Quinoa Ameliorates Hepatic Steatosis, Oxidative Stress, Inflammation and Regulates the Gut Microbiota in Nonalcoholic Fatty Liver Disease Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Experiment
2.2.1. Experimental Animals and Treatment
2.2.2. Determination of the Liver Index and Spleen Index
2.2.3. Histological Analysis
2.2.4. Quantification of Hepatic Lipid
2.2.5. Determination of NEFA, TG and TC Contents in Liver or Perirenal Adipose Tissues
2.2.6. Analysis of Levels of Hepatic Antioxidative Parameters and Cytokines
2.2.7. Determination of Liver Function Parameters
2.2.8. Gut Microbiome Analysis Using 16S Ribosomal RNA (rRNA) Gene Sequencing
2.2.9. Analysis of SCFAs Using Gas Chromatography-Mass Spectrometer (GC–MS)
2.3. Statistical Analysis
3. Results
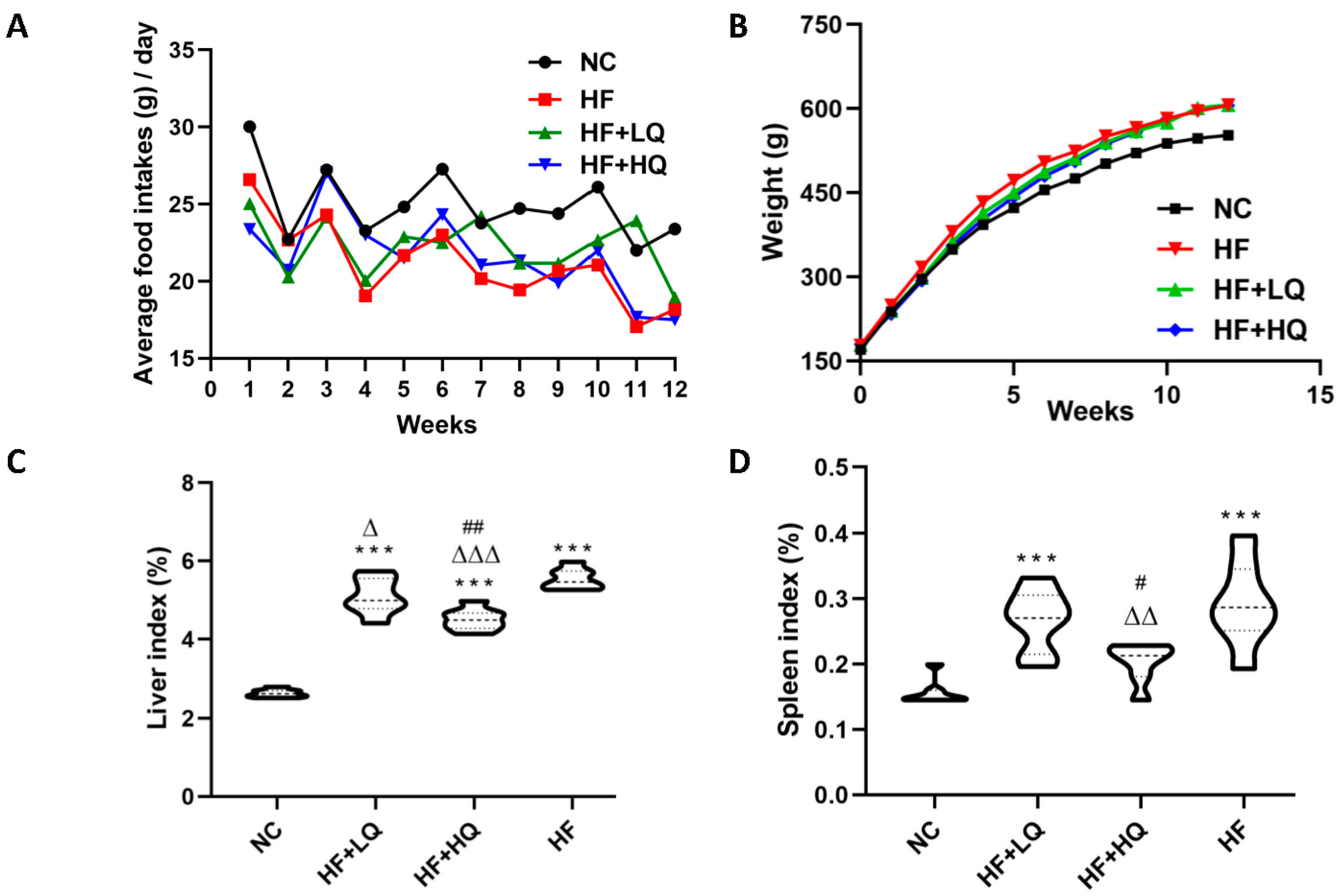
3.1. The Effect of Different Quinoa Intake Levels on the Food Intake and Weight of Rats Fed a High-Fat Diet
3.2. The Effect of Different Quinoa Intake Levels on the Liver Index and Spleen Index of Rats Fed a High-Fat Diet
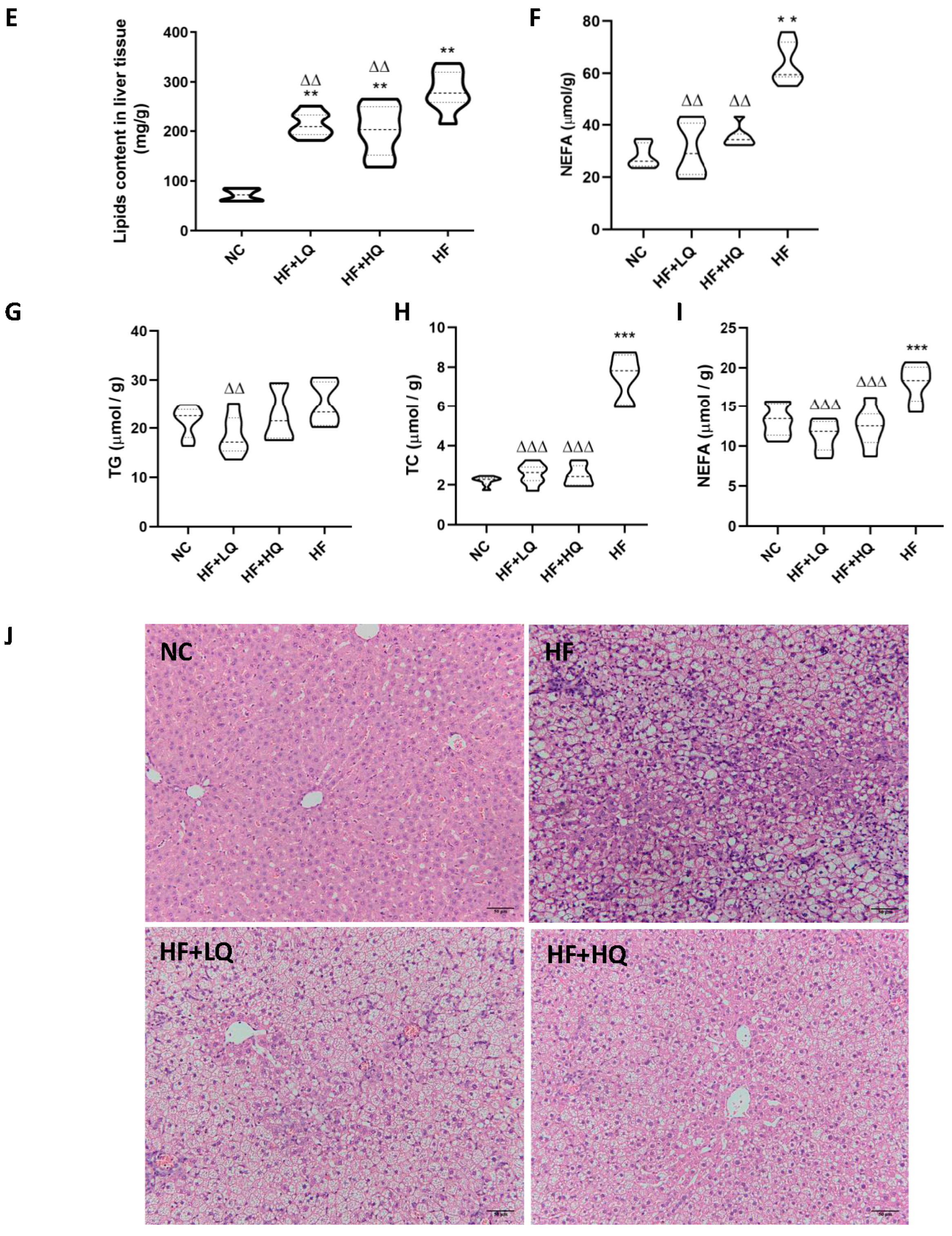
3.3. The Effect of Different Quinoa Intake Levels on the Lipid Content in Liver and Adipose Tissue and Pathological Changes in Rats Fed High-Fat Diet
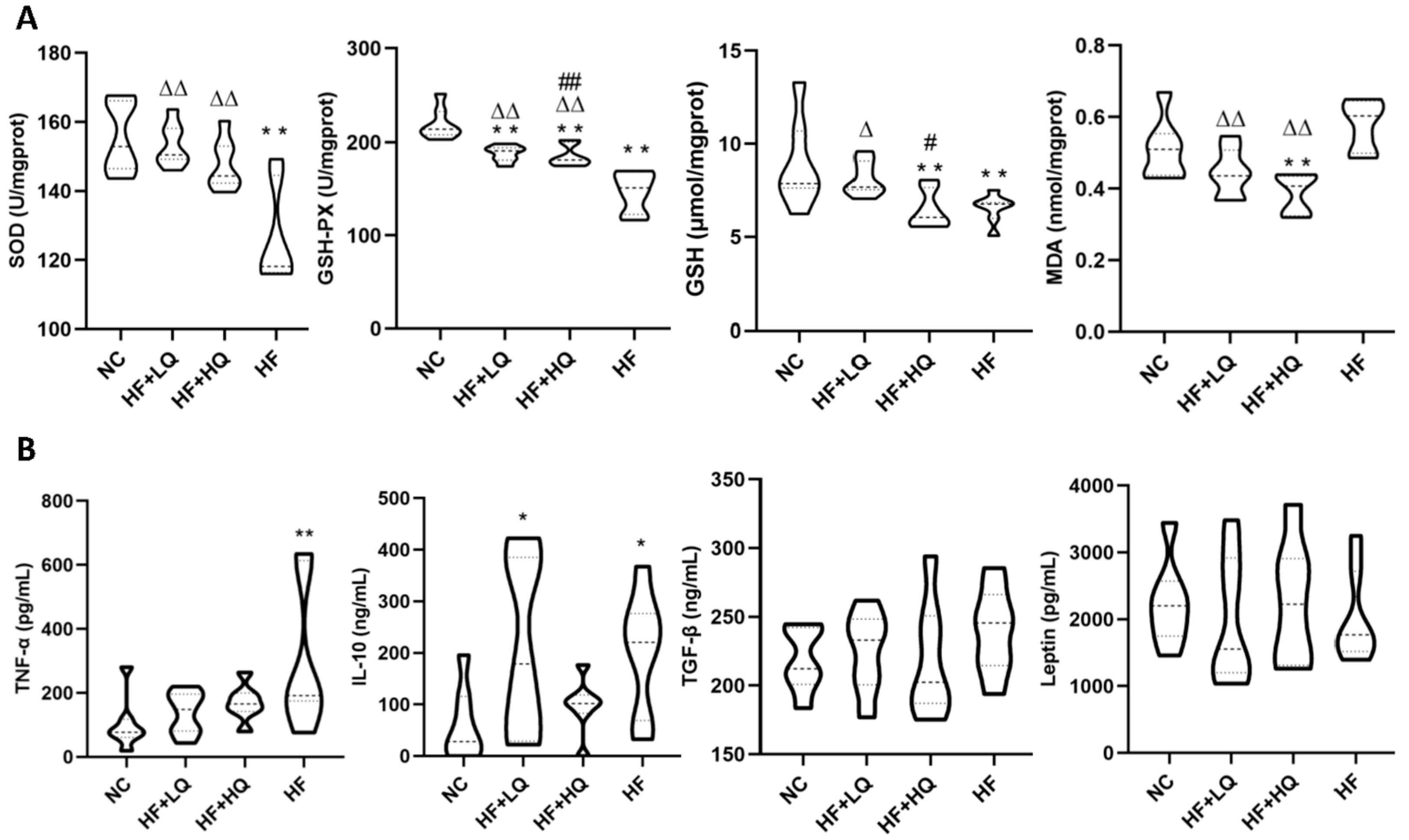
3.4. The Effect of Different Quinoa Intake Levels on Hepatic Antioxidative Parameters and Serum Cytokines of Rats Fed a High-Fat Diet
3.5. The Effect of Different Quinoa Intake Levels on Liver Function Indexes of Rats Fed a High-Fat Diet
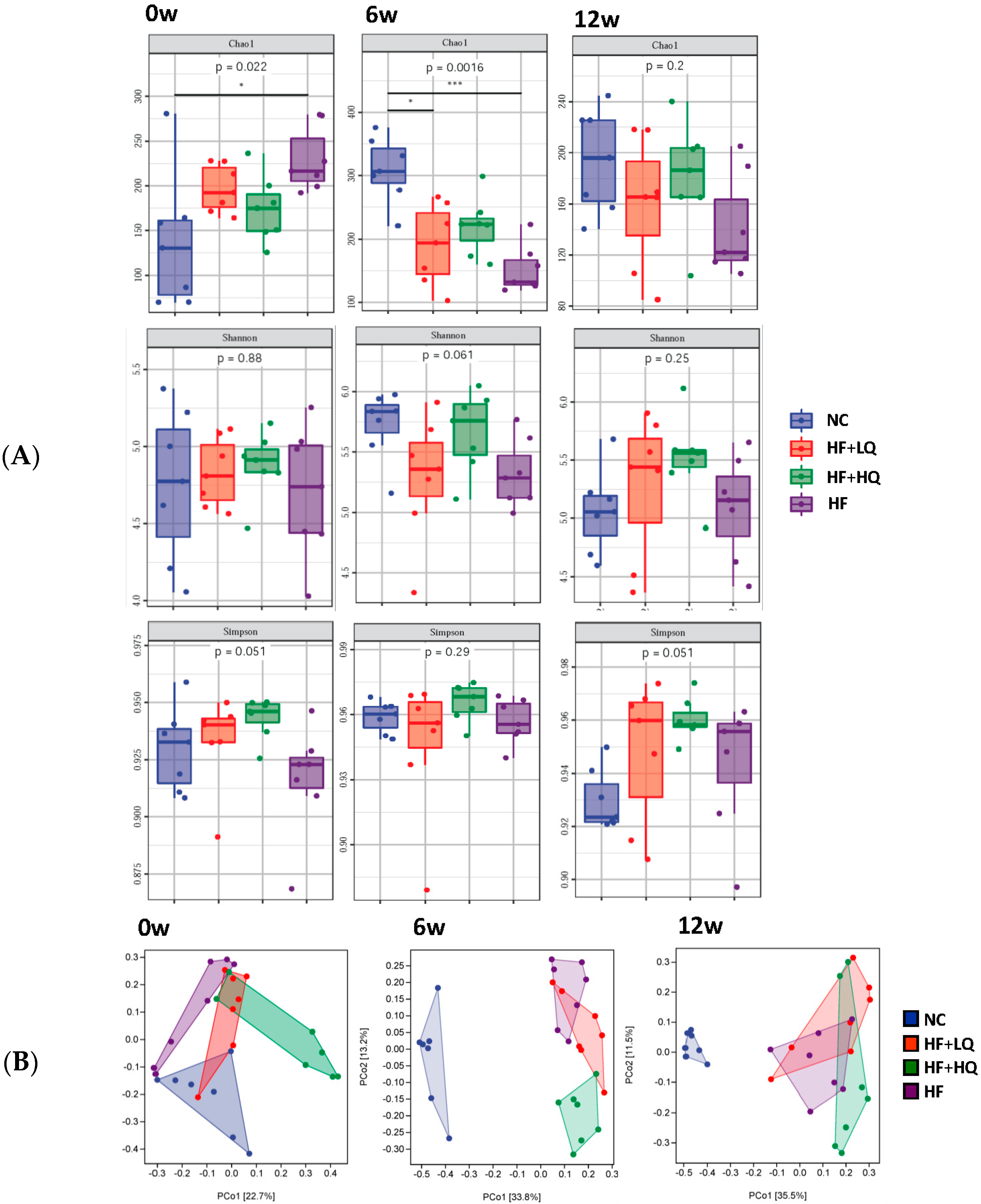
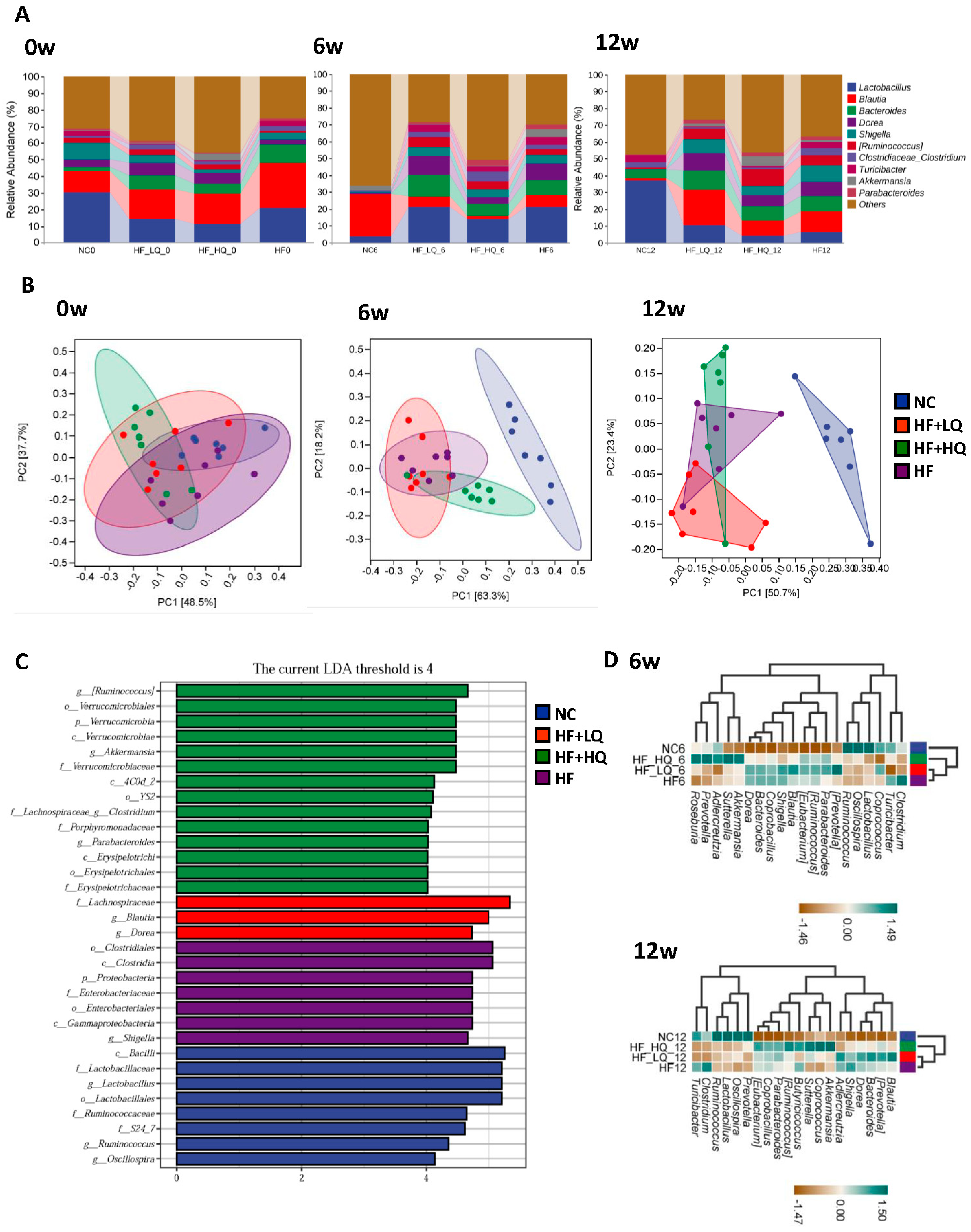
3.6. The Effect of Different Quinoa Intake Levels on the Gut Microbiota Diversity of Rats Fed a High-Fat Diet
