Impedimetric Polyaniline-Based Aptasensor for Aflatoxin B1 Determination in Agricultural Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus and Electrodes
2.2. Chemicals
2.3. Synthesis of Polyaniline
2.4. Preparation of Aptamer and AFB1 Analyte Solutions
2.5. Fabrication of Aptasensor
2.6. Electrochemical Measurements
2.7. Validation of PAni-Based Impedimetric AFB1 Aptasensor
3. Results and Discussion
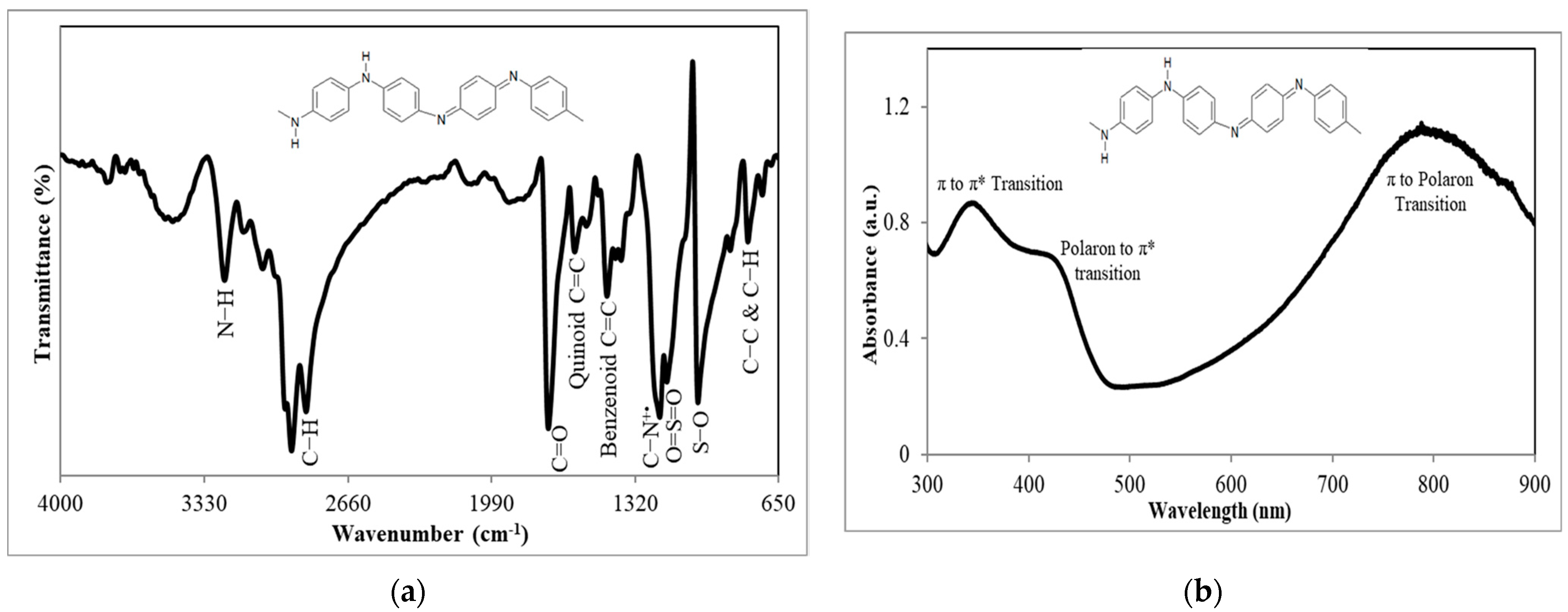
3.1. FTIR and UV–Vis Spectrophotometric Characterizations of PAni Substrate
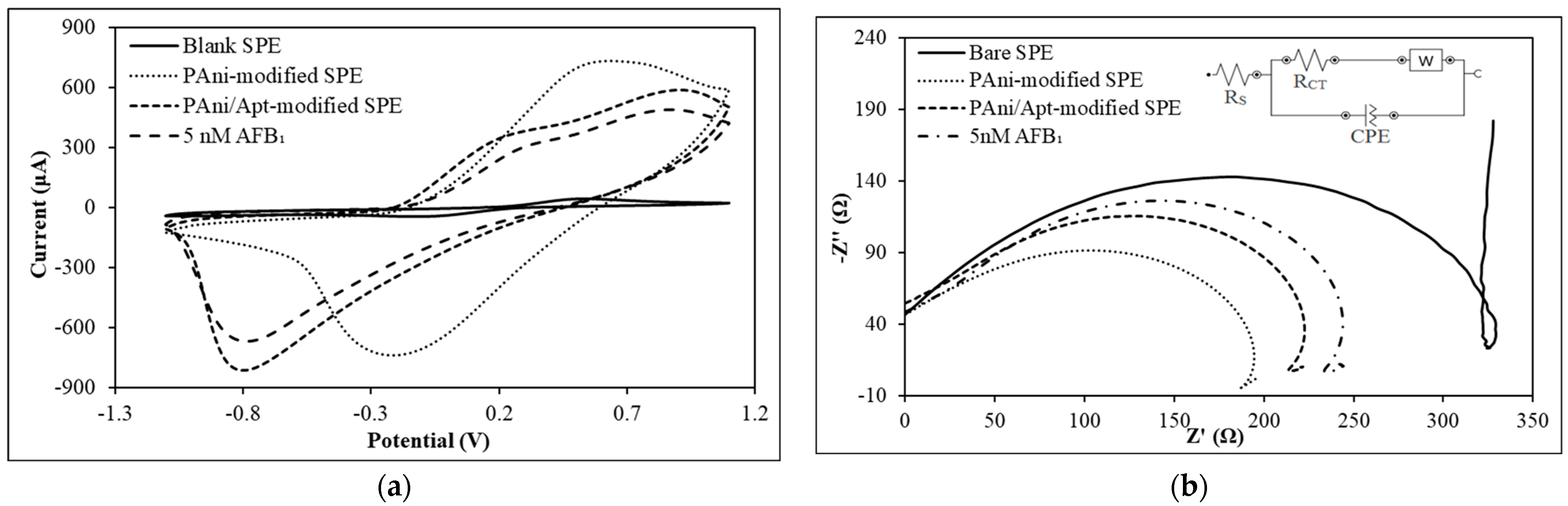
3.2. Electrochemical Characterization of PAni-Film-Modified Electrode
3.3. Electrochemical Interrogation of PAni-Film-Based AFB1 Aptasensor
3.4. Optimization of Electrochemical PAni-Modified Aptasensor for Impedance Detection of AFB1
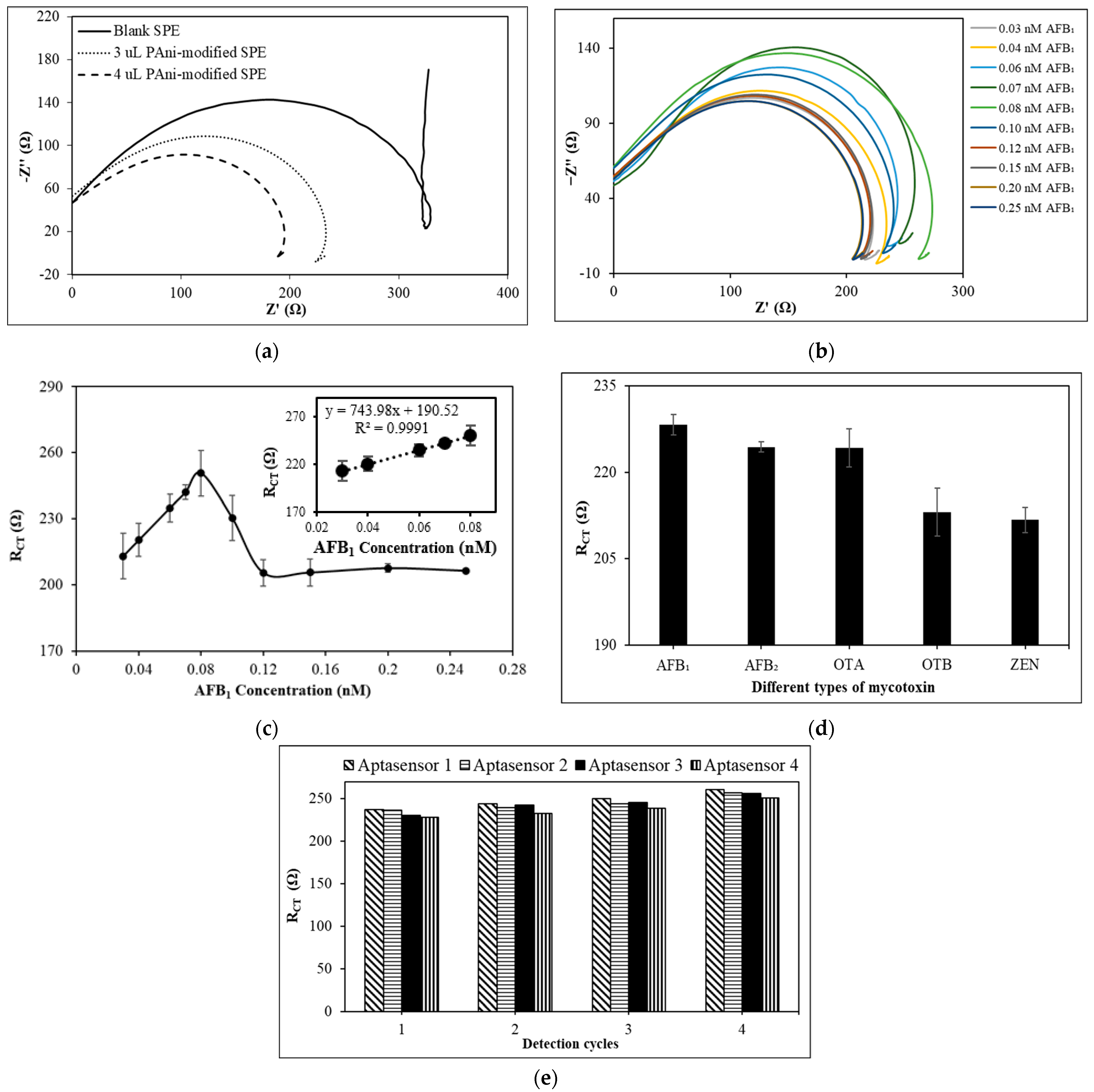
3.4.1. Optimizing the Volume of PAni on the SPE Electrode
3.4.2. Sensitivity of the PAni-Film-Based Impedimetric Aptasensor towards AFB1 Detection
