Effects of Light Shading, Fertilization, and Cultivar Type on the Stable Isotope Distribution of Hybrid Rice
Abstract
1. Introduction
2. Material and Methods
2.1. Field Experiment
2.2. Elemental Content and Isotope Ratio Measurements
2.3. Data Analysis
3. Results and Discussion
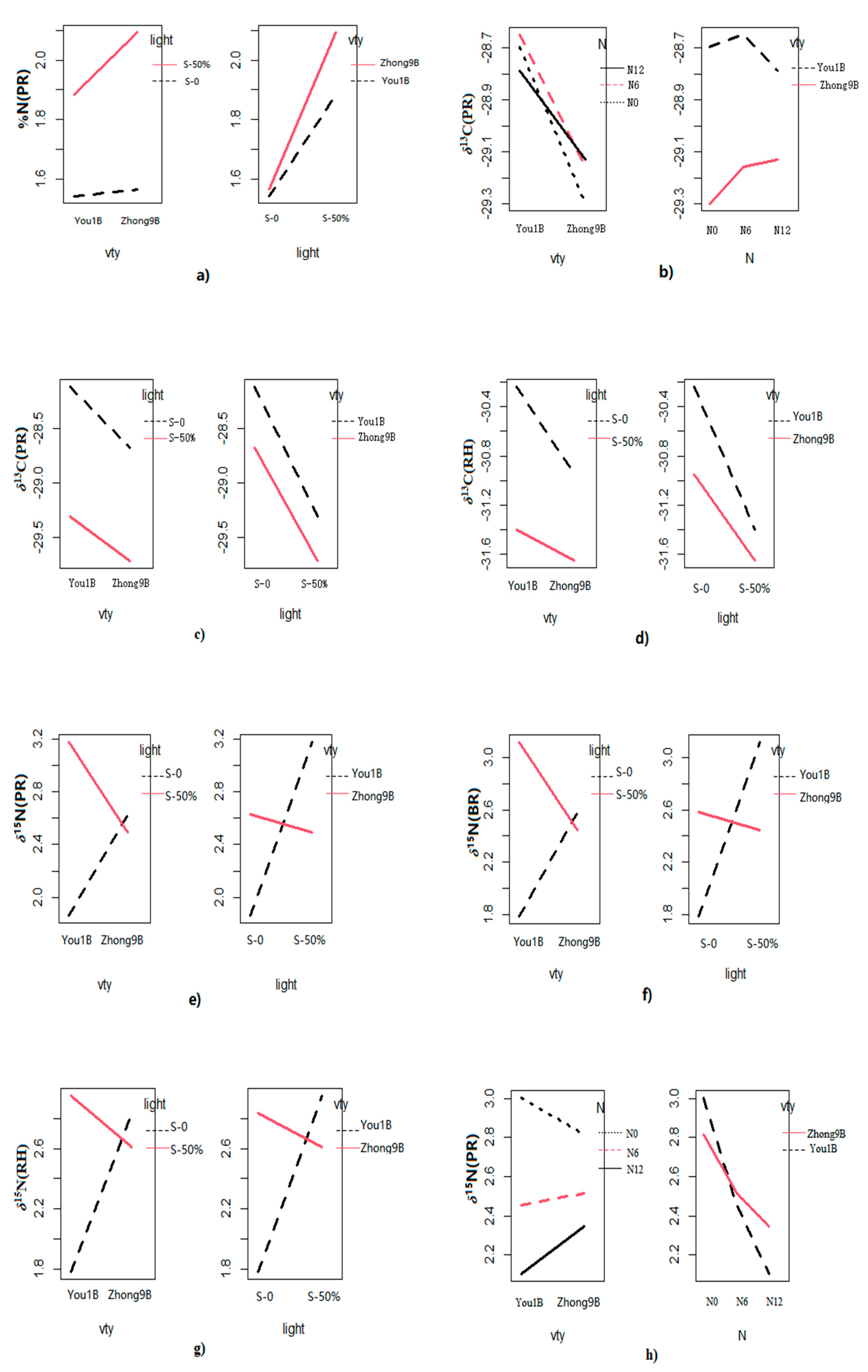
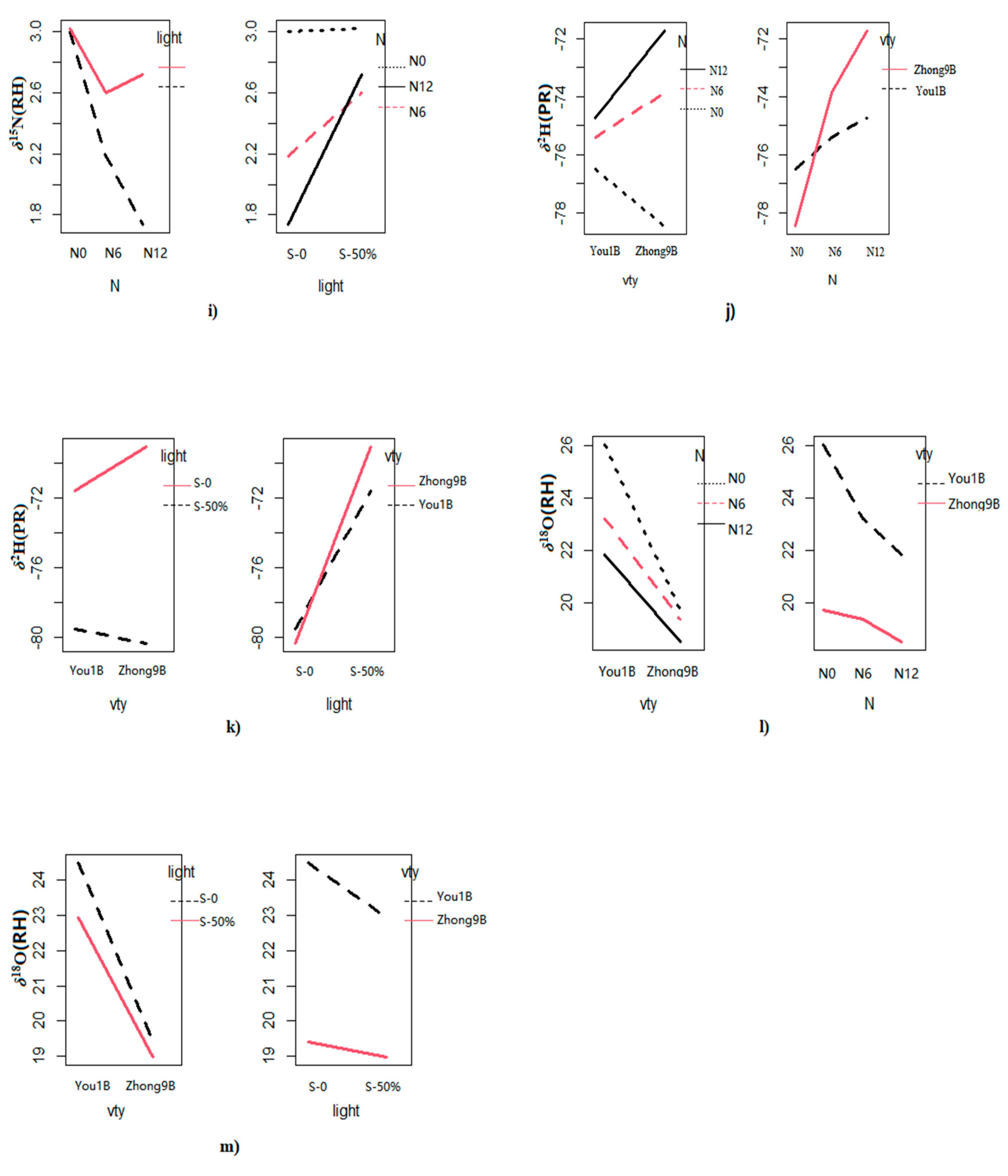
3.1. Multivariate ANOVA for Elemental Content and Isotope Ratios
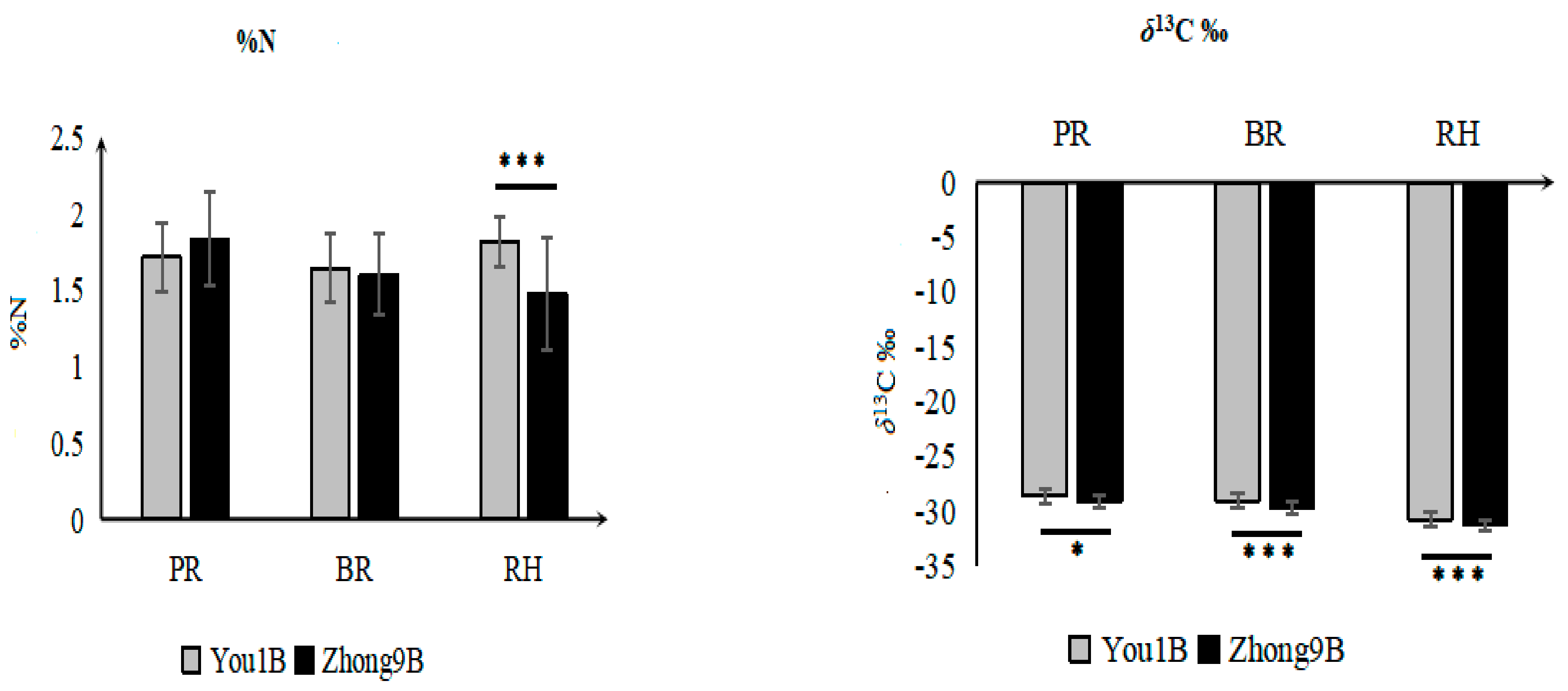
3.2. Elemental and Isotope Differences between the Rice Varieties
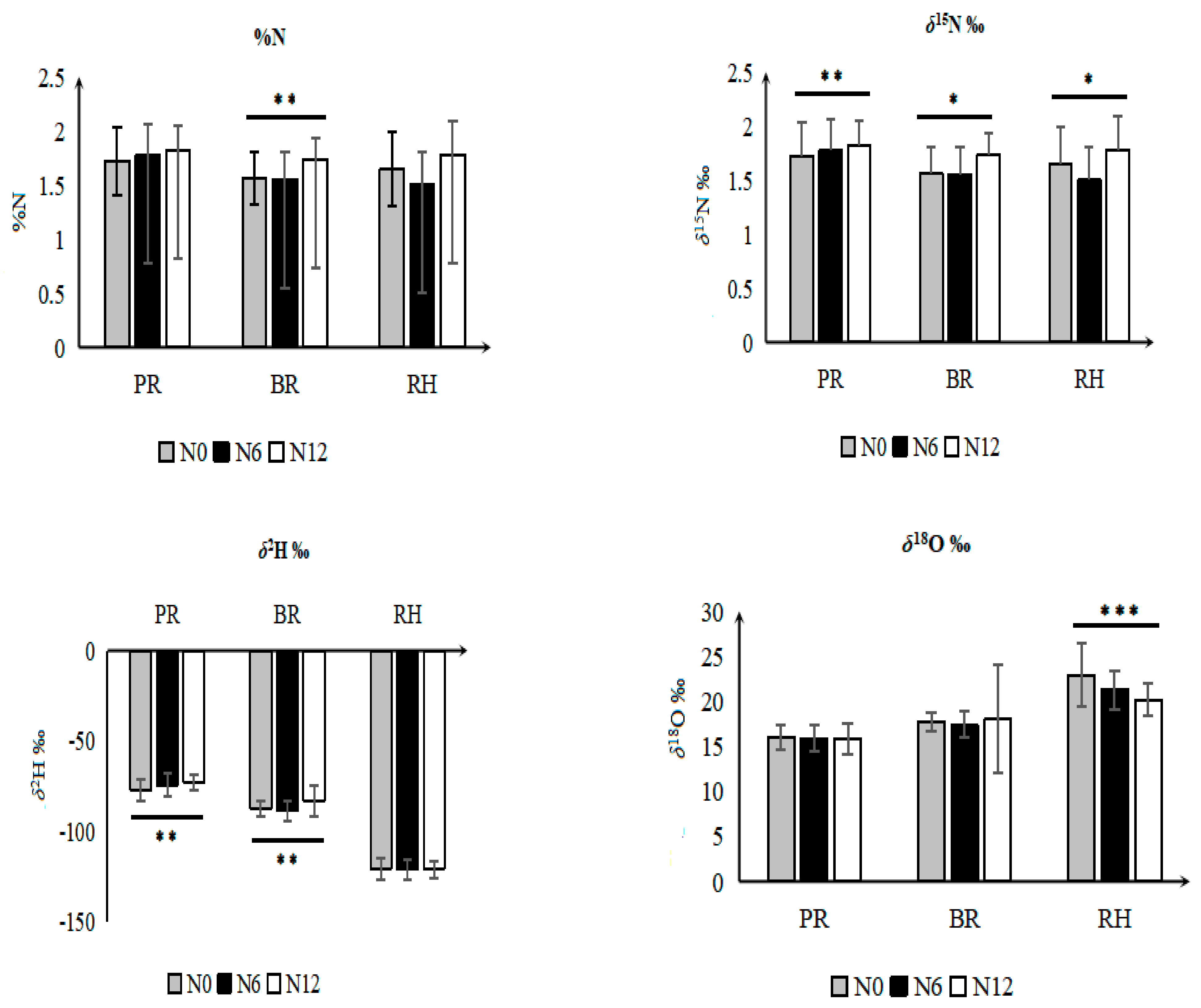
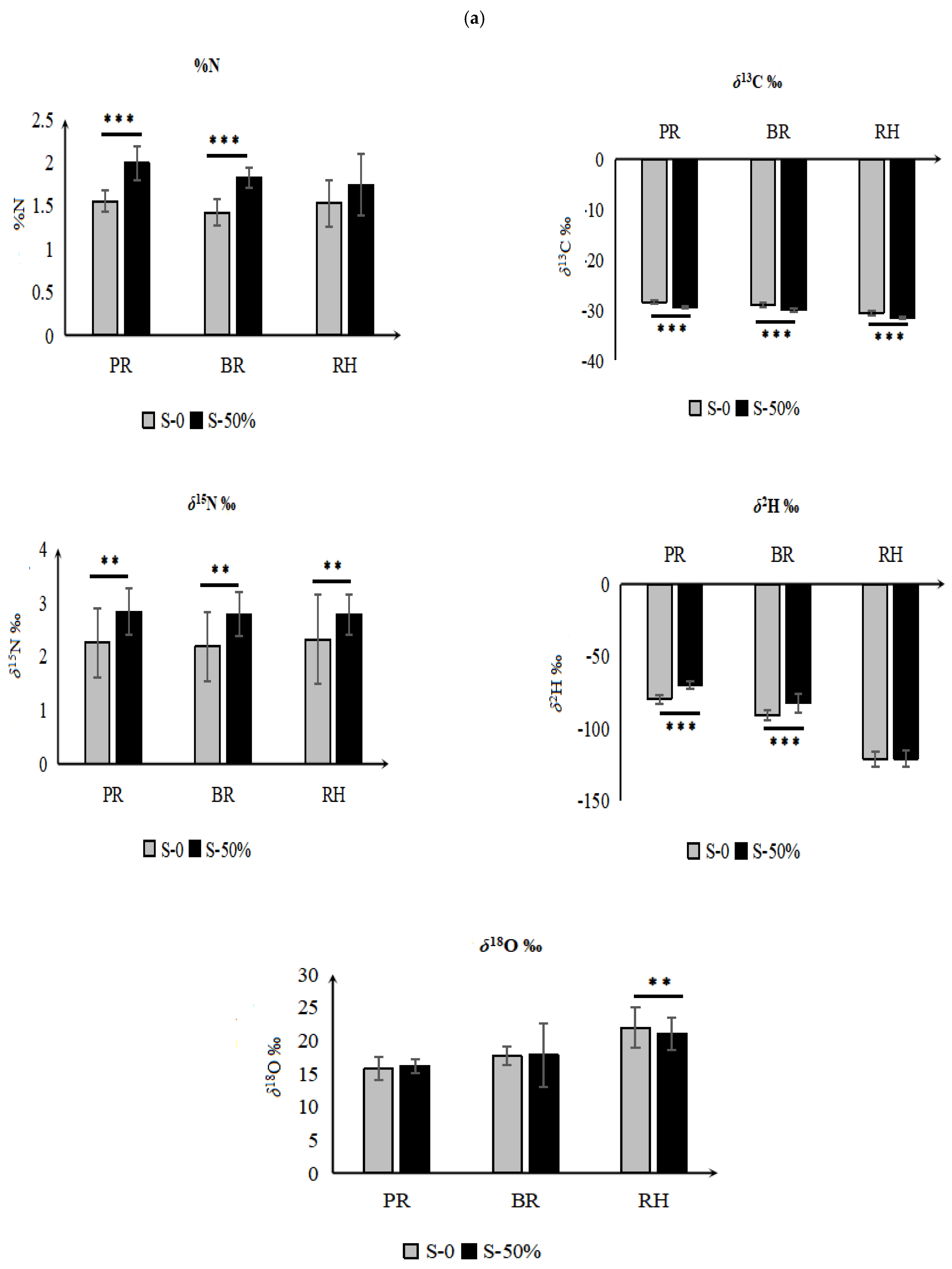
3.3. Effect of Fertilizer Regimes on Elemental and Isotope Values of Rice Fractions
3.4. Elemental and Isotopic Variations among Different Light Shading Treatments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wadood, S.A.; Nie, J.; Li, C.; Rogers, K.M.; Khan, A.; Khan, W.A.; Qamar, A.; Zhang, Y.; Yuwei, Y. Rice Authentication: An Overview of Different Analytical Techniques Combined with Multivariate Analysis. J. Food Compos. Anal. 2022, 112, 104677. [Google Scholar] [CrossRef]
- Maione, C.; Batista, B.L.; Campiglia, A.D.; Barbosa, F.; Barbosa, R.M. Classification of Geographic Origin of Rice by Data Mining and Inductively Coupled Plasma Mass Spectrometry. Comput. Electron. Agric. 2016, 121, 101–107. [Google Scholar] [CrossRef]