3.7. The Effect of Different Quinoa Intake Levels on the Gut Microbiota Distribution of Rats Fed a High-Fat Diet at the Phylum Level
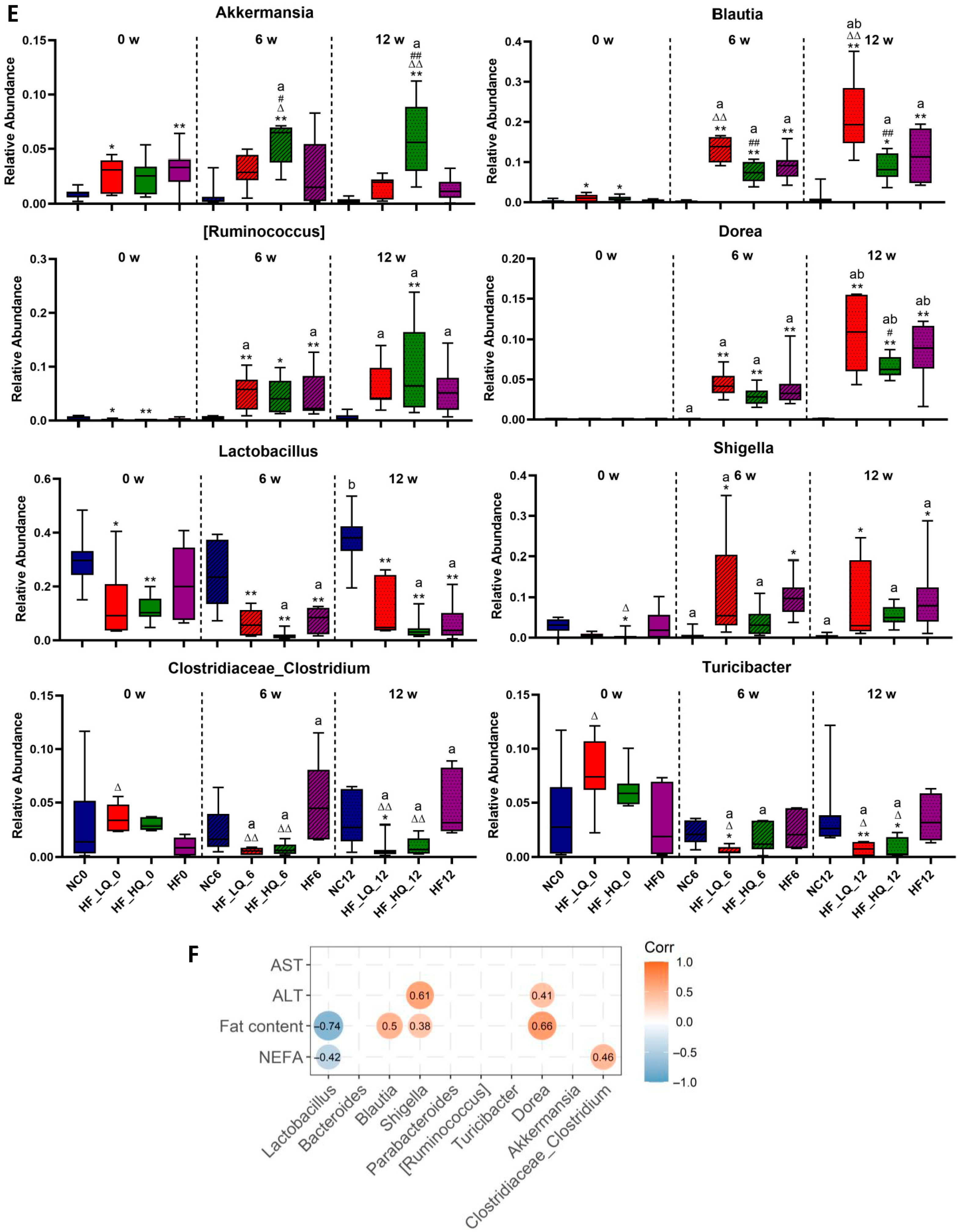
3.8. The Effect of Different Quinoa Intake Levels on the Gut Microbiota Distribution of Rats Fed a High-Fat Diet at the Genus Level
3.9. The Effect of Different Quinoa Intake Levels on SCFAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Araujo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38 (Suppl. 1), 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.H.S.; Wong, S.H. Microbiota, Obesity and NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 111–125. [Google Scholar] [PubMed]
- Abenavoli, L.; Boccuto, L. Nonalcoholic fatty liver disease in obese adolescents: The role of genetic polymorphisms. HepatoBiliary Surg. Nutr. 2019, 8, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Filho, A.M.; Pirozi, M.R.; Borges, J.T.D.S.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Alasalvar, C.; Chang, S.K.; Bolling, B.; Oh, W.Y.; Shahidi, F. Specialty seeds: Nutrients, bioactives, bioavailability, and health benefits: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2382–2427. [Google Scholar] [CrossRef]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M.; Gawlik, M.; Gorinstein, S. Effect of diet supplemented with quinoa seeds on oxidative status in plasma and selected tissues of high fructose-fed rats. Plant Foods Hum. Nutr. 2010, 65, 146–151. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014, 79, H1018–H1023. [Google Scholar] [CrossRef]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clement, K. Nonalcoholic Fatty Liver Disease: Modulating Gut Microbiota to Improve Severity? Gastroenterology 2020, 158, 1881–1898. [Google Scholar] [CrossRef] [PubMed]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y. Dietary quinoa (Chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Fan, X.; Richel, A.; Everaert, N.; Yang, X.; Ren, G. Administration with Quinoa Protein Reduces the Blood Pressure in Spontaneously Hypertensive Rats and Modifies the Fecal Microbiota. Nutrients 2021, 13, 2446. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Qiu, B.; Fan, S.; Ding, H.; Liu, Z. Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice. Sci. Rep. 2018, 8, 14916. [Google Scholar] [CrossRef]
- Zhang, R.; Zhai, Q.; Yu, Y.; Li, X.; Zhang, F.; Hou, Z.; Cao, Y.; Feng, J.; Xue, P. Safety assessment of crude saponins from Chenopodium quinoa willd. husks: 90-day oral toxicity and gut microbiota & metabonomics study in rats. Food Chem. 2022, 375, 131655. [Google Scholar]
- Maciejewska, D.; Łukomska, A.; Dec, K.; Skonieczna-Żydecka, K.; Gutowska, I.; Skórka-Majewicz, M.; Styburski, D.; Misiakiewicz-Has, K.; Pilutin, A.; Palma, J.; et al. Diet-Induced Rat Model of Gradual Development of Non-Alcoholic Fatty Liver Disease (NAFLD) with Lipopolysaccharides (LPS) Secretion. Diagnostics 2019, 9, 205. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, X.; Jin, S.; Ma, Z.; Wang, H.; Ao, N.; Yang, J.; Du, J. Vitamin D ameliorates high-fat-diet-induced hepatic injury via inhibiting pyroptosis and alters gut microbiota in rats. Arch. Biochem. Biophys. 2021, 705, 108894. [Google Scholar] [CrossRef]