3.4.3. Response Time of PAni-Film-Based AFB1 Aptasensor
3.4.4. Selectivity of the PAni-Film-Based Electrochemical Aptasensor
3.4.5. Long-Term Stability of the PAni-Film-Based Aptasensor
3.4.6. Repeatability of the PAni-Film-Based Impedimetric Aptasensor
3.5. Comparison of the Analytical Performance of the Developed PAni-Film-Based Impedimetric Aptasensor with Other Electrochemical Sensors/Biosensors for AFB1 Detection
3.6. Detection of AFB1 in Real Samples with the PAni-Film-Based Aptasensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabet, F.S.; Hosseini, M.; Khabbaz, H.; Dadmehr, M.; Ganjali, M.R. FRET-based aptamer biosensor for selective and sensitive detection. Food Chem. 2017, 220, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Bai, X.; Liu, Y.; Yang, L.; Liao, X. Simultaneous determination of trace aflatoxin B1 and ochratoxin A by aptamer-based microchip capillary electrophoresis in food samples. J. Chromatogr. A 2018, 1569, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Sarreshtehdar Emrani, A.; Zolfaghari, R.; Taghdisi, S.M. A new amplified π-shape electrochemical aptasensor for ultrasensitive detection of aflatoxin B1. Biosens. Bioelectron. 2017, 94, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Chen, J.H.; Cao, H.; Yao, D.S.; Liu, D.L. Amperometric biosensor for aflatoxin B1 based on aflatoxin-oxidase immobilized on multiwalled carbon nanotubes. Food Control 2011, 22, 43–49. [Google Scholar] [CrossRef]
- Shim, W.; Kim, M.J.; Mun, H.; Kim, M. An aptemer-based dipstick assay for the rapid and simple detection of aflatoxin B1. Biosens. Bioelectrocn. 2014, 62, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Spinella, K.; Poturnayova, A.; Snejdarkova, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Ross Macdonald, J.; Johnson, W.B. Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Prodromidis, M.I. Impedimetric Biosensors and Immunosensors. Pak. J. Anal. Environ. Chem. 2007, 8, 69–71. Available online: http://www.pjaec.pk/index.php/pjaec/article/view/115/96 (accessed on 25 July 2019).
