Dose-Dependent Effect of Hyperoside on the Physicochemical and Gel Properties of Porcine Myofibrillar Proteins at Different NaCl Concentrations under Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MPs Extraction
2.3. Preparation of Oxidative MPs Samples
2.4. Determination of Carbonyls
2.5. Determination of Free Amines Level
2.6. Determination of Total Sulphydryl (SH) Content
2.7. Determination of Surface Hydrophobicity
2.8. Gel Strength and Water Holding Capacity (WHC)
2.9. Cryo-Scanning Electron Microscopy (Cryo-SEM)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Determination of Carbonyl Content
3.2. Determination of Free Amines
3.3. Determination of SH Content
3.4. Determination of Surface Hydrophobicity
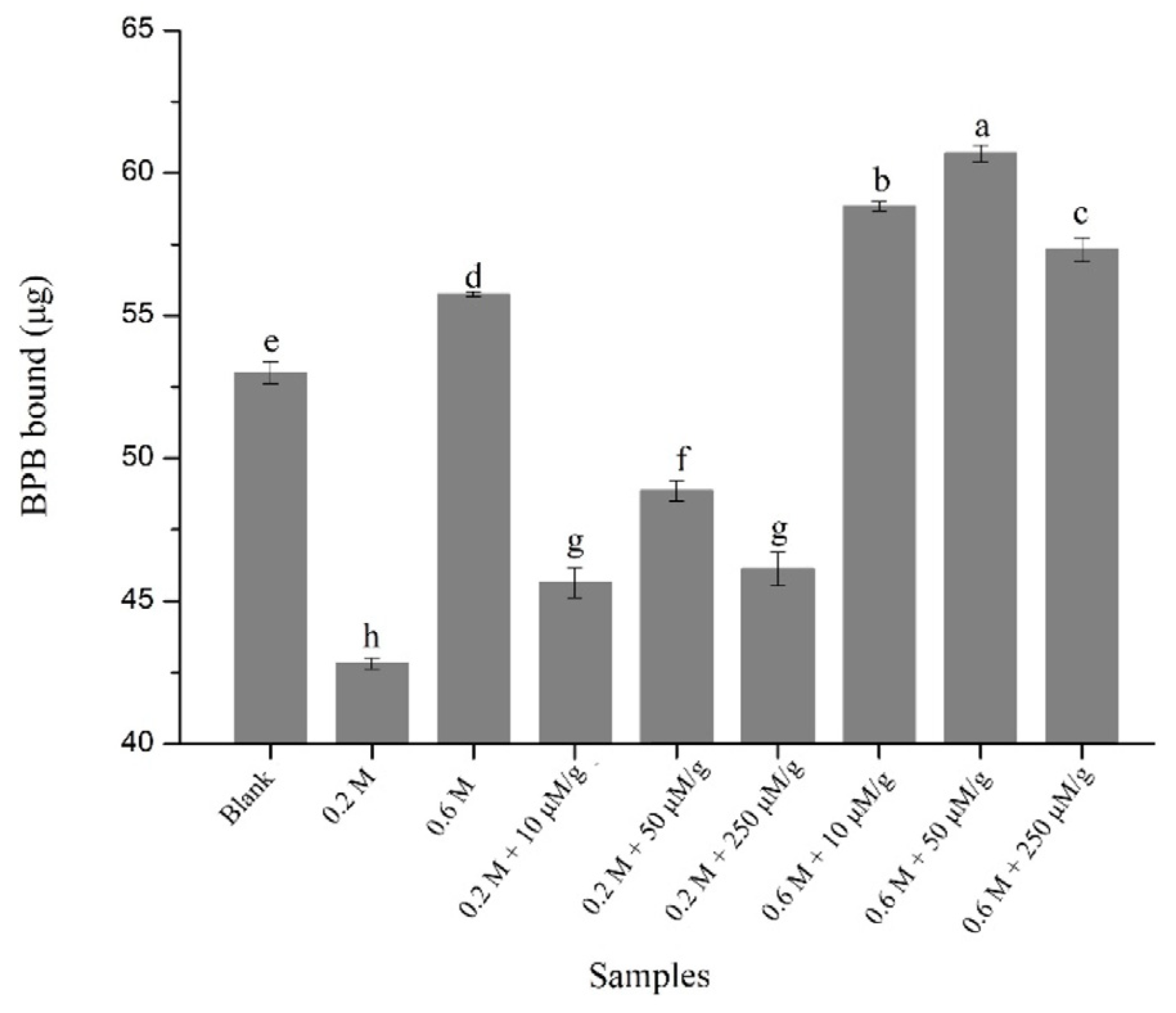
3.5. Determination of Gel Strength and WHC
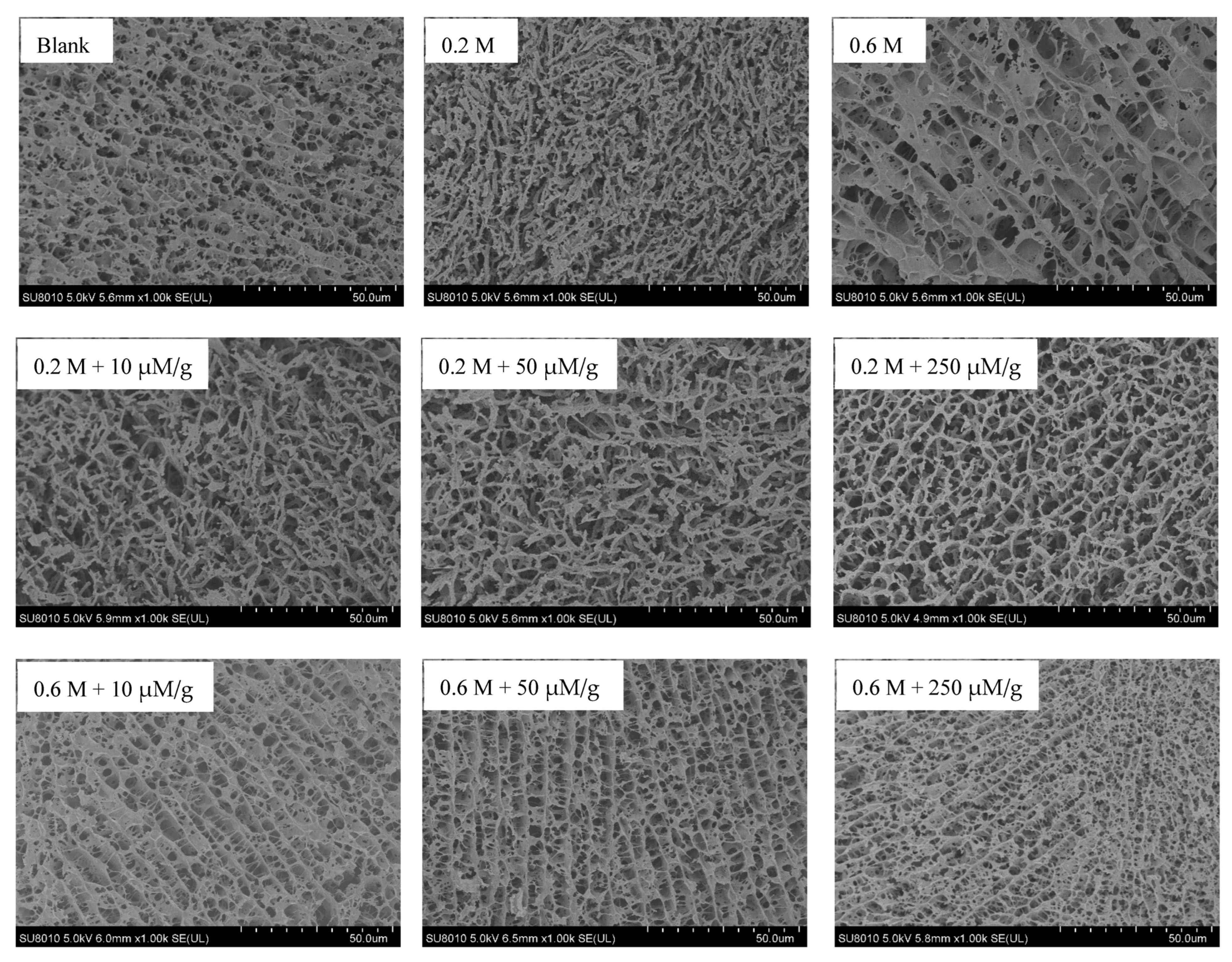
3.6. Microstructure of MPs Gel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, W.; Ma, L.; Chen, X.; Li, X.; Zhang, Y. Physicochemical and structural properties of composite gels prepared with myofibrillar protein and lecithin at various ionic strengths. Food Hydrocoll. 2018, 82, 135–143. [Google Scholar] [CrossRef]
- Pomélie, D.L.P.; Santé-Lhoutellier, V.; Sayd, T.; Gatellier, P. Oxidation and nitrosation of meat proteins under gastro-intestinal conditions: Consequences in terms of nutritional and health values of meat. Food Chem. 2018, 243, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Jiang, M.; Rui, X.; Li, W.; Chen, X.H.; Dong, M.S. Effect of rose polyphenols on oxidation, biogenic amines and microbial diversity in naturally dry fermented sausages. Food Control 2017, 78, 324–330. [Google Scholar] [CrossRef]
- García-Lomillo, J.; Gonzalez-SanJose, M.L.; Pino-García, R.D.; Ortega-Heras, M.; Muñiz-Rodríguez, P. Antioxidant effect of seasonings derived from wine pomace on lipid oxidation in refrigerated and frozen beef patties. LWT-Food Sci. Technol. 2017, 77, 85–91. [Google Scholar] [CrossRef]
- Jiao, Y.; Quek, S.Y.; Gu, M.H.; Guo, Y.R.; Liu, Y.F. Polyphenols from thinned young kiwifruit as natural antioxidant: Protective effects on beef oxidation, physicochemical and sensory properties during storage. Food Control 2020, 108, 106870. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Xiang, R.; Cheng, J.; Zhu, M.; Liu, X. Effect of mulberry (Morus alba) polyphenols as antioxidant on physiochemical properties, oxidation and bio-safety in Cantonese sausages. LWT-Food Sci. Technol. 2019, 116, 108504. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef]
