The Properties of Different Starches under the Influence of Glucono-Delta-Lactone at Different Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GDL Solution
2.3. Pasting Analysis
2.4. Starch Paste pH Values Determination
2.5. Flow Analysis
2.6. Starch Gel Syneresis Analysis
2.7. Crystallinity Changes Observation by X-ray Diffractometry (XRD)
2.8. Gel Permeation Chromatography
2.9. Statistical Analysis
3. Results and Discussion
3.1. Gel Permeation Chromatography Analysis
3.2. Pasting Properties
3.3. Starch Pastes’ pH Values
3.4. Flow Properties
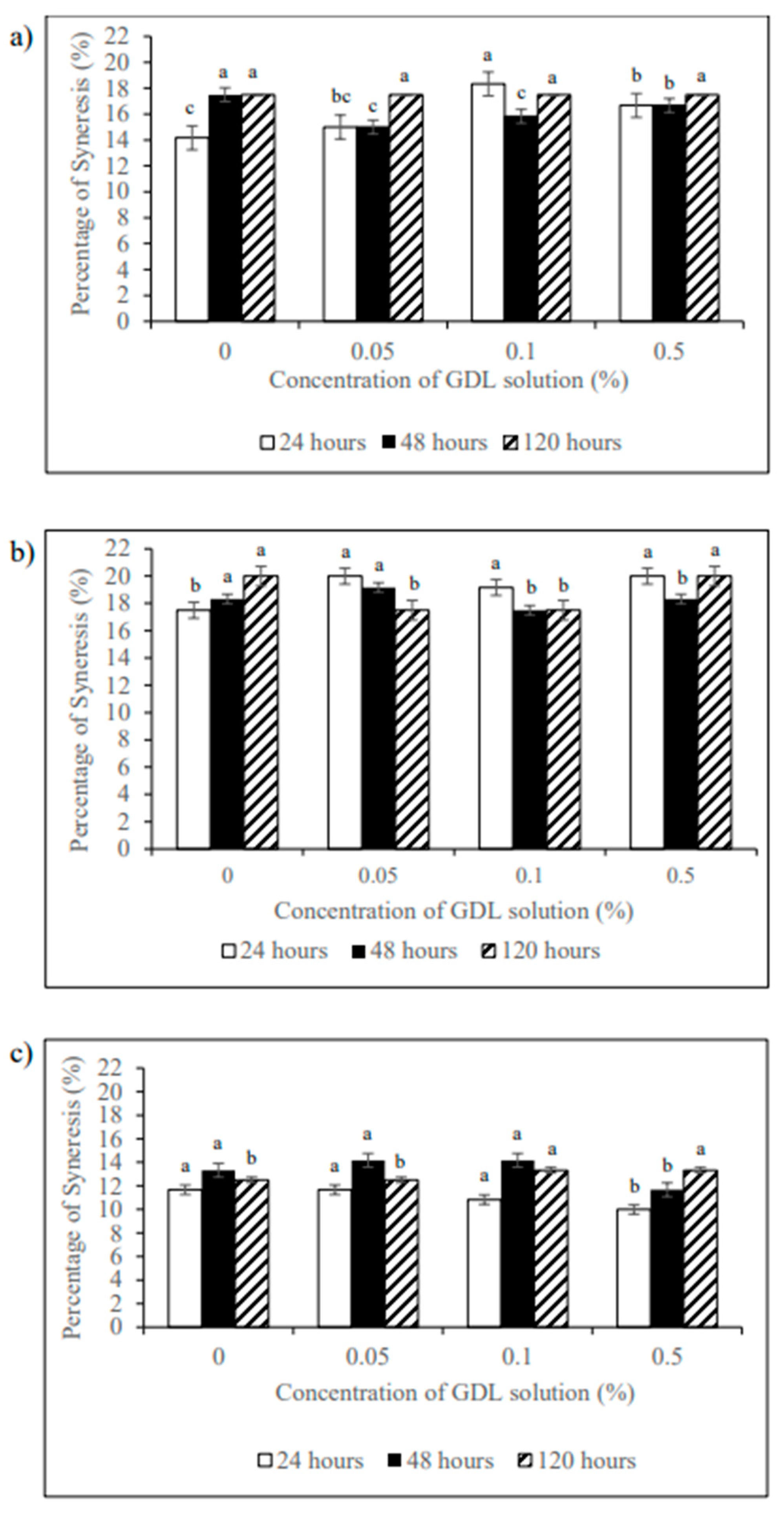
3.5. Gel Syneresis Properties
3.6. Gel Crystalline Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crosser, N. Plant-Based Meat, Eggs, and Dairy: 2019 U.S. State of the Industry Report; Good Food Institute: Washington, DC, USA, 2020; pp. 1–72. [Google Scholar]
- Hemler, E.C.; Hu, F.B. Plant-based diets for cardiovascular disease prevention: All plant foods are not created equal. Curr. Atheroscler. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Plant-based foods and prevention of cardiovascular disease: An overview. Am. J. Clin. Nutr. 2003, 78, 544S–551S. [Google Scholar] [CrossRef]
- Possidónio, C.; Prada, M.; Graça, J.; Piazza, J. Consumer perceptions of conventional and alternative protein sources: A mixed-methods approach with meal and product framing. Appetite 2021, 156, 104860. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.F.; Winslow, G.R. Meatless Diet: A Moral Imperative; Vegetarian Nutrition; CRC Press: Boca Raton, MA, USA, 2001; pp. 463–481. [Google Scholar]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. Available online: https://www.science.org/doi/10.1126/science.aaq0216 (accessed on 15 February 2023). [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Hermansson, A.M.; Svegmark, K. Developments in the understanding of starch functionality. Trends Food Sci. Technol. 1996, 7, 345–353. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Introduction to food hydrocolloids. In Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2021; pp. 3–26. [Google Scholar]
- Yazid, N.S.M.; Abdullah, N.; Muhammad, N.; Matias-Peralta, H.M. Application of starch and starch-based products in food industry. J. Sci. Technol. 2018, 10, 144–174. [Google Scholar]
- Bühler, J.M.; Schlangen, M.; Möller, A.C.; Bruins, M.E.; van der Goot, A.J. Starch in plant-based meat replacers: A new approach to using endogenous starch from cereals and legumes. Starch/Stärke 2022, 74, 2100157. [Google Scholar] [CrossRef]