- Pinkova Gajdosova, V.; Lorencova, L.; Blsakova, A.; Kasak, P.; Bertok, T.; Tkac, J. Challenges for impedimetric affinity sensors targeting protein detection. Curr. Opin. Electrochem. 2021, 28, 100717. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Seraphim, P.M.; Teixeira, M.F.S. Methylated DNA impedimetric immunosensor based on azo-polymer-AuNPs dots and 5-methylcytosine antibody using dissolved oxygen as a redox probe. Electrochem. Commun. 2022, 136, 107242. [Google Scholar] [CrossRef]
- Wen, X.H.; Zhao, X.F.; Peng, B.F.; Yuan, K.P.; Li, X.X.; Zhu, L.Y.; Lu, H.L. Facile preparation of an electrochemical aptasensor based on Au NPs/graphene sponge for detection of homocysteine. Appl. Surf. Sci. 2021, 556, 149735. [Google Scholar] [CrossRef]
- Lu, M.; Cao, C.Y.; Wang, F.; Liu, G.C. A polyethyleneimine reduced graphene oxide/gold nanocubes based electrochemical aptasensor for chloramphenicol detection using single-stranded DNA-binding protein. Mater. Des. 2021, 199, 109409. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, Y.Y.; Wang, F.Z.; Chi, H.; Zhao, G.H.; Zhang, S.; Li, T.D.; Wei, Q. Electrochemical aptasensor based on gold modified thiol graphene as sensing platform and gold-palladium modified zirconium metal-organic frameworks nanozyme as signal enhancer for ultrasensitive detection of mercury ions. J. Colloid Interface Sci. 2022, 606, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.P.; Singh, P.; Azad, U.P.; Prakasj, R. Impedimetric immunosensor for the NS1 dengue biomarker based on the gold nanorod decorated graphitic carbon nitride modified electrode. Electrochim. Acta 2022, 411, 140069. [Google Scholar] [CrossRef]
- Tertis, M.; Ciui, B.; Suciu, M.; Sandulescu, R.; Cristea, C. Label-free electrochemical aptasensor based on gold and polypyrrole nanoparticles for interleukin 6 detection. Electrochim. Acta 2017, 258, 1208–1218. [Google Scholar] [CrossRef]
- Mejri-Omrani, N.; Miodek, A.; Zribi, B.; Marrakchi, M.; Hamdi, M.; Marty, J.L.; Korri-Youssoufi, H. Direct detection of OTA by impedimetric aptasensor based on modified polypyrrole-dendrimers. Anal. Chim. Acta 2016, 920, 37–46. [Google Scholar] [CrossRef]
- Centane, S.; Nyokong, T. Impedimetric aptasensor for HER2 biomarker using graphene quantum dots, polypyrrole and cobalt phthalocyanine modified electrodes. Sens. Bio-Sens. Res. 2021, 34, 100467. [Google Scholar] [CrossRef]
- Gong, Q.; Han, H.; Yang, H.; Zhang, M.; Sun, X.; Liang, Y.; Liu, Z.; Zhang, W.; Qiao, J. Sensitive electrochemical DNA sensor for the detection of HIV based on a polyaniline/graphene nanocomposite. J. Meteriomics 2019, 5, 313–319. [Google Scholar] [CrossRef]
- Yoon, S.B.; Yoon, E.H.; Kim, K.B. Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J. Power Sources 2011, 196, 10791–10797. [Google Scholar] [CrossRef]
- Kuang, J.; Cao, Q.; Wang, X.; Jing, B.; Wang, Q.; Zhou, L. Influence of the reaction temperature on polyaniline morphology and evaluation of their performance as supercapacitor electrode. J. Appl. Polym. Sci. 2013, 130, 3753–3758. [Google Scholar] [CrossRef]
- Arora, R.; Dahshan, A.; Sharma, P. A study of ac conductivity of nano TiO2–polyaniline based film. Mater. Today Proc. 2020, 26, 341–343. [Google Scholar] [CrossRef]