- Teye, E.; Amuah, C.L.Y.; McGrath, T.; Elliott, C. Innovative and Rapid Analysis for Rice Authenticity Using Hand-Held NIR Spectrometry and Chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 147–154. [Google Scholar] [CrossRef]
- Long, N.P.; Lim, D.K.; Mo, C.; Kim, G.; Kwon, S.W. Development and Assessment of a Lysophospholipid-Based Deep Learning Model to Discriminate Geographical Origins of White Rice. Sci. Rep. 2017, 7, 8552. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Yang, W.T.; Kwon, Y.S.; Kim, H.N.; Kim, K.M.; Kim, D.H. Assessment of the Genetic Diversity of Rice Germplasms Characterized by Black-Purple and Red Pericarp Color Using Simple Sequence Repeat Markers. Plants 2019, 8, 471. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, Y.J.; Moon, H.S.; Chi, H.Y.; Kim, S.H. Long-Term Isotopic Model Study for Ecofriendly Rice (Oryza sativa L.) Authentication: Updating a Case Study in South Korea. Food Chem. 2021, 362, 130215. [Google Scholar] [CrossRef]
- Li, C.; Nie, J.; Zhang, Y.; Shao, S.; Liu, Z.; Rogers, K.M.; Zhang, W.; Yuan, Y. Geographical Origin Modeling of Chinese Rice Using Stable Isotopes and Trace Elements. Food Control 2022, 138, 108997. [Google Scholar] [CrossRef]
- Wadood, S.A.; Guo, B.; Liu, H.; Wei, S.; Bao, X.; Wei, Y. Study on the Variation of Stable Isotopic Fingerprints of Wheat Kernel along with Milling Processing. Food Control 2018, 91, 427–433. [Google Scholar] [CrossRef]