- Luo, M.; Yan, J.; Wu, L.; Wu, J.; Chen, Z.; Jiang, J.; Chen, Z.; He, B. Probiotics Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats via Gut Microbiota/FXR/FGF15 Signaling Pathway. J. Immunol. Res. 2021, 2021, 2264737. [Google Scholar] [CrossRef]
- Song, C.; Lv, W.; Li, Y.; Nie, P.; Lu, J.; Geng, Y.; Heng, Z.; Song, L. Alleviating the effect of quinoa and the underlying mechanism on hepatic steatosis in high-fat diet-fed rats. Nutr. Metab. 2021, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Yu, Y.; Li, L.; Xia, H.; Dong, X.; Li, Y.; Chen, H.; Duan, W. Uricase deficiency causes mild and multiple organ injuries in rats. PLoS ONE 2021, 16, e0256594. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guan, Q.; Song, C.; Zhong, L.; Ding, X.; Zeng, H.; Nie, P.; Song, L. Regulatory effects of Lactobacillus fermented black barley on intestinal microbiota of NAFLD rats. Food Res. Int. 2021, 147, 110467. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, L.; Pastori, D.; Baratta, F.; Tozzi, G.; Novo, M.; Vicinanza, R.; Troisi, G.; Pannitteri, G.; Ceci, F.; Scardella, L.; et al. Spleen dimensions are inversely associated with lysosomal acid lipase activity in patients with non-alcoholic fatty liver disease. Intern. Emerg. Med. 2017, 12, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, Y.; Endo, K. Spleen enlargement in patients with nonalcoholic fatty liver: Correlation between degree of fatty infiltration in liver and size of spleen. Dig. Dis. Sci. 2000, 45, 196–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, X. Curcumae Ameliorates Diethylnitrosamine-Induced Hepatocellular Carcinoma via Alteration of Oxidative Stress, Inflammation and Gut Microbiota. J. Inflamm. Res. 2021, 14, 5551–5566. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Kohli, R.; Gores, G.J. Mechanisms of lipotoxicity in NAFLD and clinical implications. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 131–140. [Google Scholar] [CrossRef]