- Nagendran, B.; Sundram, K.; Samir, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar]
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Yi, G.; Grabez, V.; Bjelanovic, M.; Slinde, E.; Olsen, K.; Langsrud, O.; Phung, V.T.; Haug, A.; Oostindjer, M.; Egelandsdal, B. Lipid oxidation in minced beef meat with added Krebs cycle substrates to stabilise colour. Food Chem. 2015, 187, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Zamuz, S.; Lopez-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Dominguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Li, R.; Tan, J.; Jiang, Z.T. Polyphenolic composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei China and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hui, T.; Wang, F.L.; Li, S.; Cui, B.; Cui, Y.; Peng, Z. Chinese red pepper (Zanthoxylum bungeanum Maxim.) leaf extract as natural antioxidants in salted silver carp (Hypophthalmichthys molitrix) in dorsal and ventral muscles during processing. Food Control 2015, 56, 9–17. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Fu, L.; Liu, F.; Wang, H.; Li, M.; Jiang, C.; Yin, B. The protective effect of hyperin on LPS-induced acute lung injury in mice. Microb. Pathog. 2019, 127, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, F.L.; Li, S.; Peng, Z.Q. Effects of pepper (Zanthoxylum bungeanum maxim.) leaf extract on the antioxidant enzyme activities of salted silver carp (Hypophthalmichthys molitrix) during processing. J. Funct. Foods 2015, 18, 1179–1190. [Google Scholar] [CrossRef]
- Guo, X.Y.; Wu, J.J.; Meng, X.R.; Zhang, Y.W.; Peng, Z.Q. Oxidative characteristics and gel properties of porcine myofibrillar proteins affected by l-lysine and l-histidine in a dose-dependent manner at a low and high salt concentration. Int. J. Food Sci. Technol. 2022, 57, 2556–2567. [Google Scholar] [CrossRef]
- Gornall, A.G. Determination of serum proteins by means off the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Su, G.; Cui, C.; Sun, W. Gelation of salted myofibrillar protein under malondialdehyde-induced oxidative stress. Food Hydrocoll. 2014, 40, 153–162. [Google Scholar] [CrossRef]