- Akamatsu, F.; Okuda, M.; Fujii, T. Long-Term Responses to Climate Change of the Carbon and Oxygen Stable Isotopic Compositions and Gelatinization Temperature of Rice. Food Chem. 2020, 315, 126239. [Google Scholar] [CrossRef] [PubMed]
- Raveh, E.; Cohen, S.; Raz, T.; Yakir, D.; Grava, A.; Goldschmidt, E.E. Increased Growth of Young Citrus Trees under Reduced Radiation Load in a Semi-Arid Climate1. J. Exp. Bot. 2003, 54, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weedon, M.M. Modelling Photosynthetic Responses to Temperature of Grapevine (Vitis vinifera Cv. Semillon) Leaves on Vines Grown in a Hot Climate. Plant. Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Li, C.; Nie, J.; Shao, S.; Rogers, K.M.; Zhang, Y.; Li, Z.; Yuan, Y. Stable Isotope and Photosynthetic Response of Tea Grown under Different Temperature and Light Conditions. Food Chem. 2022, 368, 130771. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, D.; Shree, P.; Singh, D.K. Stable Isotope Ratio Analysis in Determining the Geographical Traceability of Indian Wheat. Food Control 2017, 79, 169–176. [Google Scholar] [CrossRef]
- Xu, Y.; This, D.; Pausch, R.C.; Vonhof, W.M.; Coburn, J.R.; Comstock, J.P.; McCouch, S.R. Leaf-Level Water Use Efficiency Determined by Carbon Isotope Discrimination in Rice Seedlings: Genetic Variation Associated with Population Structure and QTL Mapping. Theor. Appl. Genet. 