Physical Blending of Fractionated Bambangan Kernel Fat Stearin and Palm Oil Mid-Fraction to Formulate Cocoa Butter Equivalent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CBE via Blending BKF-SS and POMF
2.3. Determination of Physicochemical Properties of BKF-SS: POMF Blends
2.4. Determination of Fatty Acid Profiles
2.5. Determination of TAG Profiles
2.6. Melting Behaviour
2.7. SFC Analysis
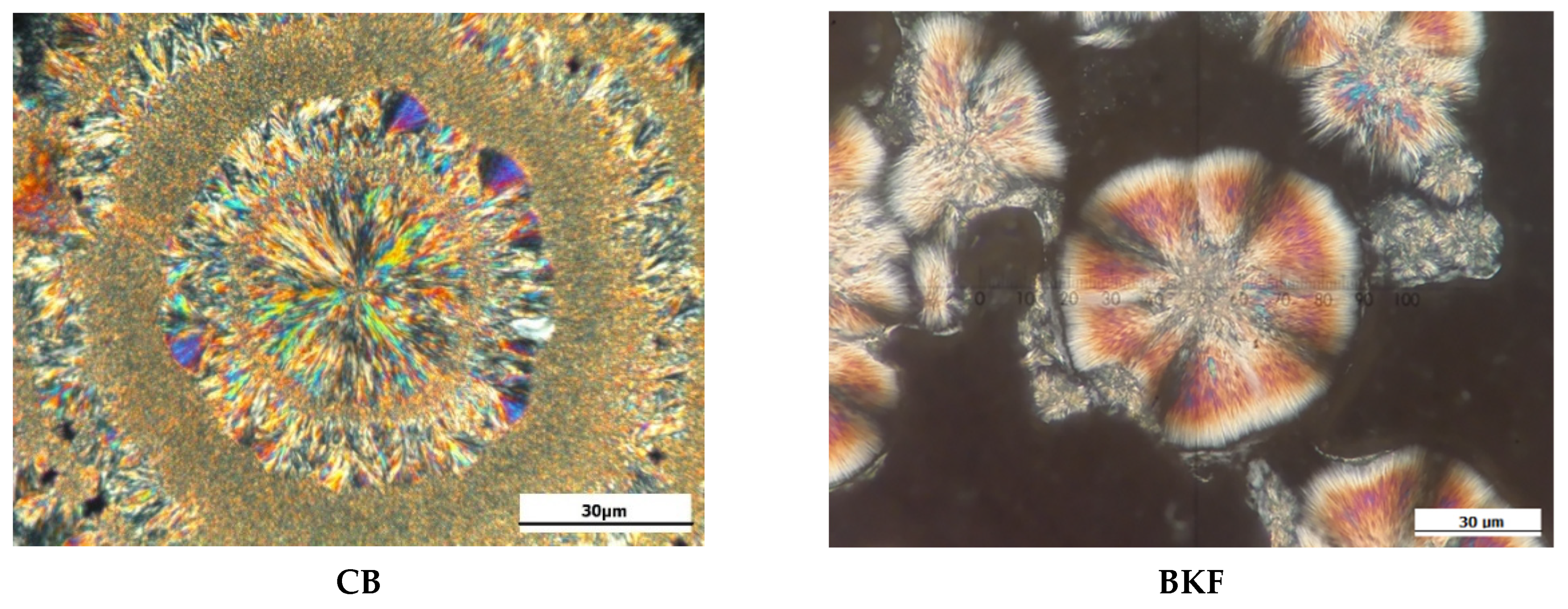
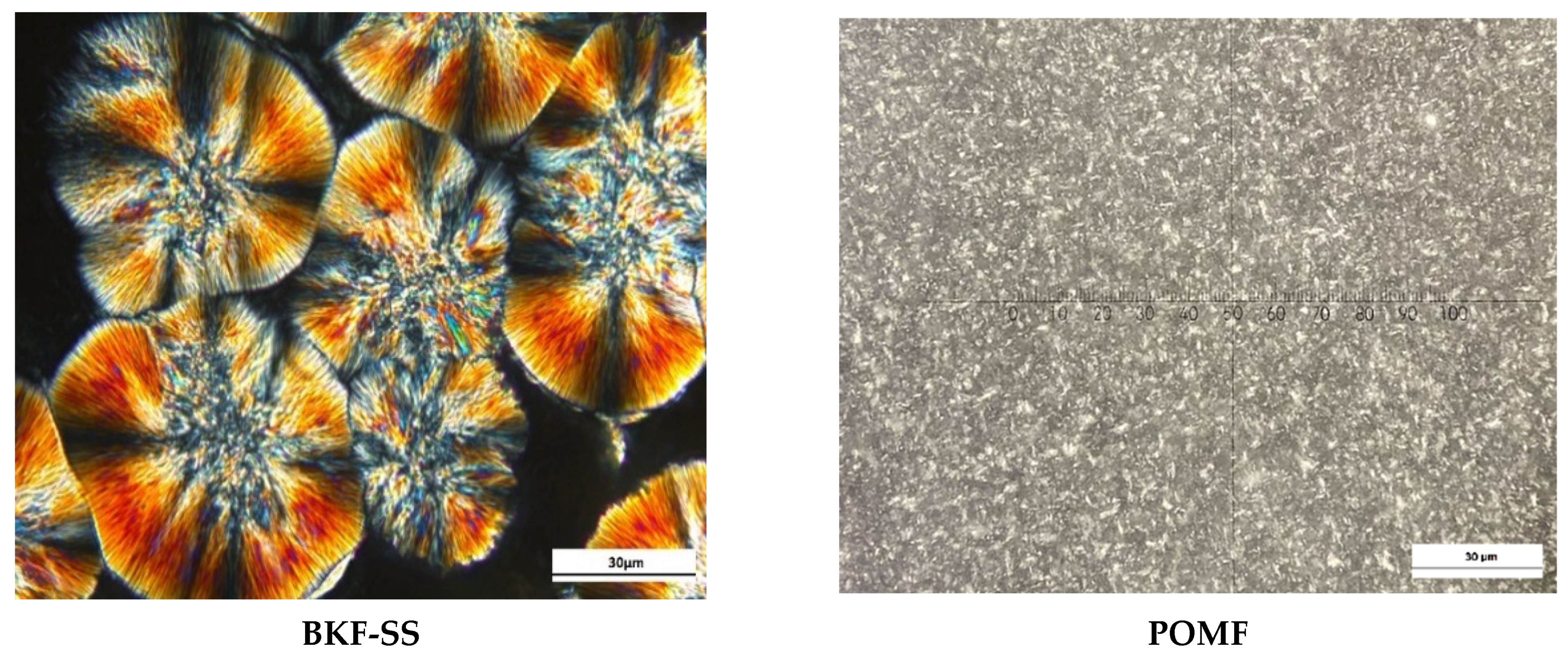
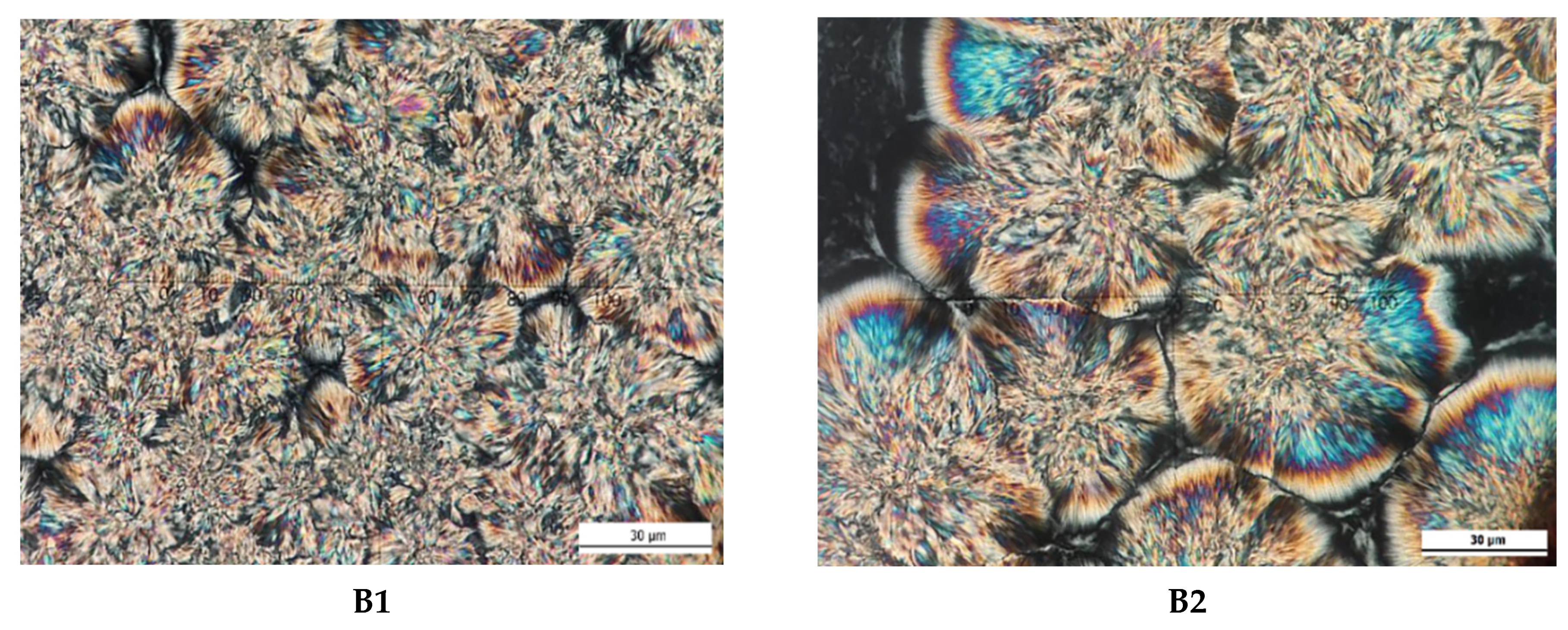
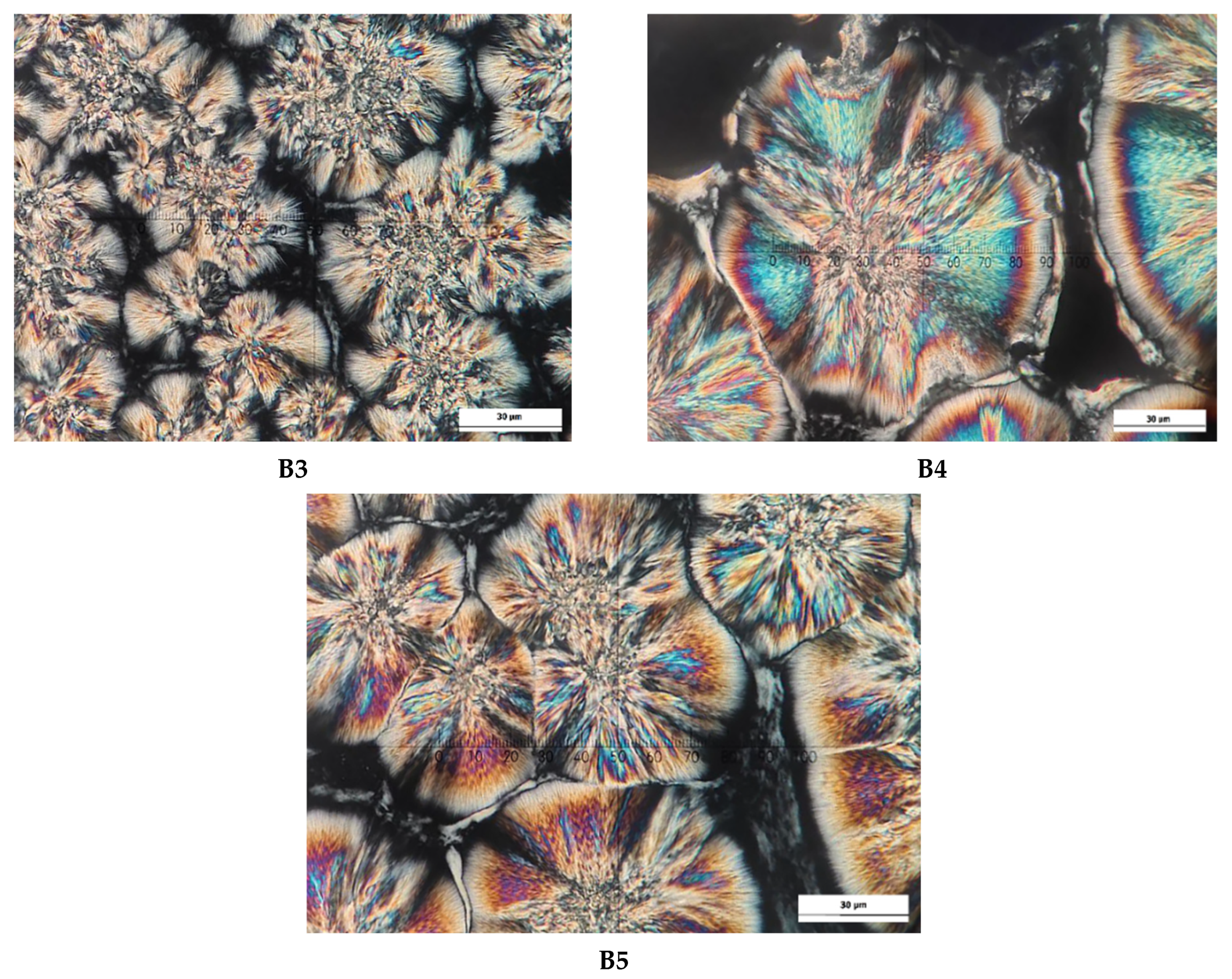
2.8. Crystal Morphology
2.9. Compatibility Analysis
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Properties of BKF-SS: POMF Blends
3.2. Fatty Acid and TAG Composition
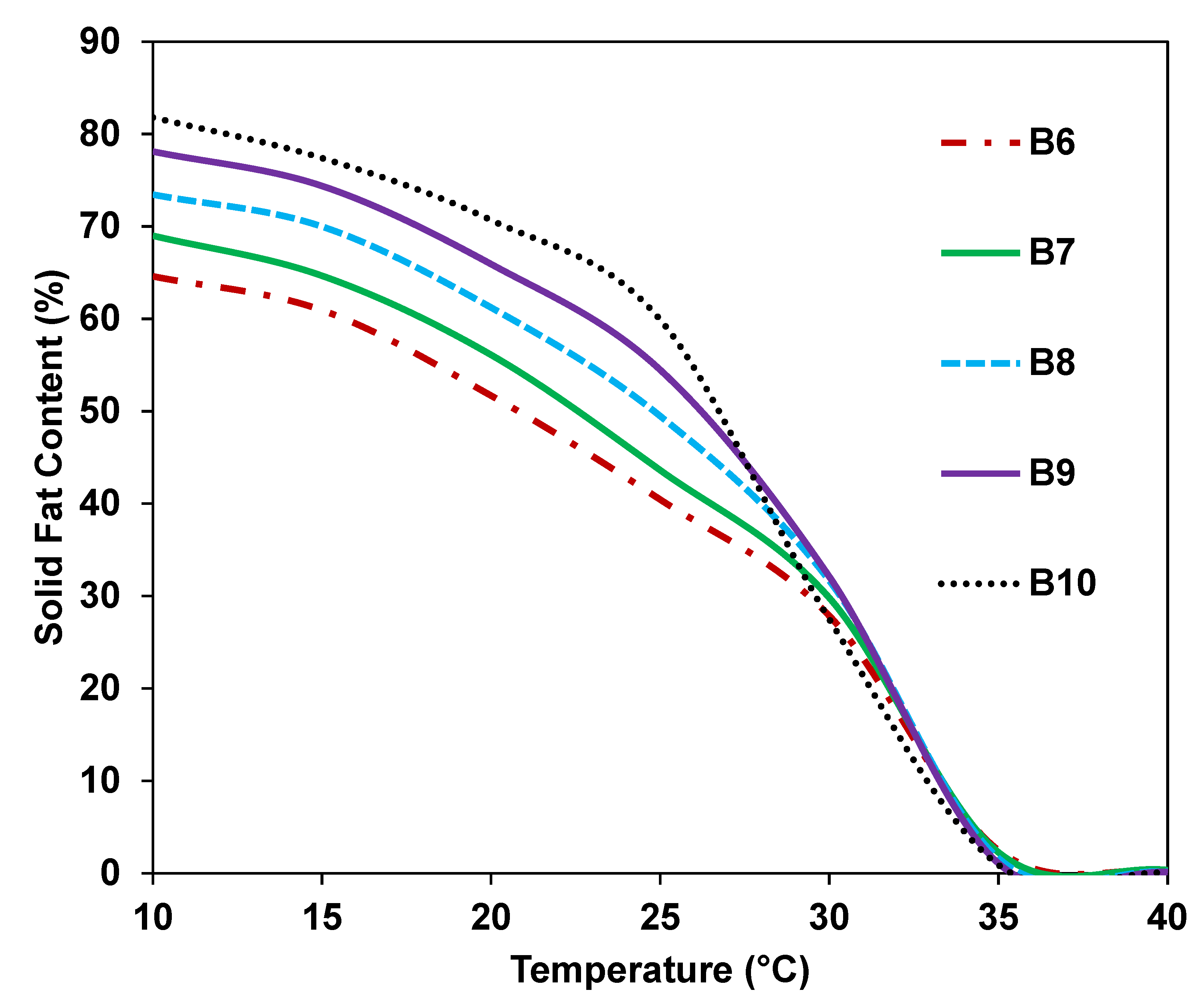
3.3. Melting Properties
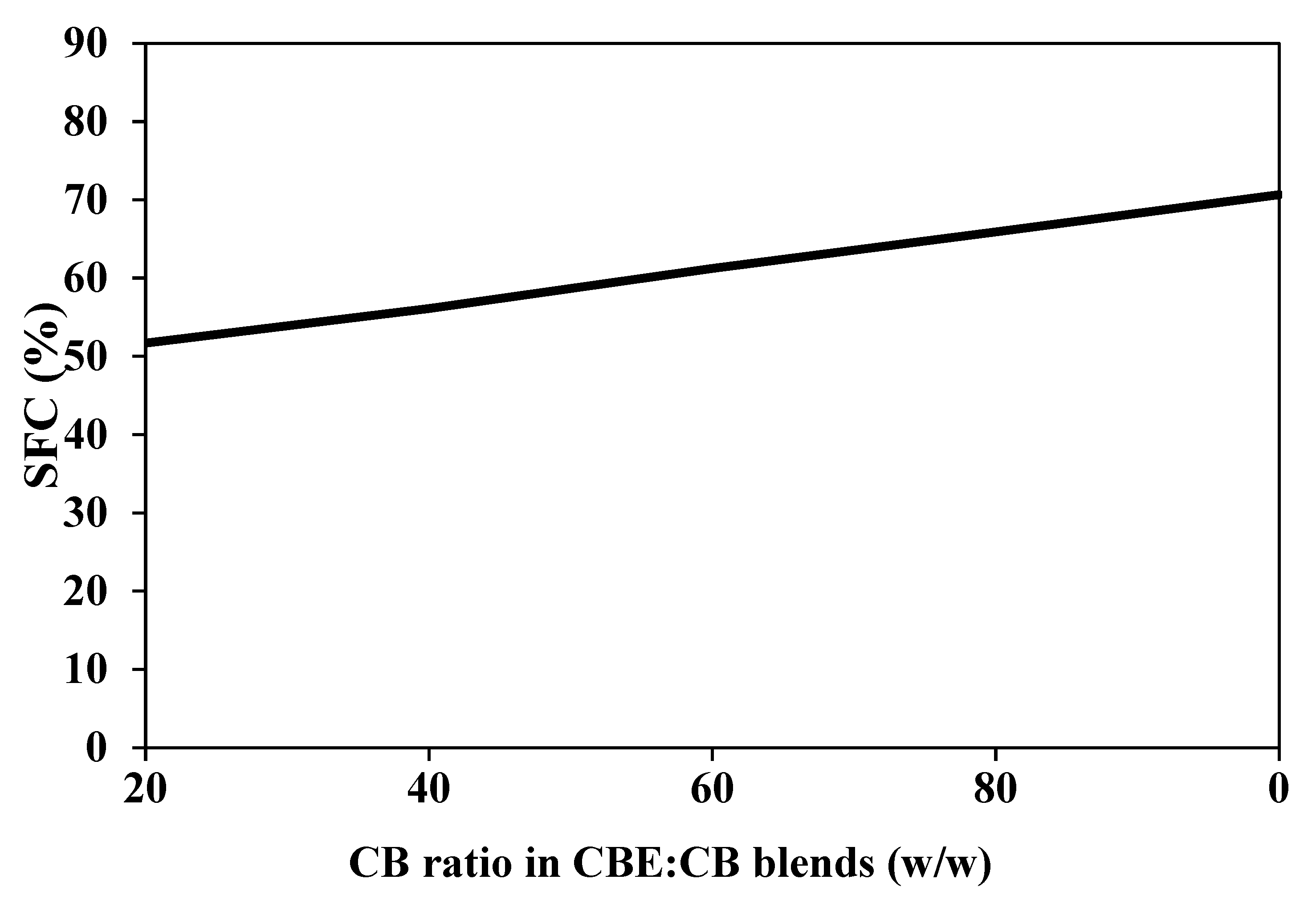
3.4. SFC
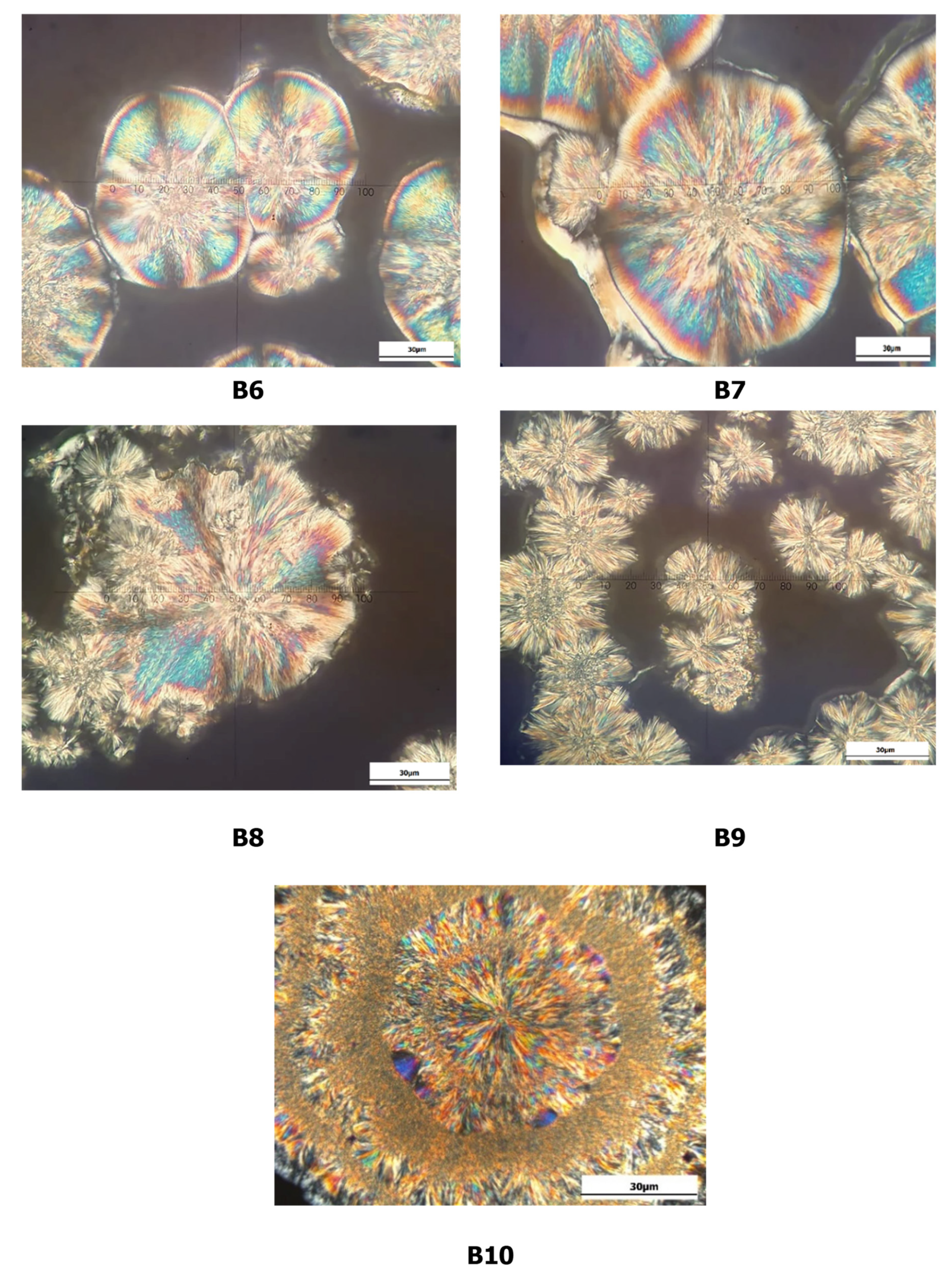
3.5. Crystal Microstructure
3.6. Compatibility between CBE and CB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahari, A.; Akoh, C.C. Texture, rheology and fat bloom study of chocolates made from cocoa butter equivalent synthesised from illipe butter and palm mid-fraction. LWT 2018, 97, 349–354. [Google Scholar] [CrossRef]
- Biswas, N.; Cheow, Y.L.; Tan, C.P.; Siow, L.F. Physicochemical properties of enzymatically produced palm-oil-based cocoa butter substitutes (CBS) with cocoa butter. Eur. J. Lipid Sci. Technol. 2018, 120, 1700205. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Ping, L.L.; Sharifudin, M.S.; Hamadi, M.; Mansoor, A.H.; Lee, J.S.; Noorakmar, B.W.; Amir, H.M.S.; Jinap, S.; Omar, A.K.M.; et al. Thermal properties, triglycerides and crystal morphology of bambangan (Mangifera pajang) kernel fat and palm stearin blends as cocoa butter alternatives. LWT 2019, 107, 64–71. [Google Scholar] [CrossRef]
- Sonwai, S.; Kaphuekngam, P.; Adrian, F. Blending of mango kernel fat and palm oil mid-fraction to obtain cocoa butter equivalent. J. Food Sci. Techol. 2014, 51, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.M.; Mazzanti, G. Rheo-NMR measurements of cocoa butter crystalised under shear flow. Cryst. Growth Des. 2009, 9, 3111–3118. [Google Scholar] [CrossRef]
- Campos, R.; Marangoni, A.G. Molecular composition dynamics and structure of cocoa butter. In Cocoa Butter and Related Compounds; Garti, N., Widlak, N., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 103–150. [Google Scholar]