- Sidheekha, M.P.; Nufaira, K.; Shabeeba, A.K.; Rajan, L.; Ismail, Y.A. Characterization of polyanilines synthesized at different pH for electrochemical sensing and supercapacitor applications. Mater. Today Proc. 2022, 51, 2286–2292. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, Z.; Li, B.; Liu, Z.; Jia, L.; Zuo, L.; Chen, L.; Zhu, Z.; Shan, G.; Luo, S. A direct determination of AFBs in vinegar by aptamer-based surface plasmon resonance biosensor. Toxicon 2018, 146, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Karaoglan, N.; Bindal, C. Synthesis and optical characterization of benzene sulfonic acid doped polyaniline. Eng. Sci. Technol. Int. J. 2018, 21, 1152–1158. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Nagaraju, D.H.; Geetha, B.R.; Werasak, S.; Ramakrishnappa, T.; Avinash, B.S. Hydrogels of polyaniline with graphene oxide for highly sensitive electrochemical determination of lead ions. Anal. Chim. Acta 2017, 990, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Munyati, M.O.; Mbozi, A.; Siamwiza, M.N.; Diale, M.M. Polyaniline nanoparticles for the selective recognition of aldrin: Synthesis, characterization, and adsorption properties. Synth. Met. 2017, 233, 79–85. [Google Scholar] [CrossRef]
- Mustapa, R.; Abu Mansor, Z.I.; Sambasevam, K.P. Fabrication of polyaniline based chemical sensor for ammonia gas detection. J. Phys. Sci. 2018, 29, 9–16. [Google Scholar] [CrossRef]
- Shao, W.W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The effect of a small amount of water on the structure and electrochemical properties of solid-state synthesized polyaniline. Materials 2012, 5, 1811–1825. [Google Scholar] [CrossRef]
- Irma Zulayka, M.A.; Sulaiman, W.H.; Gan, S.N.; Phang, S.W. Polyaniline (PAni) optical sensor in chloroform detection. Sens. Actuators B Chem. 2018, 261, 97–105. [Google Scholar] [CrossRef]
- Silva, D.B.R.S.; Júnior, L.P.C.; Aguiar, M.F.; Melo, C.P.; Alves, K.G.B. Preparation and characterization of nanofibers of polyvinyl alcohol/polyaniline-montmorillonite clay. J. Mol. Liq. 2018, 272, 1070–1076. [Google Scholar] [CrossRef]
- Steven, J.F.B. Basic Theory and Phenomenology of Polarons. Department of Physics, University of California at Berkeley, Berkeley, CA 94720. 2008. Available online: https://sjbyrnes.com/FinalPaper--Polarons.pdf (accessed on 25 July 2019).
- Dennany, L.; Innis, P.C.; McGovern, S.T.; Wallace, G.G.; Robert, J.F. Electronic interactions within composites of polyanilines formed under acidic and alkaline conditions. Conductivity, ESR, Raman, UV-vis and fluorescence studies. Phys. Chem. Chem. Phys. 2011, 13, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- Kavirajaa, P.S.; Sharifah, M.; Phang, S.W. Enhancement of polyaniline properties by different polymerization temperatures in hydrazine detection. J. Appl. Polym. Sci. 2015, 132, 41746. [Google Scholar] [CrossRef]
- Martins, J.C.; Neto, J.C.M.; Passos, R.R.; Pocrifka, L.A. Electrochemical behavior of polyaniline: A study by electrochemical impedance spectroscopy (EIS) in low-frequency. Solid State Ion. 2020, 346, 115198. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Low, S.B.; Lee, Y.H.; Bohari, M.Y.; Musa, A. Photocurable ferrocene-containing poly(2-hydroxyl ethyl methacrylate) films for mediated amperometric glucose biosensor. Thin Solid Film. 2005, 477, 104–110. [Google Scholar] [CrossRef]
- Low, S.B.; Lee, Y.H.; Bohari, M.Y.; Musa, A. The electrochemical behaviour of ferrocene in a photocurable poly(methyl methacrylate-co-2-hydroxylethyl methacrylate) film for a glucose biosensor. Bioelectrochemistry 2005, 65, 157–162. [Google Scholar] [CrossRef]
- García-Gabaldón, M.; Carrillo-Abad, J.; Ortega-Navarro, E.; Pérez-Herranz, V. Electrochemical Study of a Simulated Spent Pickling Solution. Int. J. Electrochem. Sci. 2011, 6, 506–519. Available online: http://www.electrochemsci.org/papers/vol6/6020506.pdf (accessed on 25 July 2019).