- Landerito, N.A.; Wang, Y.J. Preparation and properties of starch phosphates using waxy, common, and high-amylose corn starches II. Reactive extrusion method. Cereal Chem. 2005, 82, 271–276. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1094/CC-82-0271 (accessed on 15 February 2023). [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J. Plant-based food and protein trends from a business perspective: Markets, consumers, and challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef]
- 21CFR 184.1318. Food and Drugs; Part 184-Direct Food Substances Affirmed as Generally Recognized as Safe; Subpart B-Listing of Specific Substances Affirmed as GRAS; 184.1318-Glucono Delta Lactone. 1986. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1318 (accessed on 15 February 2023).
- Sumitra, R.; Pierre, F.; Ashok, P.A.; Christian, L. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Romeo, C.; Brenes, M.; García-Serrano, P.; Montano, A.; Medina, E.; García-García, P. Packing black ripe olives in acid conditions. Food Chem. 2021, 337, e127751. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Novel processing methods: Updates on acidified vegetables thermal processing. Curr. Opin. Food Sci. 2018, 23, 64–69. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, S.H.; Lee, J.W.; Lee, C.Y.; Byun, M.-W. Effect of glucono delta-lactone on the quality of cooked rice. J. Korean Soc. Food Sci. Nutr. 2004, 33, 1698–1702. [Google Scholar] [CrossRef]
- Low, Y.K.; Effarizah, M.E.; Cheng, L.H. The impact of glucono-delta-lactone (GDL) on rice flour pasting properties and GDL’s dipping effects on the quality of rice noodles. J. Food Process. Preserv. 2020, 45, e14944. [Google Scholar] [CrossRef]
- Singh, H.; Punia, R.; Ganesh, A.; Duttagupta, A.; Kaur, A.; Blennow, A. Modification of moth bean starch using mixture of organic acids under dry heating. Starch/Stärke 2019, 71, e1900061. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T.J.S.S. Studies on the relationship between water-saturated state and crystallinity by the diffraction method for moistened potato starch. Starch/Stärke 1983, 35, 111–114. [Google Scholar] [CrossRef]
- Chong, W.T.; Uthumporn, U.; Karim, A.A.; Cheng, L.H. The influence of ultrasound on the degree of oxidation of hypochlorite-oxidized corn starch. LWT-Food Sci. Technol. 2013, 50, 439–443. [Google Scholar] [CrossRef]
- Moody, R.T. High-performance size exclusion chromatography. In Column Handbook for Size Exclusion Chromatography; Academic Press: San Diego, CA, USA, 1999; p. 75. [Google Scholar]
- Karim, A.A.; Sufha, E.H.; Zaidul, I.S.M. Dual modification of starch via partial enzymatic hydrolysis in the granular state and subsequent hydroxypropylation. J. Agric. Food Chem. 2008, 56, 10901–10907. [Google Scholar] [CrossRef]
- Abdorreza, M.N.; Robal, M.; Cheng, L.H.; Tajul, A.Y.; Karim, A.A. Physicochemical, thermal, and rheological properties of acid-hydrolyzed sago (Metroxylon sagu) starch. LWT-Food Sci. Technol. 2012, 46, 135–141. [Google Scholar] [CrossRef]