- Talbot, G. Properties of cocoa butter and vegetable fats. In Becketts Industrial Chocolate Manufacture and Use; Beckett, S., Fowler, M., Ziegler, G., Eds.; Wiley-Blackwell: Chichester, UK, 2017; pp. 153–184. [Google Scholar]
- Beckett, S.T. The Science of Chocolate, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Rina, Y.C.Q.; Elaine, W.Y.P.; Christiani, J.H. Effects of cocoa butter and cocoa butter equivalent in a chocolate confectionery on human blood triglycerides, glusoce and insulin. Foods 2020, 9, 455. [Google Scholar]
- Norazlina, M.R.; Jahurul, M.H.A.; Hasmadi, M.; Mansoor, A.H.; Norliza, J.; Patricia, M.; Ramlah, G.; Noorakmar, A.W.; Lee, J.S.; Fan, H.Y. Trends in blending vegetables fats and oils for cocoa butter alternative application: A review. Trends Food Sci. Technol. 2021, 116, 102–114. [Google Scholar] [CrossRef]
- Huang, Z.; Gua, Z.; Xie, D.; Cao, Z.; Chen, L.; Wang, H.; Jiang, L.; Shen, Q. Rhizomucor meihei lipase-catalysed synthesis of cocoa butter equivalent from palm mid-fraction and stearic acid: Characteristics and feasibility as cocoa butter alternative. Food Chem. 2021, 343, 128407. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Kamaruzzaman, B.Y.; Ghafoor, K.; Omar, A.K.M. Cocoa butter replacers from blends of mango seed fat extracted by supercritical carbon dioxide and palm stearin. Food Res. Int. 2014, 65, 401–406. [Google Scholar] [CrossRef]
- Watanabe, S.; Yoshikawa, S.; Sato, K. Formation and properties of dark chocolate prepared using mixtures of cocoa butter and symmetric/asymmetric stearic-oleic mixed-acid triaclyglicerides: Impact of molecular compounds crystals. Food Chem. 2021, 339, 127808. [Google Scholar] [CrossRef]
- De Clercq, N.; Danthine, S.; Nguyen, M.T.; Gibon, V.; Dewettinck, K. Enzymatic interesterification of palm oil and fractions: Monitoring the degree of interesterification using different methods. J. Am. Oil Chem. Soc. 2012, 89, 219–229. [Google Scholar] [CrossRef]
- Norazura, A.M.H.; Sivaruby, K.; Noor Lida, H.M.D. Blended palm fraction as confectionery fats: A preliminary study. J. Oil Palm Res. 2020, 33, 360–380. [Google Scholar]
- Bootello, M.A.; Hartel, R.W.; Garcés, R.; Martínez-Force, E.; Salas, J.J. Evaluation of high oleic-high stearic sunflower hard stearins for cocoa butter formulation. Food Chem. 2012, 134, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Cheow, Y.L.; Tan, C.P.; Kanagaratnam, S.; Siow, L.F. Cocoa butter substitute (CBS) produced from palm mid-fraction/palm kernel oil/palm stearin for confectionery fillings. J. Am. Oil Chem. Soc. 2017, 94, 235–245. [Google Scholar] [CrossRef]