- Elugoke, S.E.; Fayemi, O.E.; Adekunle, A.S.; Mamba, B.B.; Nkambule, T.T.I.; Ebenso, E.E. Electrochemical sensor for the detection of dopamine using carbon quantum dots/copper oxide nanocomposite modified electrode. FlatChem 2022, 33, 100372. [Google Scholar] [CrossRef]
- Akkapol, S.N.; Philip, D.H.; Claire, E.S.; Andrew, J.M. An Exonuclease I-Assisted Silver-Metallized Electrochemical Aptasensor for Ochratoxin A Detection. ACS Sens. 2019, 4, 1560–1568. [Google Scholar] [CrossRef]
- Zainudin, N.; Hairul, A.R.M.; Yusoff, M.M.; Tan, L.L.; Chong, K.F. Impedimetric graphene-based biosensor for the detection of Escherichia coli DNA. Anal. Methods 2014, 6, 7935–7941. [Google Scholar] [CrossRef]
- Scott, K. Electrochemical Principles and Characterization of Bioelectrochemical Systems. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing: Cambridge, UK, 2016; pp. 29–66. [Google Scholar] [CrossRef]
- Scotto, J.; Florit, M.I.; Posadas, D. About the species formed during the electrochemical half oxidation of polyaniline: Polaron-bipolaron equilibrium. Electrochim. Acta 2018, 268, 187–194. [Google Scholar] [CrossRef]
- Mikhailenko, S.D.; Rodrigues, M.A.S.; Celso, F.; Muller, F.; Ferreira, C.A.; Kaliaguine, S. Impedance analysis of polyaniline in comparison with some conventional solid electrolytes. J. Phys. Chem. B 2018, 122, 7764–7774. [Google Scholar] [CrossRef] [PubMed]
- Abalyaeva, V.V.; Efimov, O.N. Effect of the doping anion replacement on the polyaniline electrochemical behavior. Russ. J. Electrochem. 2019, 55, 953–961. [Google Scholar] [CrossRef]
- Wu, L.; Ding, F.; Yin, W.M.; Ma, J.; Wang, B.R.; Nie, A.X.; Han, H.Y. From electrochemistry to electroluminescence: Development and application in ratiometric aptasensor for aflatoxin B1. Anal. Chem. 2017, 89, 7578–7585. [Google Scholar] [CrossRef] [PubMed]
- Erol, A.; Ozge, K. Determination of calcium in milk and water samples by using catalase enzyme electrode. Food Chem. 2009, 115, 347–351. [Google Scholar] [CrossRef]
- Erol, A.; Gizem, O.; Ezgi, C.; Gorkem, O. A new polyaniline-catalase-glutaraldehyde modified biosensor for hydrogen peroxide detection. Prep. Biochem. Biotechnol. 2016, 47, 1532–2297. [Google Scholar] [CrossRef]
- Kellenberger, A.; Ambros, D.; Plesu, N. Scan Rate Dependent Morphology of Polyaniline Films Electrochemically Deposited on Nickel. Int. J. Electrochem. Sci. 2014, 9, 6821–6833. Available online: http://www.electrochemsci.org/papers/vol9/91206821.pdf (accessed on 25 July 2019).
- Nguyen, B.H.; Tran, L.D.; Do, Q.P.; Nguyen, H.L.; Tran, N.H.; Nguyen, P.X. Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater. Sci. Eng. C 2013, 33, 2229–2234. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 15–24. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 24 August 2020).
- European Commission. Commission Regulation (EC) 1126/2007 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Fusarium Toxins in Maize and Maize Products. Off. J. Eur. Union 2007, 255, 16–17. Available online: https://www.fsai.ie/uploadedFiles/Commission_Regulation_EC_No_1126_2007.pdf (accessed on 14 August 2020).
- Republic of China, Laws & Regulations Database of The Republic of China. Sanitation Standard for Contaminants and Toxins in Food. 2021. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0040138 (accessed on 11 December 2021).
- Food Regulations, Revised ed.; Sale of Food Act (Chapter 283), Singapore. 2005, pp. 1–229. Available online: https://leap.unep.org/countries/sg/national-legislation/sale-food-act-chapter-283#:~:text=An%20Act%20for%20securing%20wholesomeness,the%20regulation%20of%20food%20establishments (accessed on 24 August 2020).
- European Commission. Commission Recommendation 2006/576/EC on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Off. J. Eur. Union 2006, 229, 7–9. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:229:0007:0009:EN:PDF (accessed on 24 August 2020).
- European Commission. Directive 2002/32/EC on Undesirable Substances in Animal Feed. Off. J. Eur. Union 2006, 140, 1–27. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2002L0032:20061020:EN:PDF (accessed on 24 August 2020).