- Boden, G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab. Rep. 2006, 6, 177–181. [Google Scholar] [CrossRef]
- Harmon, D.B.; Wu, C.; Dedousis, N.; Sipula, I.J.; Stefanovic-Racic, M.; Schoiswohl, G.; O’Donnell, C.; Alonso, L.C.; Kershaw, E.E.; Kelley, E.E.; et al. Adipose tissue-derived free fatty acids initiate myeloid cell accumulation in mouse liver in states of lipid oversupply. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E758–E770. [Google Scholar] [CrossRef]
- Santana, L.F.; Inada, A.C.; Santo, B.L.S.D.E.; Filiú, W.F.O.; Pott, A.; Alves, F.M.; Guimarães, R.D.C.A.; Freitas, K.D.C.; Hiane, P.A. Nutraceutical Potential of Carica papaya in Metabolic Syndrome. Nutrients 2019, 11, 1608. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Diniz, T.A.; Junior, E.A.D.L.; Teixeira, A.A.; Biondo, L.A.; da Rocha, L.A.F.; Valadão, I.C.; Silveira, L.S.; Cabral-Santos, C.; de Souza, C.O.; Neto, J.C.R. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-alpha signaling in obese mice. Life Sci. 2021, 266, 118868. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T.; Kondro, M.; Beregova, T.; Bodnar, P.; Shcherbakov, O.; Bubnov, R.; Caprnda, M.; Delev, D.; et al. Cerium dioxide nanoparticles possess anti-inflammatory properties in the conditions of the obesity-associated NAFLD in rats. Biomed. Pharmacother. 2017, 90, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Nong, S.; Huang, X.; Lu, Y.; Zhao, H.; Lin, Y.; Man, Y.; Wang, S.; Yang, J.; Li, J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J. Biol. Chem. 2010, 285, 29965–29973. [Google Scholar] [CrossRef]
