 38c, 171–175. [Google Scholar] [CrossRef]
- Márquez, A.L.; Wagner, J.R.; Palazolo, G.G. Cream-like Emulsions Prepared with Soy Milk 1: Stability Studies and Formulation. Grasas Aceites 2005, 56, 59–66. [Google Scholar] [CrossRef]
- Fallourd, M.J.; Viscione, L. Ingredient Selection for Stabilisation and Texture Optimisation of Functional Beverages and the Inclusion of Dietary Fibre. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2009; pp. 3–38. ISBN 978-1-84569-342-8. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version 2020. Available online: http://www.infostat.com.ar (accessed on 16 March 2023).
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Schettino, O.; Dini, A. Studies on the Constituents of Lupinus mutabilis (Fabaceae). Isolation and Characterization of Two New Isoflavonoid Derivatives. J. Agric. Food Chem. 1998, 46, 5089–5092. [Google Scholar] [CrossRef]
- Kachlicki, P.; Marczak, Ł.; Kerhoas, L.; Einhorn, J.; Stobiecki, M. Profiling Isoflavone Conjugates in Root Extracts of Lupine Species with LC/ESI/MSn Systems. J. Mass. Spectrom. 2005, 40, 1088–1103. [Google Scholar] [CrossRef]
- Wojakowska, A.; Piasecka, A.; García-López, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, Ł.; Kachlicki, P.; Stobiecki, M. Structural Analysis and Profiling of Phenolic Secondary Metabolites of Mexican Lupine Species Using LC–MS Techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef]
- Chirinos-Arias, M.C. Andean Lupin (Lupinus mutabilis Sweet) a Plant with Nutraceutical and Medicinal Potential. Rev. Bio Cienc. 2015, 3, 163–172. [Google Scholar]
- Rossnerova, A.; Izzotti, A.; Pulliero, A.; Bast, A.; Rattan, S.I.S.; Rossner, P. The Molecular Mechanisms of Adaptive Response Related to Environmental Stress. Int. J. Mol. Sci. 2020, 21, 7053. [Google Scholar] [CrossRef]
- Hansen, M.M.; Olivieri, I.; Waller, D.M.; Nielsen, E.E. Monitoring Adaptive Genetic Responses to Environmental Change. Mol. Ecol. 2012, 21, 1311–1329. [Google Scholar] [CrossRef]
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.-J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; van Haren, R.J.F.; Jóhannsson, M.H.; Mateos, C.; et al. State and Progress of Andean Lupin Cultivation in Europe: A Review. Agronomy 2020, 10, 1038. [Google Scholar] [CrossRef]
- Guilengue, N.; Alves, S.; Talhinhas, P.; Neves-Martins, J. Genetic and Genomic Diversity in a Tarwi (Lupinus mutabilis Sweet) Germplasm Collection and Adaptability to Mediterranean Climate Conditions. Agronomy 2020, 10, 21. [Google Scholar] [CrossRef]
- Barda, M.S.; Chatzigeorgiou, T.; Papadopoulos, G.K.; Bebeli, P.J. Agro-Morphological Evaluation of Lupinus mutabilis in Two Locations in Greece and Association with Insect Pollinators. Agriculture 2021, 11, 236. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Weiler, M.; Hertzler, S.R.; Dvoretskiy, S. Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake? Nutrients 2023, 15, 838. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Hag, L.V.; Haritos, V.S.; Dhital, S. Lupin Proteins: Structure, Isolation and Application. Trends Food Sci. Technol. 2021, 116, 928–939. [Google Scholar] [CrossRef]