- Peng, H.; Hui, Y.; Ren, R.; Wang, B.; Song, S.; He, Y.; Zhang, F. A sensitive electrochemical aptasensor based on MB-anchored GO for the rapid detection of Cronobacter sakazakii. J. Solid State Electrochem. 2019, 23, 3391–3398. [Google Scholar] [CrossRef]
- Chen, Q.; Du, M.; Xu, X. A label-free and selective electrochemical aptasensor for ultrasensitive detection of Di(2-ethylhexyl) phthalate based on self-assembled DNA nanostructure amplification. J. Electroanal. Chem. 2011, 914, 116300. [Google Scholar] [CrossRef]
- Mousivand, M.; Bagherzadeh, K.; Anfossi, L.; Javan-Nikkhah, M. Key criteria for engineeringmycotoxin binding aptamers via computational simulations: Aflatoxin B1 as a case study. Biotechnol. J. 2021, 17, 2100280. [Google Scholar] [CrossRef]
- Khan, R.; Aissa, S.B.; Sherazi, T.A.; Catanante, G.; Hayat, A.; Marty, J.L. Development of an impedimetric aptasensor for label free detection of patulin in apple juice. Molecules 2019, 24, 1017. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, G.; Liu, P.; Li, Z.; Yu, B. Recent development of aptamer sensors for the quantification of aflatoxin B1. Appl. Sci. 2019, 9, 2364. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.P.; Zhao, Q. A signal-on electrochemical aptasensor for rapid detection of aflatoxin B1 based on competition with complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, J.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ennifar, E.; Nakamura, Y. Thermodynamic study of aptamers binding to their target proteins. Biochimie 2019, 145, 91–97. [Google Scholar] [CrossRef]
- Krittayavathananon, A.; Sawangphruk, M. Impedimetric sensor of ss-HSDNA/reduced graphene oxide aerogel electrode toward aflatoxin B1 detection: Effects of Redox Mediator Charges and Hydrodynamic Diffusion. Anal. Chem. 2017, 89, 13283–13289. [Google Scholar] [CrossRef] [PubMed]
- Chokkareddy, R.; Redhi, G.G. Fe3O4 Nanorods-RGO-ionic Liquid Nanocomposite Based Electrochemical Sensor for Aflatoxin B1 in Ground Paprika. Electroanalysis 2022, 34, 501–511. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z.; Benvidi, A. Development of an electrochemical aptasensor based on Au nanoparticles decorated on metal–organic framework nanosheets and p-biphenol electroactive label for the measurement of aflatoxin B1 in a rice flour sample. Anal. Bioanal. Chem. 2022, 414, 1973–1985. [Google Scholar] [CrossRef]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A simple detection platform based on molecularly imprinted polymer for AFB1 and FuB1 mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Liu, B.; Peng, J.; Wu, Q.; Zhao, Y.; Shang, H.; Wang, S. A novel screening on the specific peptide by molecular simulation and development of the electrochemical immunosensor for aflatoxin B1 in grains. Food Chem. 2022, 372, 131322. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, W.; Wei, Y.; Sheng, Q.; Shang, Y. A dual-signaling electrochemical aptasensor based on an in-plane gold nanoparticles–black phosphorus heterostructure for the sensitive detection of patulin. Foods 2023, 12, 846. [Google Scholar] [CrossRef]
- Yao, X.; Yang, Q.; Wang, Y.; Bi, C.; Du, H.; Wu, W. Dual-enzyme-based signal-amplified aptasensor for zearalenone detection by using CRISPR-Cas12a and Nt.AlwI. Foods 2022, 11, 487. [Google Scholar] [CrossRef]
- Guo, W.; Yang, H.; Zhang, Y.; Wu, H.; Lu, X.; Tan, J.; Zhang, W. A Novel Fluorescent Aptasensor Based on Real-Time Fluorescence and Strand Displacement Amplification for the Detection of Ochratoxin A. Foods 2022, 11, 2443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Li, M.; She, Y.; Zhu, C.; Yan, M. An alkyne-mediated SERS aptasensor for anti-interference ochratoxin A detection in real samples. Foods 2022, 11, 3407. [Google Scholar] [CrossRef]
- Karaman, C.; Karaman, O.; Yola, B.B.; Ulker, I.; Atar, N.; Yola, M.L. A novel electrochemical aflatoxin B1 immunosensor based on gold nanoparticle-decorated porous graphene nanoribbon and Ag nanocubeincorporated MoS2 nanosheets. New J. Chem. 2021, 45, 11222–11233. [Google Scholar] [CrossRef]
- Iphigenia, N.; Satu, T. Guidance for the Validation of Analytical Methodology and Calibration of Equipment Used for Testing of Illicit Drugs in Seized Materials and Biological Specimens; United Nations Office on Drugs and Crime: New York, NY, USA, 2009.