2009, 118, 1065–1081. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, J.K.; Prabakaran, M.; Yang, J.H.; Kim, S.H. Authenticity of Rice (Oryza sativa L.) Geographical Origin Based on Analysis of C, N, O and S Stable Isotope Ratios: A Preliminary Case Report in Korea, China and Philippine. J. Sci. Food Agric. 2016, 96, 2433–2439. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; García-Romero, E. Effect of Irrigation and Variety on Oxygen (Δ18O) and Carbon (Δ13C) Stable Isotope Composition of Grapes Cultivated in a Warm Climate. Aust. J. Grape Wine Res. 2010, 16, 283–289. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, W.; Zhang, Y.; Liu, Z.; Shao, S.; Zhou, L.; Rogers, K.M. Differentiating Organically Farmed Rice from Conventional and Green Rice Harvested from an Experimental Field Trial Using Stable Isotopes and Multi-Element Chemometrics. J. Agric. Food Chem. 2018, 66, 2607–2615. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Zhang, W.; Zhao, Y.; Yang, S.; Chen, A. Tracing the Geographical Origin of Rice by Stable Isotopic Analyses Combined with Chemometrics. Food Chem. 2020, 313, 126093. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Shao, S.; Chen, Z.; Nie, J.; Liu, Z.; Rogers, K.M.; Yuan, Y. Stable Isotope Effects of Biogas Slurry Applied as an Organic Fertilizer to Rice, Straw, and Soil. J. Agric. Food Chem. 2021, 69, 8090–8097. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Mendizábal, T.; Estavillo, J.M.; Duñabeitia, M.K.; Huérfano, X.; Castellón, A.; González-Murua, C.; Aizpurua, A.; González-Moro, M.B. 15N Natural Abundance Evidences a Better Use of N Sources by Late Nitrogen Application in Bread Wheat. Front. Plant Sci. 2018, 9, 853. [Google Scholar] [CrossRef]
- Robinson, D. Δ15N as an Integrator of the Nitrogen Cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kalcsits, L.A.; Buschhaus, H.A.; Guy, R.D. Nitrogen Isotope Discrimination as an Integrated Measure of Nitrogen Fluxes, Assimilation and Allocation in Plants. Physiol. Plant. 2014, 151, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Nehe, A.S.; Misra, S.; Murchie, E.H.; Chinnathambi, K.; Singh Tyagi, B.; Foulkes, M.J. Nitrogen Partitioning and Remobilization in Relation to Leaf Senescence, Grain Yield and Protein Concentration in Indian Wheat Cultivars. Field Crop. Res. 2020, 251, 107778. [Google Scholar] [CrossRef] [PubMed]
- Serret, M.; Ortiz-Monasterio, I.; Pardo, A.; Araus, J. The Effects of Urea Fertilisation and Genotype on Yield, Nitrogen Use Efficiency, δ 15 N and δ 13 C in Wheat. Ann. Appl. Biol. 2008, 153, 080617165316730. [Google Scholar] [CrossRef]