- Zhao, X.; Xing, T.; Wang, P.; Xu, X.; Zhou, G. Oxidative stability of isoelectric solubilisation/precipitation-isolated PSE-like chicken protein. Food Chem. 2019, 283, 646–655. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Huang, X.; Liu, L.; Wang, G.; Song, H.; Geng, F.; Luo, P. Effect of nano eggshell calcium on the structure, physicochemical, and gel properties of threadfin bream (Nemipterus virgatus) actomyosin. LWT-Food Sci. Technol. 2021, 150, 112047. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Chen, L.; Xu, X.; Zhou, G.; Li, Z.; Feng, X. Dose-dependent effects of rosmarinic acid on formation of oxidatively stressed myofibrillar protein emulsion gel at different NaCl concentrations. Food Chem. 2018, 243, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Oxidation of skeletal muscle myofibrillar proteins in oil-in-water emulsions: Interaction with lipids and effect of selected phenolic compounds. J. Agric. Food Chem. 2008, 56, 10933–10940. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Han, Y.R.; Zheng, J.B.; Zhao, M.M.; Sun, W.Z. Physicochemical characteristics and gel-forming properties of myofibrillar protein in an oxidative system affected by partial substitution of NaCl with KCl, MgCl2 or CaCl2. Food Chem. 2020, 309, 125614. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Wang, J.; Zhou, K.; Yi, S.; Zhu, W.; Li, J. Effect of hydroxyl radicals on biochemical and functional characteristics of myofibrillar protein from large yellow croaker (Pseudosciaena crocea). J. Food Biochem. 2020, 44, 3378–3387. [Google Scholar] [CrossRef]
- Zhou, F.; Sun, W.; Zhao, M. Controlled formation of emulsion gels stabilized by salted myofibrillar protein under malondialdehyde (MDA)-induced oxidative stress. J. Agric. Food Chem. 2015, 63, 3766–3777. [Google Scholar] [CrossRef]
- Pan, J.; Lian, H.; Jia, H.; Hao, R.; Wang, Y.; Ju, H.; Li, S.; Dong, X. Dose affected the role of gallic acid on mediating gelling properties of oxidatively stressed Japanese seerfish myofibrillar protein. LWT-Food Sci. Technol. 2020, 118, 108849. [Google Scholar] [CrossRef]