- Norazlina, M.R.; Jahurul, M.H.A.; Hasmadi, M.; Sharifudin, M.S.; Patricia, M.; Mansoor, A.H.; Lee, J.S. Characteristics of bambangan kernel fat fractions produced by solvent fractionation and their potential industrial applications. J. Food Proces. Preser. 2020, 44, e14446. [Google Scholar] [CrossRef]
- Norazlina, M.R.; Jahurul, M.H.A.; Hasmadi, M.; Sharifudin, M.S.; Patricia, M.; Lee, J.S.; Amir, H.M.S.; Noorakmar, A.W.; Riman, I. Effect of fractionation technique on triglycerides, melting and crystallisation and the polymorphic behavior of bambangan kernel fat as cocoa butter improver. LWT 2020, 129, 109558. [Google Scholar] [CrossRef]
- Tran, P.D.; Van de Walle, D.; Hinneh, M.; Delbaere, C.; De Clercq, N.; Tran, D.N.; Dewettinck, K. Controlling the stability of chocolates through the incorporation of soft and hard StOSt-rich fats. Eur. J. Lipid Sci. Technol. 2015, 117, 1700–1713. [Google Scholar] [CrossRef]
- Department of Agriculture. Fruits Crops by State and Type Malaysia; Department of Agriculture: Kuala Lumpur, Malaysia, 2020; pp. 7–177. [Google Scholar]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oils Chemists’ Society, 5th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2003. [Google Scholar]
- Talhat, A.M.; Lister, V.Y.; Moggridge, G.D.; Rasburn, J.R.; Wilson, D. Development of a single droplet freezing apparatus for studying crystallisation in cocoa butter droplets. J. Food Eng. 2015, 156, 67–83. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oils Chemists’ Society, 6th ed.; AOCS Press: Champaign, IL, USA, 2009. [Google Scholar]
- Jin, J.; Akoh, C.K.; Jin, Q.; Wang, X. Preparation of mango kernel fat stearin-based hard chocolate fats via physical blending and enzymatic interesterification. LWT 2018, 97, 308–316. [Google Scholar] [CrossRef]
- Jin, J.; Zheng, L.; Pembe, W.M.; Zhang, J.; Xie, D.; Wang, X.; Huang, J.; Jin, Q.; Wang, X. Production of sn-1,3-disteroyl-2-oleoyl-glycerol-rich fats from mango kernel fat by selective fractionation using 2-methylpentane based isohexane. Food Chem. 2017, 234, 46–54. [Google Scholar] [CrossRef]
- Jin, J.; Warda, P.; Qi, C.; Sun, C.; Jie, L.; Xie, D.; Huang, J.; Jin, Q.; Wang, X. Mango kernel fat-based chocolate fat with heat resistant triglycerides: Production via blending using mango kernel fat mid-fraction and palm mid-fractions produced in different fractionation paths. Rsc Adv. 2016, 6, 108981–108988. [Google Scholar] [CrossRef]