- Nati, M.; Haddad, D.; Birkenfeld, A.L.; Koch, C.A.; Chavakis, T.; Chatzigeorgiou, A. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev. Endocr. Metab. Disord. 2016, 17, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef]
- Srdić, M.; Ovčina, I.; Fotschki, B.; Haros, C.M.; Llopis, J.M.L. C. quinoa and S. hispanica L. Seeds Provide Immunonutritional Agonists to Selectively Polarize Macrophages. Cells 2020, 9, 593. [Google Scholar] [CrossRef]
- Machado, M.V.; Cortez-Pinto, H. Diet, Microbiota, Obesity, and NAFLD: A Dangerous Quartet. Int. J. Mol. Sci. 2016, 17, 481. [Google Scholar] [CrossRef]
- Dai, Z.; Lyu, W.; Xie, M.; Yuan, Q.; Ye, H.; Hu, B.; Zhou, L.; Zeng, X. Effects of alpha-Galactooligosaccharides from Chickpeas on High-Fat-Diet-Induced Metabolic Syndrome in Mice. J. Agric. Food Chem. 2017, 65, 3160–3166. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Tao, S.-Y.; Wu, Y.-X.; An, T.; Lv, B.-H.; Liu, J.-X.; Liu, Y.-T.; Jiang, G.-J. Quinoa Reduces High-Fat Diet-Induced Obesity in Mice via Potential Microbiota-Gut-Brain-Liver Interaction Mechanisms. Microbiol. Spectr. 2022, 10, e0032922. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Ebrahimzadeh, S.; Hemmati, F.; Khabbaz, A.; Hasani, A.; Gholizadeh, P. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. J. Med. Microbiol. 2021, 70, 001435. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004-19. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Ilie, I.; Oprea, L.; Picu, A.; Petcu, L.M.; Burlibasa, L.; Chifiriuc, M.-C.; Musat, M. Microbiome, Mycobiome and Related Metabolites Alterations in Patients with Metabolic Syndrome-A Pilot Study. Metabolites 2022, 12, 218. [Google Scholar] [CrossRef]