- Majzoobi, M.; Kaveh, Z.; Farahnaky, A. Effect of acetic acid on physical properties of pregelatinized wheat and corn starch gels. Food Chem. 2016, 196, 720–725. [Google Scholar] [CrossRef]
- Chan, H.T.; Leh, C.P.; Bhat, R.; Senan, C.; Williams, P.A.; Karim, A.A. Molecular structure, rheological and thermal characteristics of ozone-oxidized starch. Food Chem. 2011, 126, 1019–1024. [Google Scholar] [CrossRef]
- Yan, J.K.; Li, L.; Wang, Z.M.; Leung, P.H.; Wang, W.Q.; Wu, J.Y. Acidic degradation and enhanced antioxidant activities of exopolysaccharides from Cordyceps sinensis mycelial culture. Food Chem. 2009, 117, 641–646. [Google Scholar] [CrossRef]
- Abera, S.; Rakshit, S.K. Processing technology comparison of physicochemical and functional properties of cassava starch extracted from fresh root and dry chips. Starch/Stärke 2003, 55, 287–296. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/star.200390072 (accessed on 15 February 2023). [CrossRef]
- Putri, W.D.R.; Marseno, D.W.; Cahyanto, M.N. Role of lactic acid bacteria on structural and physicochemical properties of sour cassava starch. APCBEE Procedia 2012, 2, 104–109. [Google Scholar] [CrossRef]
- Absar, N.; Zaidul, I.S.M.; Takigawa, S.; Hashimoto, N.; Matsuura-Endo, C.; Yamauchi, H.; Noda, T. Enzymatic hydrolysis of potato starches containing different amount of phosphorus. Food Chem. 2009, 112, 57–62. [Google Scholar] [CrossRef]
- Carciofi, M.; Shaik, S.S.; Jensen, S.L.; Blennow, A.; Svensson, J.T.; Vincze, É.; Hebelstrup, K.H. Hyperphosphorylation of cereal starch. J. Cereal Sci. 2011, 54, 339–346. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch/Stärke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Kasemsuwan, T.; Jane, J.L. Quantitative method for the survey of starch phosphate derivatives and starch phospholipids by 31P nuclear magnetic resonance spectroscopy. Cereal Chem. 1996, 73, 702–707. [Google Scholar]
- Takahashi, R. Physical properties of starch granules and their uses. J. Jpn. Soc. Starch Sci. 1974, 21, 51–60. [Google Scholar]
- Barnes, H.A. The ‘yield stress myth?’ paper-21 years on. Appl. Rheol. 2007, 17, 43110-1–43110-5. [Google Scholar] [CrossRef]
- Wei, Y.P.; Wang, C.S.; Wu, J.S.B. Flow properties of fruit fillings. Food Res. Int. 2001, 34, 377–381. [Google Scholar] [CrossRef]
- Sun, A.; Gunasekaran, S. Yield stress in foods: Measurements and applications. Int. J. Food Prop. 2009, 12, 70–101. [Google Scholar] [CrossRef]
- Amani, N.G.; Kamenan, A.; Rolland-Sabaté, A.; Colonna, P. Stability of yam starch gels during processing. Afr. J. Biotechnol. 2005, 4, 94–101. [Google Scholar]
- Wang, S.; Yu, J.; Yu, J.; Chen, H.; Pang, J. The effect of acid hydrolysis on morphological and crystalline properties of Rhizoma Dioscorea starch. Food Hydrocoll. 2007, 21, 1217–1222. [Google Scholar] [CrossRef]