- Ramli, N.; Said, M.; Mizan, A.B.A.; Tan, Y.N.; Ayob, M.K. Physicochemical properties of blends of palm mid-fraction, palm stearin and olive oil. J. Food Qual. 2014, 37, 57–62. [Google Scholar] [CrossRef]
- Tena, N.; Lobo-Prieto, A.; Aparicio, R.; García-González, D.L. Storage and preservation of fats and oils. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E., Jock, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Esuoso, K.O.; Odetokun, S.M. Proximate chemical composition and possible industrial utilisation of Bliphia sapida seed and seed oils. Riv. Ital. Delle Sostanze Grasse 1995, 72, 311–313. [Google Scholar]
- Hamid, A.; Damit, A.A. Quality of Malaysian cocoa butter during storage. J. Sci. Food Agr. 2004, 84, 513–516. [Google Scholar] [CrossRef]
- Kadivar, S.; De Clercq, N.; Danthine, S.; Dewettinck, K. Crystallisation and polymorphiv behavior of enzymatically produced sunflower oil based cocoa butter equivalents. Eur. J. Lipid Sci. Technol. 2016, 118, 1521–1538. [Google Scholar] [CrossRef]
- Bootello, M.A.; Chong, P.S.; Manez, A.; Garcés, R.; Martínez-Force, E.; Salas, J.J. Characterisation of sunflower stearin-based confectionery fats in bulk and in compound coatings. J. Am. Oil Chem. Soc. 2018, 95, 1139–1150. [Google Scholar] [CrossRef]
- Koyona, T.; Hachiya, I.; Sato, K. Phase behavior of mixed system of SOS and OSO. J. Phys. Chem. 1992, 96, 10514–10520. [Google Scholar] [CrossRef]
- Arishima, T.; Sagi, N.; Mori, H.; Sato, K. Polymorphism of POS. I. Occurrence and polymorphic transformation. J. Am. Oil Chem. 1991, 68, 710–715. [Google Scholar] [CrossRef]
- Ribeiro, A.P.B.; Claro da Silva, R.; Gioielli, L.A.; De Almeida Gonçalves, M.I.; Gimaldi, R.; Gonçalves, L.A.G.; Kieckbuch, T.G. Physico-chemical properties of Brazilian cocoa butter and industrial blends: Part I: Chemical composition solid fat content and consistency. Grasas Y Aceites 2012, 63, 79–88. [Google Scholar] [CrossRef]
- Talbot, G. Fats for confectionery coatings and fillings. In Science and Technology of Enrobed and Filled Chocolate, Confectionery and Bakery Products; Woodhead Publishing: Cambridge, UK, 2009; pp. 53–79. [Google Scholar]
- Talbot, G. Vegetable fats. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S., Ed.; Willey-Blackwell: York, UK, 2009; pp. 415–433. [Google Scholar]
- Çiftçi, O.N.; Fadiloğlu, S.; Göğüş, F. Conversion of milk fat and milk fat fractions with confectionery fats. J. Diary Sci. 2009, 84, 332–344. [Google Scholar]