- Cao, Y.; True, A.D.; Chen, J.; Xiong, Y.L. Dual role (anti- and pro-oxidant) ofgallic acid in mediating myofibrillar protein gelation and gel in vitro digestion. J. Agric. Food Chem. 2016, 15, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.X.; Li, F.; Li, D.P.; Ge, F.Q.; Xu, L.H.; Wang, Y.L. Effects of ferulic acid on the oxidation stability and nitrozation of myofibrillar proteins under oxidative stress. Food Chem. Adv. 2022, 1, 100016. [Google Scholar] [CrossRef]
- Cheng, Y.; Chi, Y.; Geng, X.; Chi, Y. Effect of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) induced oxidation on the physicochemical properties, in vitro digestibility, and nutritional value of egg white protein. LWT-Food Sci. Technol. 2021, 143, 111103. [Google Scholar] [CrossRef]
- Jia, N.; Wang, L.; Shao, J.; Liu, D.; Kong, B. Changes in the structural and gel properties of pork myofibrillar protein induced by catechin modification. Meat Sci. 2017, 127, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Y.L.; Chen, J. Oxidation-induced unfolding facilitates myosin cross-linking in myofibrillar protein by microbial transglutaminase. J. Agric. Food Chem. 2012, 60, 8020–8027. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, W.; Zou, Y.; Xing, L.; Zheng, H.; Xu, X.; Zhou, G. Influence of RosA-protein adducts formation on myofibrillar protein gelation properties under oxidative stress. Food Hydrocoll. 2017, 67, 197–205. [Google Scholar] [CrossRef]
- Jongberg, S.; Torngren, M.A.; Gunvig, A.; Skibsted, L.H.; Lund, M.N. Effect of green tea or rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Sci. 2013, 93, 538–546. [Google Scholar] [CrossRef]
- Cheng, J.; Xiang, R.; Tang, D.; Zhu, M.; Liu, X. Regulation of protein oxidation in Cantonese sausages by rutin, quercetin and caffeic acid. Meat Sci. 2021, 175, 108422. [Google Scholar] [CrossRef]
- Jia, N.; Zhang, F.; Liu, Q.; Wang, L.; Lin, S.; Liu, D. The beneficial effects of rutin on myofibrillar protein gel properties and related changes in protein conformation. Food Chem. 2019, 301, 125206. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Glucose oxidase promotes gallic acid-myofibrillar protein interaction and thermal gelation. Food Chem. 2019, 293, 529–536. [Google Scholar] [CrossRef]