- Liu, H.T.; Gong, X.Y.; Schäufele, R.; Yang, F.; Hirl, R.T.; Schmidt, A.; Schnyder, H. Nitrogen Fertilization and δ 18 O of CO 2 Have No Effect on 18 O-Enrichment of Leaf Water and Cellulose in Cleistogenes Squarrosa (C 4)—Is VPD the Sole Control? Plant. Cell Environ. 2016, 39, 2701–2712. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Barbour, M.M.; Arndt, S.K.; Cheesman, A.W.; English, N.B.; Feild, T.S.; Helliker, B.R.; Holloway-Phillips, M.M.; Holtum, J.A.M.; Kahmen, A.; et al. Stable Isotopes in Leaf Water of Terrestrial Plants. Plant. Cell Environ. 2016, 39, 1087–1102. [Google Scholar] [CrossRef]
- Gao, J. Effects of Different Light Conditions on Grain Yield and Physiological Characteristics of Summer Maize and Their Regulatory Mechanisation; Shandong Agricultural University: Taian, China, 2018. [Google Scholar]
- Israeli, Y.; Schwartz, A.; Plaut, Z.; Yakir, D. Effects of Light Regime on Delta13C, Photosynthesis and Yield of Field-Grown Banana (Musa sp., Musaceae). Plant Cell Environ. 1996, 19, 225–230. [Google Scholar] [CrossRef]
- Cohen, S.; Raveh, E.; Li, Y.; Grava, A.; Goldschmidt, E.E. Physiological Responses of Leaves, Tree Growth and Fruit Yield of Grapefruit Trees under Reflective Shade Screens. Sci. Hortic. 2005, 107, 25–35. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, B.; Dong, S.; Liu, P.; Ren, B.; Zhang, J. Response of Summer Maize Photosynthate Accumulation and Distribution to Shading Stress Assessed by Using 13CO2 Stable Isotope Tracer in the Field. Front. Plant Sci. 2017, 8, 1821. [Google Scholar] [CrossRef]