- Solís-Fuentes, J.A.; del Rosario, M.; Hernandez, M.; del Carmen, M.; Durán-de-Bazúa, C. Determination of the predominant polymorphic form of mango (Mangifera indica) almond fat by differential scanning calorimetry and X-ray diffraction. Eur. J. Lipid Sci. Technol. 2005, 107, 395–401. [Google Scholar] [CrossRef]
- Biswas, N.; Cheow, Y.; Tan, C.; Siow, L. Blending of palm oil mid-fraction refined bleached deodorised palm kernel oil or palm stearin for coco abutter alternative. J. Am. Oil Chem. Soc. 2016, 93, 1415–1427. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef]
- Narine, S.S.; Marangoni, A.G. The difference between cocoa butter and salatrim lies in the microstructure of the fat crystal network. J. Am. Oil Chem. Soc. 1999, 76, 7–13. [Google Scholar] [CrossRef]
- Devi, A.; Khatkar, B.S. Physicochemical, rheological and functional properties of fats and oils in relation to cookie quality: A review. J. Food Sci. Technol. 2016, 53, 3633–3641. [Google Scholar] [CrossRef]
- Marangoni, A.G.; McGauley, S.E. Relationship between crystallisation behaviour and structure in cocoa butter. Cryst. Growth Des. 2003, 3, 95–108. [Google Scholar] [CrossRef]
- Asep, E.; Jinap, S.; Tan, T.J.; Russly, A.; Haracharan, S.; Nazimah, S. The effects of particle size, fermentation and roasting of cocoa nibs on supercritical fluid extraction of cocoa butter. J. Food Eng. 2008, 85, 450–458. [Google Scholar] [CrossRef]
- Lieb, V.M.; Schuster, L.K.; Kronmuller, A.; Schmarr, H.G.; Carle, R.; Steingass, C.B. Fatty acids, triglycerides, and thermal behavior of various mango (Mangifera Indica) kernel fats. Food Res. Int. 2018, 116, 527–537. [Google Scholar] [CrossRef]
- Gunstone, F.D. Vegetable Oils in Food Technology Composition, Properties and Use; Willy-Blackwell: Hoboken, NJ, USA; CRC Press: Boca Raton, FL, USA, 2011; pp. 291–343. [Google Scholar]
- Muraslin, M.; Terniza, Y. Cocoa butter substitute production by mixing the fraction of palm kernel stearin with tengkawang fat. Adv. Eng. Res. 2021, 205, 160–163. [Google Scholar]
- Calliuw, G.; Foubert, I.; De Grect, W.; Dijckmans, P.; Kellens, M.; Dewettinck, K. Production of cocoa butter substitute via two-stage fractionation of palm kernel oil. J. Am. Oil Chem. Soc. 2005, 82, 783–789. [Google Scholar] [CrossRef]

| Blend (100 g) | BKF-SS (%) | POMF (%) |
|---|---|---|
| B1 | 90 | 10 |
| B2 | 85 | 15 |
| B3 | 80 | 20 |
| B4 | 75 | 25 |
| B5 | 70 | 30 |
| Blend (100 g) | B5 (%) | CB (%) |
|---|---|---|
| B6 | 80 | 20 |
| B7 | 60 | 40 |
| B8 | 40 | 60 |
| B9 | 20 | 80 |
| B10 | 0 | 100 |
| Physicochemical Properties | B1 | B2 | B3 | B4 | B5 | POMF | CB |