- Feng, X.; Chen, L.; Lei, N.; Wang, S.; Xu, X.; Zhou, G.; Li, Z. Emulsifying properties of oxidatively stressed myofibrillar protein emulsion gels prepared with (−)-epigallocatechin-3-gallate and NaCl. J. Agric. Food Chem. 2017, 65, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Peng, Z.Q.; Zhang, Y.W.; Liu, B.; Cui, Y.Q. The solubility and conformational characteristics of porcine myosin as affected by the presence of l-lysine and l-histidine. Food Chem. 2015, 170, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Chen, L.; Han, B.F.; Wang, R.; Liu, Y.; Fan, X.; Lv, X.; Huang, F.; Han, M.; Kang, Z.; et al. Effects of NaCl on the interactions between neomethyl hesperidin dihydrochalcone and pork myofibrillar protein: Their relevance to gelation properties. Food Res. Int. 2022, 162, 111983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Chen, L.; Lv, Y.Q.; Wang, S.; Suo, Z.; Cheng, X.; Xu, X.; Zhou, G.; Li, Z.; Feng, X. Inhibition of interaction between epigallocatechin-3-gallate and myofibrillar protein by cyclodextrin derivatives improves gel quality under oxidative Stress. Food Res. Int. 2018, 108, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Y.L.; Chen, J. Protein oxidation at different salt concentrations affects the cross-linking and gelation of pork myofibrillar protein catalyzed by microbial transglutaminase. J. Food Sci. 2013, 78, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Stephen Elmore, J.; Zhao, M.M.; Sun, W.Z. Effect of oxidation on the gel properties of porcine myofibrillar proteins and their binding abilities with selected flavour compounds. Food Chem. 2020, 329, 127032. [Google Scholar] [CrossRef]
- Totosaus, A.; Perez-Chabela, M.L. Textural properties and microstructure of low-fat and sodium-reduced meat batters formulated with gellan gum and dicationic salts. LWT-Food Sci. Technol. 2009, 42, 563–569. [Google Scholar] [CrossRef]
- Feng, M.Q.; Pan, L.H.; Yang, X.; Sun, J.; Xu, X.L.; Zhou, G.H. Thermal gelling properties and mechanism of porcine myofibrillar protein containing flaxseed gum at different nacl concentrations. LWT-Food Sci. Technol. 2018, 87, 361–367. [Google Scholar] [CrossRef]
| Samples | Carbonyl (nmol/mg Protein) | Free Amines (nmol/mg Protein) | SH (nmol/mg Protein) |
|---|---|---|---|
| Blank | 0.33 ± 0.19 i | 80.15 ± 2.98 a | 58.40 ± 0.98 a |
| 0.2 M NaCl | 2.45 ± 0.11 c | 56.63 ± 1.29 d | 51.57 ± 0.64 b |