- Czubinski, J.; Grygier, A.; Siger, A. Lupinus mutabilis Seed Composition and Its Comparison with Other Lupin Species. J. Food Compos. Anal. 2021, 99, 103875. [Google Scholar] [CrossRef]
- Osorio, C.E.; Till, B.J. A Bitter-Sweet Story: Unraveling the Genes Involved in Quinolizidine Alkaloid Synthesis in Lupinus albus. Front. Plant. Sci. 2022, 12, 795091. [Google Scholar] [CrossRef]
- Villacrés, E.; Álvarez, J.; Rosell, C. Effects of Two Debittering Processes on the Alkaloid Content and Quality Characteristics of Lupin (Lupinus mutabilis Sweet). J. Sci. Food Agric. 2020, 100, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, J.-P.; Glorio-Paulet, P.; Yang, B.; Repo-Carrasco-Valencia, R. Profile and Content of Residual Alkaloids in Ten Ecotypes of Lupinus mutabilis Sweet after Aqueous Debittering Process. Plant Foods Hum. Nutr. 2020, 75, 184–191. [Google Scholar] [CrossRef]
- Galek, R.; Sawicka-Sienkiewicz, E.; Zalewski, D.; Stawiński, S.; Spychała, K. Searching for Low Alkaloid Forms in the Andean Lupin (Lupinus mutabilis) Collection. Czech J. Genet. Plant. Breed. 2017, 53, 55–62. [Google Scholar] [CrossRef]
- Magalhães, S.C.Q.; Fernandes, F.; Cabrita, A.R.J.; Fonseca, A.J.M.; Valentão, P.; Andrade, P.B. Alkaloids in the Valorization of European Lupinus Spp. Seeds Crop. Ind. Crops Prod. 2017, 95, 286–295. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy Proteins: A Review on Composition, Aggregation and Emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Estivi, L.; Buratti, S.; Fusi, D.; Benedetti, S.; Rodríguez, G.; Brandolini, A.; Hidalgo, A. Alkaloid Content and Taste Profile Assessed by Electronic Tongue of Lupinus albus Seeds Debittered by Different Methods. J. Food Compos. Anal. 2022, 114, 104810. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Kulshrestha, S.; Bhardwaj, K.; Jangir, R. A Review on Properties and Applications of Xanthan Gum. In Microbial Polymers: Applications and Ecological Perspectives; Vaishnav, A., Choudhary, D.K., Eds.; Springer Singapore: Singapore, 2021; pp. 87–107. ISBN 978-981-16-0045-6. [Google Scholar]
- Genovese, M.I.; Hassimotto, N.M.A.; Lajolo, F.M. Isoflavone Profile and Antioxidant Activity of Brazilian Soybean Varieties. Food Sci. Technol. Int. 2005, 11, 205–211. [Google Scholar] [CrossRef]
- Lozovaya, V.V.; Lygin, A.V.; Ulanov, A.V.; Nelson, R.L.; Daydé, J.; Widholm, J.M. Effect of Temperature and Soil Moisture Status during Seed Development on Soybean Seed Isoflavone Concentration and Composition. Crop. Sci. 2005, 45, 1934–1940. [Google Scholar] [CrossRef]
- Vamerali, T.; Barion, G.; Hewidy, M.; Mosca, G. Soybean Isoflavone Patterns in Main Stem and Branches as Affected by Water and Nitrogen Supply. Eur. J. Agron. 2012, 41, 1–10. [Google Scholar] [CrossRef]