- Benus, R.F.; van der Werf, T.S.; Welling, G.W.; Judd, P.A.; Taylor, M.A.; Harmsen, H.J.; Whelan, K. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br. J. Nutr. 2010, 104, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Gut microbiota, immune development and function. Pharmacol. Res. 2013, 69, 87–113. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]


| Primary Nutrients (g/100 g) | Elements (mg/kg) | Bioactive Components (g/100 g) | |||
|---|---|---|---|---|---|
| Protein | 13.34 | K | 6642 | Flavonoids | 0.528 |
| Moisture | 10.20 | P | 5303 | Polyphenols | 0.496 |
| Ash | 2.073 | Ca | 629 | Saponins | 0.723 |
| Starch | 56.52 | Na | 73.1 | ||
| Soluble dietary fiber | 1.760 | Fe | 46.5 | ||
| Insoluble dietary fiber | 7.912 | Zn | 28.0 | ||
| Fat | 6.805 | Mn | 17.7 | ||
| Cu | 6.44 | ||||
| Time | Indicator | NC | HF | HF + LQ | HF + HQ |
|---|---|---|---|---|---|
| t0 | ALB(g/L) | 44.43 ± 1.27 | 44.71 ± 0.76 | 45.29 ± 1.5 | 44.57 ± 0.53 |
| ALT(U/L) | 53.43 ± 7.41 | 54.86 ± 5.18 | 52.86 ± 7.03 | 53.71 ± 5.22 | |
| AST(U/L) | 203.29 ± 19.47 | 255.29 ± 57.27 * | 187.86 ± 15.32 ΔΔ | 206.71 ± 34.19 Δ | |
| ALP(U/L) | 376.43 ± 44.05 | 408.29 ± 37.14 | 386.86 ± 69.92 | 385.43 ± 68.13 | |
| TP(g/L) | 57.33 ± 2.29 | 59.11 ± 1.27 | 57.33 ± 2.06 | 59 ± 2.69 | |
| Glob(g/L) | 12.86 ± 0.9 | 14.29 ± 0.49 * | 13.71 ± 1.11 | 12.71 ± 1.6 Δ | |
| A/G | 3.43 ± 0.21 | 3.16 ± 0.15 | 3.33 ± 0.2 | 3.54 ± 0.46 Δ | |
| t12 | ALB(g/L) | 39.86 ± 1.68 | 41.14 ± 1.77 | 41 ± 1.83 | 40.14 ± 0.69 |