|---|---|---|---|---|---|---|---|
| Iodine value (g iodine/g) | 34.39 ± 0.23 a | 35.20 ± 0.57 b | 35.73 ± 0.32 b,c | 36.08 ± 0.01 c,d | 36.82 ± 0.34 d | 45.56 ± 0.23 e | 33.69 ± 0.11 a |
| Slip melting point (°C) | 34.5 ± 0.50 e,f | 34.25 ± 0.25 e | 33.67 ± 0.28 b,c | 33.75 ± 0.25 c,d | 32.67 ± 0.28 a | 33.50 ± 0.34 c,d | 33.83 ± 0.28 c,d |
| Acid value (mg KOH/g) | 2.24 ± 0.09 d | 2.90 ± 0.15 e | 1.49 ± 0.16 b | 1.31 ± 0.16 a | 1.68 ± 0.11 b,c | 2.33 ± 0.16 c | 2.81 ± 0.09 e |
| Free fatty acid: oleic (%) | 1.13 ± 0.17 d | 1.46 ± 0.08 e | 0.76 ± 0.08 b,c | 0.62 ± 0.08 a,b | 0.85 ± 0.00 c | 1.17 ± 0.07 d | 1.41 ± 0.07 e |
| Free fatty acid: palmitic (%) | 1.02 ± 0.11 d | 1.32 ± 0.08 f | 0.68 ± 0.08 b,c | 0.55 ± 0.08 a,b | 0.77 ± 0.16 c | 1.11 ± 0.08 d,e | 1.28 ± 0.11 e |
| CB | BKF | BKF-SS | POMF | B1 | B2 | B3 | B4 | B5 | |
|---|---|---|---|---|---|---|---|---|---|
| Fatty acid content (%) | |||||||||
| C16 | 25.19 ± 0.04 h | 8.40 ± 0.01 b | 7.53 ± 0.28 a | 46.97 ± 0.08 i | 10.59 ± 0.01 c | 12.49 ± 0.03 d | 14.52 ± 0.34 e | 17.78 ± 0.07 f | 18.74 ± 0.08 g |
| C18 | 36.40 ± 0.57 c | 29.16 ± 0.01 b | 45.86 ± 0.01 h | 4.77 ± 0.07 a | 47.89 ± 0.01 i | 45.39 ± 0.02 g | 43.21 ± 0.03 f | 40.26 ± 0.21 e | 38.26 ± 0.01 d |
| C18:1 | 32.47 ± 0.01 a | 48.25 ± 0.21 h | 35.93 ± 0.03 f | 38.79 ± 0.01 g | 32.94 ± 0.07 a,b | 33.36 ± 0.21 c | 33.67 ± 0.47 d | 33.56 ± 0.02 c,d | 34.05 ± 0.02 e |
| C18:2 | 2.91 ± 0.08 a | 9.24 ± 0.23 i | 4.25 ± 0.07 f,g | 6.83 ± 0.01 h | 3.29 ± 0.37 b | 3.52 ± 0.51 c | 3.72 ± 0.09 d | 3.96 ± 0.07 e | 4.14 ± 0.21 f |
| C18:3 | 0.19 ± 0.01 e | 0.44 ± 0.01 g | 0.21 ± 0.05 f | 0.04 ± 0.02 a | 0.11 ± 0.21 d | 0.11 ± 0.67 d | 0.08 ± 0.09 b | 0.10 ± 0.10 c | 0.10 ± 0.07 c |
| C20 | 1.09 ± 0.19 b | 1.75 ± 0.01 c | 2.20 ± 0.08 h | 0.38 ± 0.08 a | 2.29 ± 0.01 i | 2.11 ± 0.09 g | 2.09 ± 0.21 f | 1.96 ± 0.37 e | 1.87 ± 0.01 d |
| C22 | 0.20 ± 0.00 b | 0.33 ± 0.01 e | 0.37 ± 0.11 f | 0.07 ± 0.21 a | 0.37 ± 0.09 f | 0.33 ± 0.10 e | 0.60 ± 0.07 g | 0.32 ± 0.01 d | 0.28 ± 0.01 c |
| C24 | 0.13 ± 0.11 b | 0.69 ± 0.09 h | 0.67 ± 0.01 i | 0.11 ± 0.57 a | 0.61 ± 0.09 g | 0.54 ± 0.21 e | 0.55 ± 0.00 f | 0.52 ± 0.13 d | 0.45 ± 0.34 c |
| TAGs composition (%) | |||||||||
| OLO | - | 1.38 ± 0.24 | 0.32 ± 0.08 | 2.36 ± 0.02 | - | 1.37 ± 0.01 | 3.28 ± 0.11 | 3.98 ± 0.19 | 1.33 ± 0.01 |
| POL | 1.26 ± 0.07 c | 1.03 ± 0.01 b | 0.26 ± 0.01 a | 5.86 ± 0.11 i | 2.88 ± 0.34 d | 4.09 ± 0.09 g | 3.77 ± 0.57 e | 4.02 ± 0.02 f | 4.48 ± 0.09 h |