- Kainuma, K.; French, D. Naegeli amylodextrin and its relationship to starch granule structure. I. Preparation and properties of amylodextrins from various starch types. Biopolymers 1971, 10, 1673–1680. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T. Structures and physicochemical properties of acid-thinned corn, potato, and rice starches. Starch/Stärke 2001, 53, 570–576. [Google Scholar] [CrossRef]


| Types of Starch | GDL Concentration (%) | Fraction I | Fraction II | ||||
|---|---|---|---|---|---|---|---|
| Molecular Weight (Mw) (Da) × 106 | Polydispersity (Mw/Mn) | Intrinsic Viscosity (η, DL/g) | Molecular Weight (Mw) (Da) × 103 | Polydispersity (Mw/Mn) | Intrinsic Viscosity (η, dL/g) | ||
| Potato | 0 | 5.51 ± 0.07 a | 1.55 ± 0.04 d | 0.304 ± 0.005 a | 6.80 ± 0.91 d | 3.06 ± 0.24 b | 1.70 ± 0.001 a |
| 0.05 | 1.16 ± 0.12 b | 6.82 ± 0.16 c | 0.392 ± 0.008 b | 8.80 ± 0.01 c | 3.54 ± 0.59 b | 1.63 ± 0.006 b | |
| 0.1 | 1.16 ± 0.13 b | 7.32 ± 0.31 b | 0.390 ± 0.060 b | 9.83 ± 0.76 b | 5.29 ± 0.36 a | 1.49 ± 0.009 c | |
| 0.5 | 0.61 ± 0.05 c | 8.84 ± 0.30 a | 0.373 ± 0.006 c | 10.2 ± 0.74 a | 5.50 ± 0.34 a | 1.47 ± 0.002 c | |
| Tapioca | 0 | 4.60 ± 0.06 a | 6.39 ± 0.32 c | 0.674 ± 0.002 a | 4.16 ± 1.17 d | 4.70 ± 0.22 b | 0.678 ± 0.009 a |
| 0.05 | 2.55 ± 0.08 b | 9.05 ± 0.84 b | 0.537 ± 0.002 b | 5.23 ± 0.48 c | 4.67 ± 0.15 b | 0.665 ± 0.004 b | |
| 0.1 | 2.47 ± 0.04 b | 10.01 ± 0.02 ab | 0.519 ± 0.040 b | 5.50 ± 2.23 b | 4.51 ± 0.31 b | 0.605 ± 0.003 c | |
| 0.5 | 1.81 ± 0.06 c | 10.50 ± 0.40 a | 0.506 ± 0.002 b | 6.55 ± 0.25 a | 7.99 ± 0.03 a | 0.593 ± 0.000 d | |
| Corn | 0 | 3.15 ± 0.17 a | 1.99 ± 0.06 b | 0.489 ± 0.001 a | 3.83 ± 0.15 d | 2.86 ± 0.05 c | 1.200 ± 0.003 a |
| 0.05 | 1.40 ± 0.02 b | 2.18 ± 0.15 b | 0.154 ± 0.006 b | 4.87 ± 0.68 c | 4.15 ± 0.37 b | 0.744 ± 0.000 b | |
| 0.1 | 1.36 ± 0.03 b | 2.27 ± 0.96 b | 0.142 ± 0.003 b | 5.15 ± 0.14 b | 4.65 ± 0.30 b | 0.590 ± 0.005 c | |
| 0.5 | 1.33 ± 0.07 b | 9.91 ± 0.54 a | 0.138 ± 0.012 b | 6.14 ± 0.69 a | 5.94 ± 0.26 a | 0.538 ± 0.001 d | |
| Types of Starches | Concentration of GDL Solution (%) | pH Value | Pasting Parameters * | ||||
|---|---|---|---|---|---|---|---|
| PV (Pa.s) | TV (Pa.s) | BD (Pa.s) | FV (Pa.s) | SB (Pa.s) | |||
| 0.00 | 5.70 ± 0.03 a | 8.58 ± 0.09 a | 2.80 ± 0.02 a | 5.72 ± 0.08 a | 3.19 ± 0.05 a | 0.38 ± 0.03 b | |
| 0.05 | 3.11 ± 0.02 b | 6.63 ± 0.05 b | 2.23 ± 0.05 b | 4.40 ± 0.08 b | 2.81 ± 0.07 b | 0.58 ± 0.02 a | |
| Potato | 0.10 | 3.11 ± 0.20 b | 6.06 ± 0.02 c | 1.99 ± 0.04 c | 4.07 ± 0.05 c | 2.58 ± 0.05 c | 0.59 ± 0.02 a |
| 0.50 | 2.88 ± 0.02 c | 5.07 ± 0.07 d | 1.39 ± 0.02 d | 3.68 ± 0.05 d | 1.98 ± 0.05 d | 0.59 ± 0.04 a | |
| 0.00 | 5.85 ± 0.03 a | 3.45 ± 0.01 a | 1.61 ± 0.03 a | 1.84 ± 0.02 d | 2.54 ± 0.06 a | 0.934 ± 0.07 a | |