| ALT(U/L) | 45.00 ± 8.81 | 73.43 ± 27.26 ** | 63.86 ± 21.62 | 52.29 ± 12.26 Δ | |
| AST(U/L) | 146.14 ± 24.04 | 170.71 ± 39.8 | 174.43 ± 48.1 | 135.43 ± 4.79 # | |
| ALP(U/L) | 109.29 ± 16.87 | 168.43 ± 16.51 *** | 159.43 ± 14.73 *** | 156.57 ± 18.06 *** | |
| TP(g/L) | 53.86 ± 1.68 | 62.14 ± 2.19 *** | 61.43 ± 2.15 *** | 57.43 ± 2.64 **ΔΔΔ## | |
| Glob(g/L) | 14.14 ± 0.9 | 21.14 ± 0.9 *** | 20.71 ± 2.36 *** | 17.57 ± 2.88 **ΔΔ## | |
| A/G | 2.8 ± 0.19 | 1.97 ± 0.08 *** | 2.01 ± 0.26 *** | 2.34 ± 0.48 **Δ# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Lyu, W.; Lin, Z.; Lu, J.; Geng, Y.; Song, L.; Zhang, H. Quinoa Ameliorates Hepatic Steatosis, Oxidative Stress, Inflammation and Regulates the Gut Microbiota in Nonalcoholic Fatty Liver Disease Rats. Foods 2023, 12, 1780. https://doi.org/10.3390/foods12091780
Zhong L, Lyu W, Lin Z, Lu J, Geng Y, Song L, Zhang H. Quinoa Ameliorates Hepatic Steatosis, Oxidative Stress, Inflammation and Regulates the Gut Microbiota in Nonalcoholic Fatty Liver Disease Rats. Foods. 2023; 12(9):1780. https://doi.org/10.3390/foods12091780
Chicago/Turabian StyleZhong, Lingyue, Wei Lyu, Zihan Lin, Jun Lu, Yanlou Geng, Lihua Song, and Heng Zhang. 2023. "Quinoa Ameliorates Hepatic Steatosis, Oxidative Stress, Inflammation and Regulates the Gut Microbiota in Nonalcoholic Fatty Liver Disease Rats" Foods 12, no. 9: 1780. https://doi.org/10.3390/foods12091780
APA StyleZhong, L., Lyu, W., Lin, Z., Lu, J., Geng, Y., Song, L., & Zhang, H. (2023). Quinoa Ameliorates Hepatic Steatosis, Oxidative Stress, Inflammation and Regulates the Gut Microbiota in Nonalcoholic Fatty Liver Disease Rats. Foods, 12(9), 1780. https://doi.org/10.3390/foods12091780