| PLP | 2.43 ± 0.43 c | 0.27 ± 0.09 b | 0.07 ± 0.13 a | 7.41 ± 0.07 i | 2.86 ± 0.01 d | 3.75 ± 0.01 g | 3.60 ± 0.21 f | 3.57 ± 0.37 e | 3.78 ± 0.67 h |
| OOO | 3.60 ± 0.01 h | 6.57 ± 0.01 i | 1.26 ± 0.13 a | 3.25 ± 0.09 e | 2.75 ± 0.43 c | 2.68 ± 0.17 b | 3.44 ± 0.19 g | 2.76 ± 0.01 d | 3.39 ± 0.00 f |
| POO | 3.42 ± 0.00 c | 2.68 ± 0.13 b | 1.16 ± 0.09 a | 16.36 ± 0.11 i | 4.08 ± 0.37 d | 4.61 ± 0.07 e | 5.02 ± 0.01 f | 5.14 ± 0.01 g | 6.53 ± 0.13 h |
| POP | 14.25 ± 0.13 k | 3.83 ± 0.09 b | 0.56 ± 0.21 a | 44.91 ± 0.07 o | 6.41 ± 0.00 d | 8.69 ± 0.01 g | 9.51 ± 0.01 h | 12.24 ± 0.34 k | 15.20 ± 0.08 m |
| SOO | 4.70 ± 0.11 b | 22.10 ± 0.13 i | 11.82 ± 0.09 h | 1.00 ± 0.01 a | 5.68 ± 0.21 e | 6.45 ± 0.09 f | 5.63 ± 0.16 d | 5.47 ± 0.29 c | 6.61 ± 0.08 g |
| POS | 33.36 ± 0.23 h | 10.03 ± 0.46 a | 15.40 ± 0.08 g | 10.03 ± 0.09 a | 11.30 ± 0.19 b | 11.78 ± 0.21 e | 11.72 ± 0.67 d | 10.59 ± 0.63 c | 11.92 ± 0.12 f |
| SOS | 25.65 ± 0.11 | 41.18 ± 0.09 | 63.87 ± 0.23 | - | 35.33 ± 0.58 | 35.85 ± 0.19 | 31.02 ± 0.09 | 30.87 ± 0.09 | 29.74 ± 0.01 |
| SSS | 1.62 ± 0.01 | 3.40 ± 0.11 | 2.38 ± 0.78 | - | 7.10 ± 0.32 | 6.48 ± 0.33 | 3.49 ± 0.21 | 2.07 ± 0.16 | 3.13 ± 0.09 |
| Others | 13.12 ± 0.01 e | 7.34 ± 0.17 b | 2.90 ± 0.17 a | 8.82 ± 0.68 c | 21.61 ± 0.30 i | 14.24 ± 0.08 f | 19.53 ± 0.13 h | 19.30 ± 0.20 g | 13.89 ± 0.07 d |
| Sample | Melting Properties | |||
|---|---|---|---|---|
| Onset Temperature (°C) | Offset Temperature (°C) | Max Temperature (°C) | Enthalpy (J/g) | |
| BKF | 11.42 ± 0.13 b | 29.78 ± 0.01 a | 24.20 ± 0.00 b | 51.94 ± 0.17 f |
| BKF-SS | 22.19 ± 0.02 e | 37.82 ± 0.01 i | 31.19 ± 0.01 f | 66.14 ± 0.16 i |
| POMF | 11.34 ± 0.07 a | 30.92 ± 0.23 b | 22.67 ± 0.03 a | 53.42 ± 0.00 g |
| CB | 20.99 ± 0.58 c | 37.09 ± 0.08 g | 28.17 ± 0.11 c | 56.35 ± 0.12 h |
| B1 | 24.51 ± 0.32 g | 37.22 ± 0.11 h | 30.32 ± 0.01 e | 30.61 ± 0.12 b |
| B2 | 23.56 ± 0.01 f | 36.87 ± 0.09 f | 29.95 ± 0.07 d | 33.54 ± 0.10 c |
| B3 | 25.46 ± 0.11 h | 35.81 ± 0.07 d | 32.62 ± 0.09 h | 34.79 ± 0.08 d |
| B4 | 21.60 ± 0.43 d | 36.31 ± 0.67 e | 32.62 ± 0.07 h | 45.39 ± 0.09 e |
| B5 | 26.71 ± 0.08 i | 35.26 ± 0.19 c | 32.11 ± 0.07 g | 24.30 ± 0.12 a |
| B6 | B7 | B8 | B9 | B10 | ||
|---|---|---|---|---|---|---|
| Fatty acid (%) | C16 | 21.42 ± 0.01 f | 22.11 ± 0.23 h | 23.20 ± 0.13 j | 24.31 ± 0.01 m | 25.20 ± 0.11 o |
| C18 | 32.92 ± 0.23 a | 34.02 ± 0.09 c,d | 34.94 ± 0.68 e | 36.03 ± 0.21 f,g | 36.40 ± 0.10 g | |
| C18:1 | 36.80 ± 0.09 o | 35.88 ± 0.09 l | 34.75 ± 0.43 j | 33.88 ± 0.23 f | 32.47 ± 0.57 b | |