| Pr (>F) | |||||||
|---|---|---|---|---|---|---|---|
| Factor | %C | %N | δ13C (‰) | δ15N (‰) | δ2H (‰) | δ18O (‰) | |
| Polished Rice | Fertilizer (N) | 0.300 | 0.168 | 0.241 | 0.007 ** | 0.003 ** | 0.903 |
| Light shading (LS) | 0.624 | 0.000 *** | 0.000 *** | 0.001 ** | 0.000 *** | 0.408 | |
| Variety (vty) | 0.960 | 0.011 * | 0.000 *** | 0.541 | 0.222 | 0.004 ** | |
| N × LS | 0.945 | 0.528 | 0.291 | 0.061 | 0.190 | 0.893 | |
| Vty × N | 0.289 | 0.072 | 0.007 ** | 0.043 * | 0.028 * | 0.367 | |
| Vty × LS | 0.889 | 0.033 * | 0.016 * | 0.000 *** | 0.030 * | 0.456 | |
| Brown Rice | Fertilizer (N) | 0.061 | 0.007 ** | 0.775 | 0.014 * | 0.005 ** | 0.848 |
| Light shading (LS) | 0.208 | 0.000 *** | 0.000 *** | 0.001 ** | 0.000 *** | 0.920 | |
| Variety (vty) | 0.398 | 0.339 | 0.000 *** | 0.338 | 0.029 * | 0.683 | |
| N × LS | 0.432 | 0.630 | 0.087 | 0.073 | 0.273 | 0.954 | |
| Vty × N | 0.662 | 0.354 | 0.439 | 0.583 | 0.187 | 0.287 | |
| Vty × LS | 0.876 | 0.602 | 0.258 | 0.000 *** | 0.114 | 0.403 | |
| Rice Husk | Fertilizer (N) | 0.326 | 0.165 | 0.344 | 0.002 * | 0.974 | 0.000 *** |
| Light shading (LS) | 0.081 | 0.053 | 0.000 *** | 0.002 ** | 0.829 | 0.008 ** | |
| Variety (vty) | 0.833 | 0.000 *** | 0.000 *** | 0.004 ** | 0.000 *** | 0.000 *** | |