- Samac, D.A.; Graham, M.A. Recent Advances in Legume-Microbe Interactions: Recognition, Defense Response, and Symbiosis from a Genomic Perspective. Plant Physiol. 2007, 144, 582–587. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Kulling, S.E. Antioxidant Activity of Isoflavones and Their Major Metabolites Using Different in Vitro Assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Mujić, I.; Šertović, E.; Jokić, S.; Sarić, Z.; Alibabić, V.; Vidović, S.; Živković, S. Isoflavone Content and Antioxidant Properties of Soybean Seeds. Croat. J. Food Sci. Technol. 2011, 3, 16–20. [Google Scholar]
- Tepavčević, V.; Atanacković, M.; Miladinović, J.; Malenčić, D.; Popović, J.; Cvejić, J. Isoflavone Composition, Total Polyphenolic Content, and Antioxidant Activity in Soybeans of Different Origin. J. Med. Food 2010, 13, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Pedulli, G.F.; Cabrini, L.; Zambonin, L.; Landi, L. Solvent and PH Effects on the Antioxidant Activity of Caffeic and Other Phenolic Acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of Assay Conditions for the Determination of Antioxidant Capacity and Polyphenols in Cereal Food Components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic Content, Chemical Composition and Anti-/pro-Oxidant Activity of Gold Milenium and Papierowka Apple Peel Extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Vera-Vega, M.; Jimenez-Davalos, J.; Zolla, G. The Micronutrient Content in Underutilized Crops: The Lupinus mutabilis Sweet Case. Sci. Rep. 2022, 12, 15162. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Vaquero, M.P. Iron Bioavailability from Food Fortification to Precision Nutrition. A Review. Innov. Food Sci. Emerg. Technol. 2019, 51, 126–138. [Google Scholar] [CrossRef]
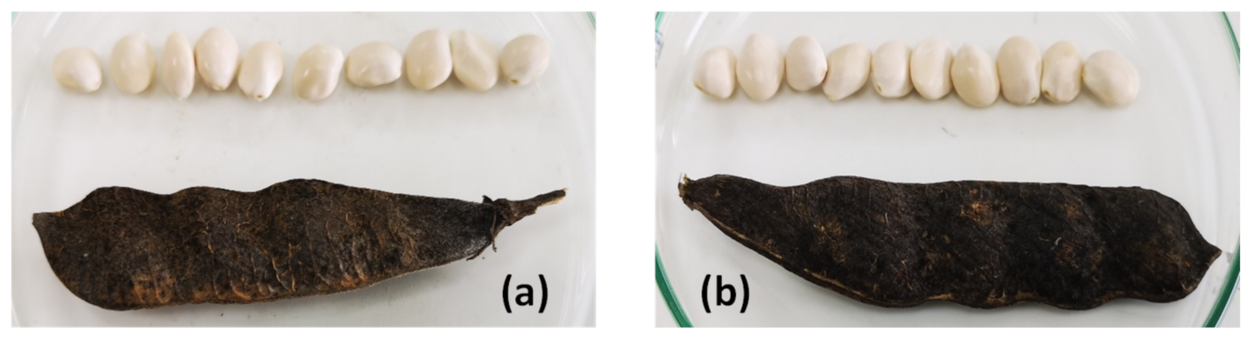

| Silty Loam (sl) Soil | Sandy Clay Loam (scl) Soil | |||
|---|---|---|---|---|
| LC a | LP b | LC a | LP b | |
| Pod size (cm) | 8.1 ± 1.8 A | 6.5 ± 1.3 A | 7.3 ± 1.6 A | 5.7 ± 1.4 A |
| Seed dry weight (g) | 0.39 ± 0.04 A | 0.31 ± 0.03 B | 0.33 ± 0.02 AB | 0.25 ± 0.02 C |
| Seed diameter (mm) | 9.9 ± 0.3 A | 9.2 ± 0.6 AB | 9.7 ± 0.4 AB | 8.9 ± 0.4 B |
| Samples a | Ext b | RCT c | WV d | PCi e | PCf f | PL g | DML h | TACi i | TACf j |
|---|---|---|---|---|---|---|---|---|---|
| LC-sl | W | 195 | 7.0 | 48.9 ± 0.7 | 39.1 ± 1.7 | 9.8 | 27.5 ± 1.3 | 236.5 ± 2.4 | <LoD k |
| LC-scl | W | 187 | 6.7 | 47.1 ± 0.7 | 37.7 ± 1.6 | 9.4 | 27.4 ± 0.9 | 190.5 ± 1.9 | <LoD k |
| LP-sl | W | 187 | 6.7 | 47.4 ± 0.8 | 38.8 ± 1.1 | 8.6 | 32.1 ± 1.1 | 187.6 ± 2.5 | <LoD k |
| LP-scl | W | 187 | 6.7 | 45.3 ± 0.7 | 36.6 ± 1.5 | 8.7 | 31.3 ± 1.3 | 175.4 ± 2.1 | <LoD k |
| LC-sl + A | A | 139 | 4.9 | 48.9 ± 0.7 | 24.3 ± 0.7 | 24.6 | 38.1 ± 1.5 | 206.5 ± 2.4 | <LoD k |
| # a | tR (min) b | [M + H]+ m/z | [M − H]− m/z | Accurate Mass | Error c | Formula | λmax d | Annotation e |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.8 | 305 | 303 | 303.0512 | 2.3 | C15H10O7 | 275,329 | dihydroxygenistein |