| C18:2 | 4.63 ± 0.01 p | 4.20 ± 0.00 k | 3.79 ± 0.23 f | 3.38 ± 0.47 b | 2.91 ± 0.01 a | |
| C18:3 | 0.14 ± 0.07 c | 0.16 ± 0.11 d | 0.17 ± 0.01 e | 0.18 ± 0.01 f | 0.18 ± 0.07 g | |
| C20 | 1.60 ± 0.11 i | 1.49 ± 0.57 g | 1.35 ± 0.11 e | 1.21 ± 0.07 b | 1.09 ± 0.27 a | |
| C22 | 0.28 ± 0.13 h | 0.26 ± 0.03 f | 0.24 ± 0.09 e | 0.22 ± 0.01 c | 0.20 ± 0.11 b | |
| C24 | 0.48 ± 0.02 j | 0.41 ± 0.08 h | 0.32 ± 0.11 e | 0.23 ± 0.01 b | 0.13 ± 0.01 a | |
| TAG (%) | OLO | 2.80 ± 0.21 | 1.09 ± 0.01 | 0.83 ± 0.11 | - | - |
| POL | 4.91 ± 0.68 l | 2.34 ± 0.01 g | 1.71 ± 0.67 c | 1.24± 0.10 a | 1.26 ± 0.23 b | |
| PLP | 4.26 ± 0.23 j | 2.51 ± 0.67 e | 2.81 ± 0.56 g | 2.44± 0.02 d | 2.43 ± 0.31 d | |
| OOO | 3.90 ± 0.09 l | 2.66 ± 0.43 g | 1.92 ± 0.13 b | 1.61 ± 0.02 a | 3.60 ± 0.11 k | |
| POO | 6.85 ± 0.09 m | 5.52 ± 0.12 l | 4.51 ± 0.07 i | 3.89 ± 0.13 e | 3.42 ± 0.01 c | |
| POP | 13.58 ± 0.11 i | 14.75± 0.03 l | 14.00 ± 0.03 k | 13.73 ± 0.03 j | 14.25 ± 0.08 k | |
| SOO | 10.36 ± 0.43 l | 7.42 ± 0.01 h | 5.90 ± 0.01 d | 5.04 ± 0.43 b | 4.70 ± 0.10 a | |
| POS | 16.11 ± 0.11 c | 21.09 ± 0.01 f | 25.00 ± 0.10 k | 29.58 ± 0.03 l | 33.36 ± 0.11 l | |
| SOS | 24.96 ± 0.02 c | 30.17 ± 0.11 m | 29.94 ± 0.02 i | 26.89 ± 0.12 e | 25.65 ± 0.34 d | |
| SSS | 2.29 ± 0.01 c | 3.46 ± 0.23 h | 1.19 ± 0.02 a | 3.31 ± 0.23 e | 1.62 ± 0.03 b | |
| Others | 10.00± 0.13 b | 8.99 ± 0.43 a | 13.19 ± 0.01 h | 12.72 ± 0.56 e | 13.12 ± 0.06 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad Ridwan, N.; Mamat, H.; Haque Akanda, M.J. Physical Blending of Fractionated Bambangan Kernel Fat Stearin and Palm Oil Mid-Fraction to Formulate Cocoa Butter Equivalent. Foods 2023, 12, 1744. https://doi.org/10.3390/foods12091744
Mohammad Ridwan N, Mamat H, Haque Akanda MJ. Physical Blending of Fractionated Bambangan Kernel Fat Stearin and Palm Oil Mid-Fraction to Formulate Cocoa Butter Equivalent. Foods. 2023; 12(9):1744. https://doi.org/10.3390/foods12091744
Chicago/Turabian StyleMohammad Ridwan, Norazlina, Hasmadi Mamat, and Md Jahurul Haque Akanda. 2023. "Physical Blending of Fractionated Bambangan Kernel Fat Stearin and Palm Oil Mid-Fraction to Formulate Cocoa Butter Equivalent" Foods 12, no. 9: 1744. https://doi.org/10.3390/foods12091744
APA StyleMohammad Ridwan, N., Mamat, H., & Haque Akanda, M. J. (2023). Physical Blending of Fractionated Bambangan Kernel Fat Stearin and Palm Oil Mid-Fraction to Formulate Cocoa Butter Equivalent. Foods, 12(9), 1744. https://doi.org/10.3390/foods12091744