| N × LS | 0.208 | 0.581 | 0.153 | 0.015 * | 0.541 | 0.283 | |
| Vty × N | 0.476 | 0.217 | 0.279 | 0.695 | 0.253 | 0.000 *** | |
| Vty × LS | 0.586 | 0.363 | 0.025 * | 0.000 *** | 0.708 | 0.000 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadood, S.A.; Jiang, Y.; Nie, J.; Li, C.; Rogers, K.M.; Liu, H.; Zhang, Y.; Zhang, W.; Yuan, Y. Effects of Light Shading, Fertilization, and Cultivar Type on the Stable Isotope Distribution of Hybrid Rice. Foods 2023, 12, 1832. https://doi.org/10.3390/foods12091832
Wadood SA, Jiang Y, Nie J, Li C, Rogers KM, Liu H, Zhang Y, Zhang W, Yuan Y. Effects of Light Shading, Fertilization, and Cultivar Type on the Stable Isotope Distribution of Hybrid Rice. Foods. 2023; 12(9):1832. https://doi.org/10.3390/foods12091832
Chicago/Turabian StyleWadood, Syed Abdul, Yunzhu Jiang, Jing Nie, Chunlin Li, Karyne M. Rogers, Hongyan Liu, Yongzhi Zhang, Weixing Zhang, and Yuwei Yuan. 2023. "Effects of Light Shading, Fertilization, and Cultivar Type on the Stable Isotope Distribution of Hybrid Rice" Foods 12, no. 9: 1832. https://doi.org/10.3390/foods12091832
APA StyleWadood, S. A., Jiang, Y., Nie, J., Li, C., Rogers, K. M., Liu, H., Zhang, Y., Zhang, W., & Yuan, Y. (2023). Effects of Light Shading, Fertilization, and Cultivar Type on the Stable Isotope Distribution of Hybrid Rice. Foods, 12(9), 1832. https://doi.org/10.3390/foods12091832