| 2 | 2.9 | 319 | 317 | 317.0674 | 4.1 | C16H12O7 | 271,331 | dihydroxymutabilein |
| 3 | 4.5 | 289 | 287 | 287.0569 | 4.5 | C15H10O6 | 269,329 | hydroxygenistein |
| 4 | 8.8 | 273 | 271 | 271.0614 | 3.0 | C15H10O5 | 267,339 | apigenin |
| 5 | 14.4 | 273 | 271 | 271.0611 | 1.8 | C15H10O5 | 262,328 | genistein |
| 6 | 16.3 | 303 | 301 | 301.0721 | 3.0 | C16H12O6 | 270,326 | hydroxymutabilein |
| 7 | 16.5 | 317 | 315 | 315.0855 | −4.4 | C17H14O6 | 264,323 | methoxymutabilein |
| 8 | 17.6 | 287 | 285 | 285.0751 | −4.2 | C16H12O5 | 266,325 | mutabilein |
| 9 | 21.8 | 373 | 371 | 371.1146 | 4.0 | C20H18O7 | 277,329 | lupinisoflavone D or B |
| 10 | 22.0 | 357 | 355 | 355.1173 | −2.5 | C20H18O6 | 278,331 | luteone |
| 11 | 32.6 | 341 | 339 | 339.1246 | 4.1 | C20H18O5 | 278,330 | lupiwighteone |
| # a | LCaf-sl b | LCaf-scl b | LPaf-sl b | LPaf-scl b | LCaf-sl + A b | |
|---|---|---|---|---|---|---|
| 1 | 23.2 ± 1.0 B | 18.5 ± 0.6 D | 25.5 ± 1.1 A | 21.5 ± 1.1 C | 17.4 ± 0.5 D | |
| 2 | 12.2 ± 0.6 A | 9.6 ± 0.3 C | 12.4 ± 0.4 A | 10.7 ± 0.3 B | 9.2 ± 0.2 C | Hm c |
| 3 | 4.2 ± 0.1 A | 3.5 ± 0.1C | 4.4 ± 0.2 A | 3.8 ± 0.1 B | 1.9 ± 0.1 D | H |
| 4 | 4.8 ± 0.1 C | 6.0 ± 0.2 B | 5.0 ± 0.2 C | 6.7 ± 0.2 A | 4.9 ± 0.2 C | |
| 5 | 15.5 ± 0.7 C | 16.3 ± 0.7 C | 18.4 ± 0.6 B | 18.9 ± 0.6 B | 22.4 ± 0.5 A | |
| 6 | 3.2 ± 0.1 C | 1.4 ± 0.1 D | 4.4 ± 0.1 B | 3.0 ± 0.1 C | 6.2 ± 0.3 A | M |
| 7 | 5.1 ± 0.1 C | 2.7 ± 0.1 E | 7.8 ± 0.4 B | 4.0 ± 0.1 D | 17.1 ± 0.6 A | |
| 8 | 51.0 ± 3.0 C | 38.3 ± 1.6 E | 61.0 ± 1.6 B | 47.5 ± 1.3 D | 104.3 ± 4.6 A | |
| 9 | 2.4 ± 0.1 B | 1.3 ± 0.1 C | 3.2 ± 0.1 A | 2.4 ± 0.1 B | 0.8 ± 0.1 D | L |
| 10 | 5.6 ± 0.2 B | 3.5 ± 0.1 C | 7.0 ± 0.1 A | 5.5 ± 0.1 B | 3.1 ± 0.1 D | |
| 11 | 26.7 ± 0.5 B | 22.6 ± 1.1 C | 32.1 ± 1.2 A | 26.5 ± 1.0 B | 11.0 ± 0.3 D | |
| Total d | 154.0 ± 6.6 C | 123.6 ± 4.8 D | 181.4 ± 5.6 B | 150.6 ± 4.9 C | 198.3 ± 7.3 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urrego-Pava, F.; Coy-Barrera, E. Isoflavone Content and Nutritional-Related Properties of Debittered Seeds from Two Andean Lupin (Lupinus mutabilis Sweet) Ecotypes Propagated in Two Soils. Foods 2023, 12, 1841. https://doi.org/10.3390/foods12091841
Urrego-Pava F, Coy-Barrera E. Isoflavone Content and Nutritional-Related Properties of Debittered Seeds from Two Andean Lupin (Lupinus mutabilis Sweet) Ecotypes Propagated in Two Soils. Foods. 2023; 12(9):1841. https://doi.org/10.3390/foods12091841
Chicago/Turabian StyleUrrego-Pava, Francisco, and Ericsson Coy-Barrera. 2023. "Isoflavone Content and Nutritional-Related Properties of Debittered Seeds from Two Andean Lupin (Lupinus mutabilis Sweet) Ecotypes Propagated in Two Soils" Foods 12, no. 9: 1841. https://doi.org/10.3390/foods12091841
APA StyleUrrego-Pava, F., & Coy-Barrera, E. (2023). Isoflavone Content and Nutritional-Related Properties of Debittered Seeds from Two Andean Lupin (Lupinus mutabilis Sweet) Ecotypes Propagated in Two Soils. Foods, 12(9), 1841. https://doi.org/10.3390/foods12091841