| EC Technique | Types of Working Electrode | Electrode Surface Modification | Mycotoxin | Real Sample | LOD (nM) | Linear Range (nM) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| EIS | Glassy carbon rotating disk electrode | Reduced graphene oxide aerogel (rGOae) Single stranded thiol-modified DNA (ss-HSDNA) | AFB1 | UHT milk | 0.128 | 0.320–224.158 | 86.0–96.0 | [66] |
| DPV | Glassy carbon electrode | Methyltrioctylammonium chloride ionic liquid (IL) Fe3O4 nanorods rGO | AFB1 | Ground paprika | 0.096 | 0.064– 1.057 | 98.6–101.6 | [67] |
| DPV | Glassy carbon electrode | AuNP Nickel-based metal–organic framework nanosheets (Ni-MOF) 3-Mercaptopropionic acid (MPA) Apt cDNA 4,4′-Biphenol (PBP) | AFB1 | Rice flour | 0.003 | 0.016–480.338 | 98.4–101.3 | [68] |
| DPV | Glassy carbon electrode | Porous AuNP Specific peptide | AFB1 | Glutinous rice, corn, rice | 0.003 | 0.032– 64.045 | 88.4–102.0 | [70] |
| DPV | Indium tin oxide (ITO)-coated glass | PAni film as MIP | AFB1 FUM B1 | Corn | 0.001 | 0.003–1601.128 0.001– 692.684 | 91.6–108.6 86.2–102.4 | [69] |
| EIS | Screen-printed carbon paste electrode (SPE) | PAni film Glutaraldehyde Apt | AFB1 | Pistachio nut, cinnamon, clove, soybean, corn | 0.010 | 0.030– 0.080 | 87.9–94.7 | This work |
| Type of Samples | Standard AFB1 Concentration Spiked into Samples (nM) | Mean of Found AFB1 Concentration (nM), n = 3 | Mean of Aptasensor RCT Response (Ω), n = 3 | t Value (At CL = 95%, tcritical = 2.776) | Recovery (%) | Precision (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Negative sample (distilled water) | - | 0.030 ± 0.005 | 212.774 ± 3.898 | - | - | - | - |
| Corn | - | 0.038 ± 0.003 | 218.875 ± 1.931 | - | - | 7.9 | - |
| Pistachio nut | 0.06 | 0.053 ± 0.003 | 229.755 ± 2.278 | 0.002 | 87.9 | 5.8 | 12.1 |
| Cinnamon | 0.06 | 0.057 ± 0.008 | 232.804 ± 5.696 | 0.001 | 94.7 | 13.5 | 5.3 |
| Clove | 0.06 | 0.054 ± 0.004 | 230.365 ± 3.011 | 0.002 | 89.3 | 7.6 | 10.7 |
| Soybean | 0.06 | 0.054 ± 0.006 | 230.394 ± 4.102 | 0.001 | 89.3 | 10.3 | 10.7 |
| Corn | 0.06 | 0.054 ± 0.005 | 230.588 ± 3.638 | 0.001 | 89.8 | 9.1 | 10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, J.Y.; Phang, S.-W.; Goh, C.T.; Pike, A.; Tan, L.L. Impedimetric Polyaniline-Based Aptasensor for Aflatoxin B1 Determination in Agricultural Products. Foods 2023, 12, 1698. https://doi.org/10.3390/foods12081698
Ong JY, Phang S-W, Goh CT, Pike A, Tan LL. Impedimetric Polyaniline-Based Aptasensor for Aflatoxin B1 Determination in Agricultural Products. Foods. 2023; 12(8):1698. https://doi.org/10.3390/foods12081698
Chicago/Turabian StyleOng, Jing Yi, Sook-Wai Phang, Choo Ta Goh, Andrew Pike, and Ling Ling Tan. 2023. "Impedimetric Polyaniline-Based Aptasensor for Aflatoxin B1 Determination in Agricultural Products" Foods 12, no. 8: 1698. https://doi.org/10.3390/foods12081698
APA StyleOng, J. Y., Phang, S.-W., Goh, C. T., Pike, A., & Tan, L. L. (2023). Impedimetric Polyaniline-Based Aptasensor for Aflatoxin B1 Determination in Agricultural Products. Foods, 12(8), 1698. https://doi.org/10.3390/foods12081698