| 0.6 M NaCl | 3.03 ± 0.08 a | 51.87 ± 2.13 e | 46.47 ± 0.61 c |
| 0.2 M NaCl + 10 μM/g HYP | 2.12 ± 0.08 d | 59.03 ± 1.42 c | 43.79 ± 0.33 d |
| 0.2 M NaCl + 50 μM/g HYP | 1.77 ± 0.12 e | 62.67 ± 1.38 b | 38.99 ± 0.84 e |
| 0.2 M NaCl + 250 μM/g HYP | 1.39 ± 0.04 g | 65.28 ± 2.03 b | 31.42 ± 0.98 f |
| 0.6 M NaCl + 10 μM/g HYP | 2.75 ± 0.12 b | 53.66 ± 2.03 de | 36.65 ± 1.51 e |
| 0.6 M NaCl + 50 μM/g HYP | 1.57 ± 0.16 f | 55.92 ± 1.67 d | 31.55 ± 2.01 f |
| 0.6 M NaCl + 250 μM/g HYP | 1.11 ± 0.04 h | 59.25 ± 2.04 c | 24.24 ± 1.50 g |
| Samples | Gel Strength (g·mm) | WHC (%) |
|---|---|---|
| Blank | 0.25 ± 0.010 c | 67.14 ± 1.56 b |
| 0.2 M NaCl | 0.066 ± 0.013 h | 27.23 ± 1.33 g |
| 0.6 M NaCl | 0.18 ± 0.012 d | 59.72 ± 0.29 d |
| 0.2 M NaCl + 10 μM/g HYP | 0.094 ± 0.013 g | 29.93 ± 0.24 g |
| 0.2 M NaCl + 50 μM/g HYP | 0.12 ± 0.0065 f | 36.73 ± 0.65 f |
| 0.2 M NaCl + 250 μM/g HYP | 0.15 ± 0.011 e | 41.09 ± 3.43 e |
| 0.6 M NaCl + 10 μM/g HYP | 0.26 ± 0.013 c | 64.24 ± 2.21 c |
| 0.6 M NaCl + 50 μM/g HYP | 0.30 ± 0.019 b | 69.10 ± 1.15 b |
| 0.6 M NaCl + 250 μM/g HYP | 0.36 ± 0.014 a | 75.91 ± 2.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Xu, S.; Meng, X.; Peng, Z. Dose-Dependent Effect of Hyperoside on the Physicochemical and Gel Properties of Porcine Myofibrillar Proteins at Different NaCl Concentrations under Oxidative Stress. Foods 2023, 12, 1684. https://doi.org/10.3390/foods12081684
Guo X, Xu S, Meng X, Peng Z. Dose-Dependent Effect of Hyperoside on the Physicochemical and Gel Properties of Porcine Myofibrillar Proteins at Different NaCl Concentrations under Oxidative Stress. Foods. 2023; 12(8):1684. https://doi.org/10.3390/foods12081684
Chicago/Turabian StyleGuo, Xiuyun, Shuangyi Xu, Xiangren Meng, and Zengqi Peng. 2023. "Dose-Dependent Effect of Hyperoside on the Physicochemical and Gel Properties of Porcine Myofibrillar Proteins at Different NaCl Concentrations under Oxidative Stress" Foods 12, no. 8: 1684. https://doi.org/10.3390/foods12081684
APA StyleGuo, X., Xu, S., Meng, X., & Peng, Z. (2023). Dose-Dependent Effect of Hyperoside on the Physicochemical and Gel Properties of Porcine Myofibrillar Proteins at Different NaCl Concentrations under Oxidative Stress. Foods, 12(8), 1684. https://doi.org/10.3390/foods12081684