| 0.05 | 3.14 ± 0.02 b | 3.37 ± 0.03 b | 1.20 ± 0.02 b | 2.17 ±0.02 c | 1.78 ± 0.03 b | 0.577 ± 0.09 b | |
| Tapioca | 0.10 | 3.12 ± 0.04 b | 3.35 ± 0.04 bc | 1.07 ± 0.03 c | 2.28 ± 0.02 b | 1.60 ± 0.05 c | 0.527 ± 0.03 c |
| 0.50 | 2.87 ± 0.09 c | 3.34 ± 0.03 c | 0.64 ± 0.02 d | 2.70 ±0.02 a | 0.99 ± 0.02 d | 0.356 ± 0.06 d | |
| 0.00 | 5.81 ± 0.01 a | 1.73 ± 0.03 a | 1.46 ± 0.01 a | 0.28 ± 0.02 d | 1.93 ± 0.02 a | 0.578 ± 0.01 a | |
| 0.05 | 3.21 ± 0.14 b | 1.72 ± 0.02 a | 1.28 ± 0.03 b | 0.44 ± 0.01 c | 1.87 ± 0.02 b | 0.597 ±0.01 a | |
| Corn | 0.10 | 3.19 ± 0.01 b | 1.67 ± 0.03 b | 1.19 ± 0.03 c | 0.48 ± 0.02 b | 1.72 ± 0.07 c | 0.536 ± 0.06 b |
| 0.50 | 2.82 ± 0.03 c | 1.65 ± 0.01 b | 0.96 ± 0.01 d | 0.70 ± 0.01 a | 1.52 ± 0.02 d | 0.548 ± 0.02 b | |
| Types of Starch | GDL Concentration (%) | Herschel–Bulkley Model Parameters * | |||
|---|---|---|---|---|---|
| Yield Stress (σο, Pa) | Consistency (K, Pa sn) | Flow Index (n) | R2 | ||
| Potato starch | 0 | 19.33 ± 0.10 a | 5.05 ± 0.04 a | 0.428 ± 0.003 a | 0.9937 |
| 0.05 | 3.34 ± 1.84 b | 4.24 ± 0.12 b | 0.409 ± 0.009 b | 0.9976 | |
| 0.1 | 2.54 ±1.63 b | 4.23 ± 0.34 b | 0.412 ± 0.009 b | 0.9928 | |
| 0.5 | 2.60 ± 0.89 b | 3.83 ± 0.02 c | 0.402 ± 0.006 b | 0.9955 | |
| Tapioca starch | 0 | 7.50 ± 0.53 a | 4.50 ± 0.34 a | 0.472 ± 0.019 b | 0.9900 |
| 0.05 | 6.56 ± 0.12 a | 3.75 ± 0.27 b | 0.546 ± 0.015 a | 0.9977 | |
| 0.1 | 5.77 ± 2.75 a | 3.99 ± 0.30 b | 0.533 ± 0.014 a | 0.9906 | |
| 0.5 | 4.34 ± 0.41 a | 2.97 ± 0.12 c | 0.563 ± 0.009 a | 0.9967 | |
| Corn starch | 0 | 36.33 ± 1.62 b | 3.49 ± 0.45 a | 0.448 ± 0.020 a | 0.9925 |
| 0.05 | 35.99 ± 2.79 b | 3.49 ± 0.22 a | 0.352 ± 0.030 a | 0.9973 | |
| 0.1 | 41.81 ± 0.78 a | 3.49 ± 0.09 a | 0.453 ± 0.015 a | 0.9903 | |
| 0.5 | 41.98 ± 1.42 a | 3.22 ± 0.06 a | 0.459 ± 0.007 a | 0.9923 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Shukri, A.; Cheng, L.-H. The Properties of Different Starches under the Influence of Glucono-Delta-Lactone at Different Concentrations. Foods 2023, 12, 1770. https://doi.org/10.3390/foods12091770
Mohd Shukri A, Cheng L-H. The Properties of Different Starches under the Influence of Glucono-Delta-Lactone at Different Concentrations. Foods. 2023; 12(9):1770. https://doi.org/10.3390/foods12091770
Chicago/Turabian StyleMohd Shukri, Afirah, and Lai-Hoong Cheng. 2023. "The Properties of Different Starches under the Influence of Glucono-Delta-Lactone at Different Concentrations" Foods 12, no. 9: 1770. https://doi.org/10.3390/foods12091770
APA StyleMohd Shukri, A., & Cheng, L.-H. (2023). The Properties of Different Starches under the Influence of Glucono-Delta-Lactone at Different Concentrations. Foods, 12(9), 1770. https://doi.org/10.3390/foods12091770







