Short-Term Effects of PJE Administration on Metabolic Parameters in Diet-Induced Obesity Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Extraction
2.1.2. HPLC Analysis
2.1.3. Cell Culture
2.1.4. Cell Viability Assay
2.1.5. Animals
2.1.6. In Vivo DIO Mice Model and Treatment Groups
2.1.7. Liver and Fat Tissue Histology
2.1.8. Biochemical Analysis
2.1.9. Statistical Analysis
3. Results
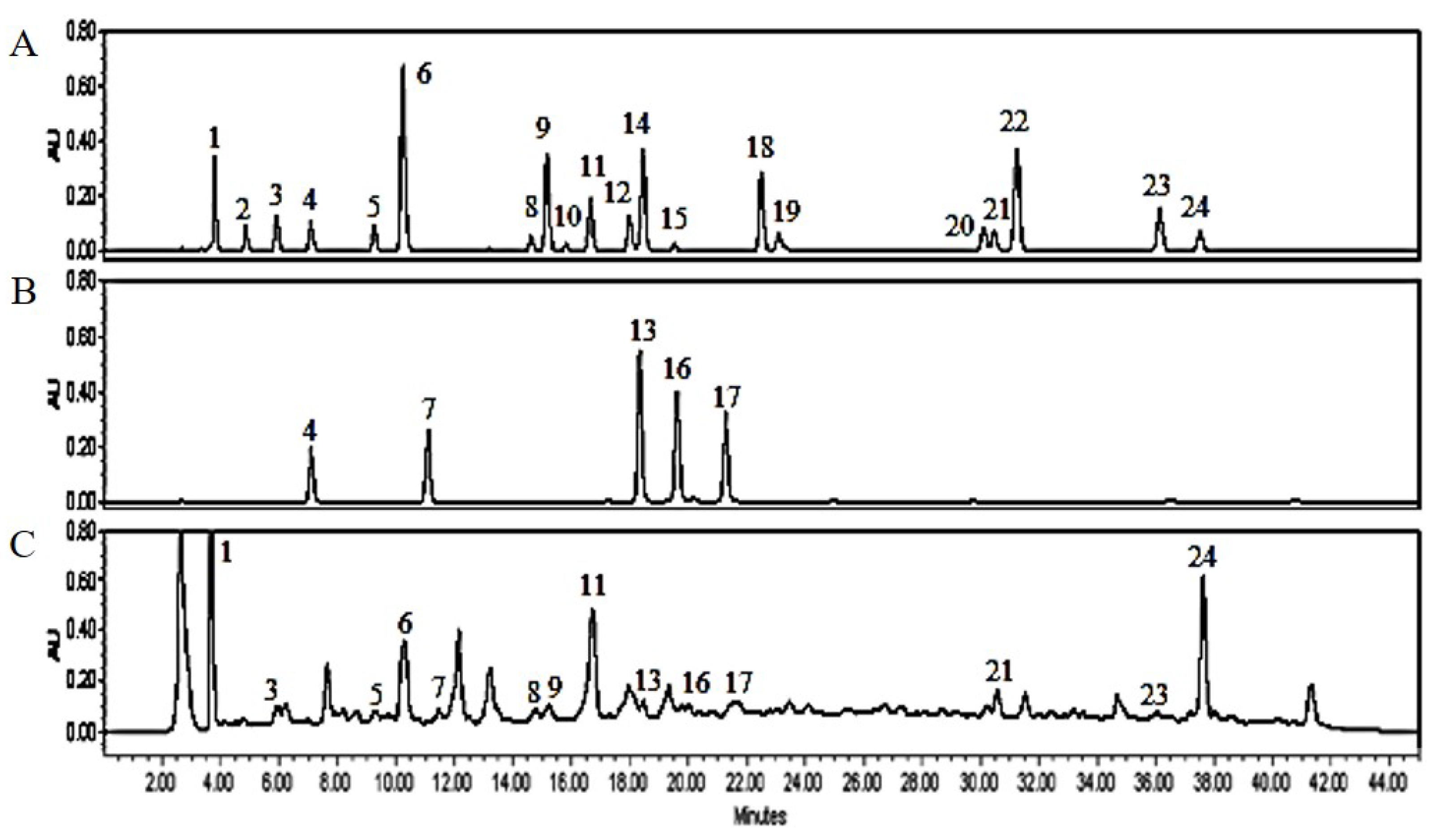
3.1. PJE Contains Polyphenolic Compounds
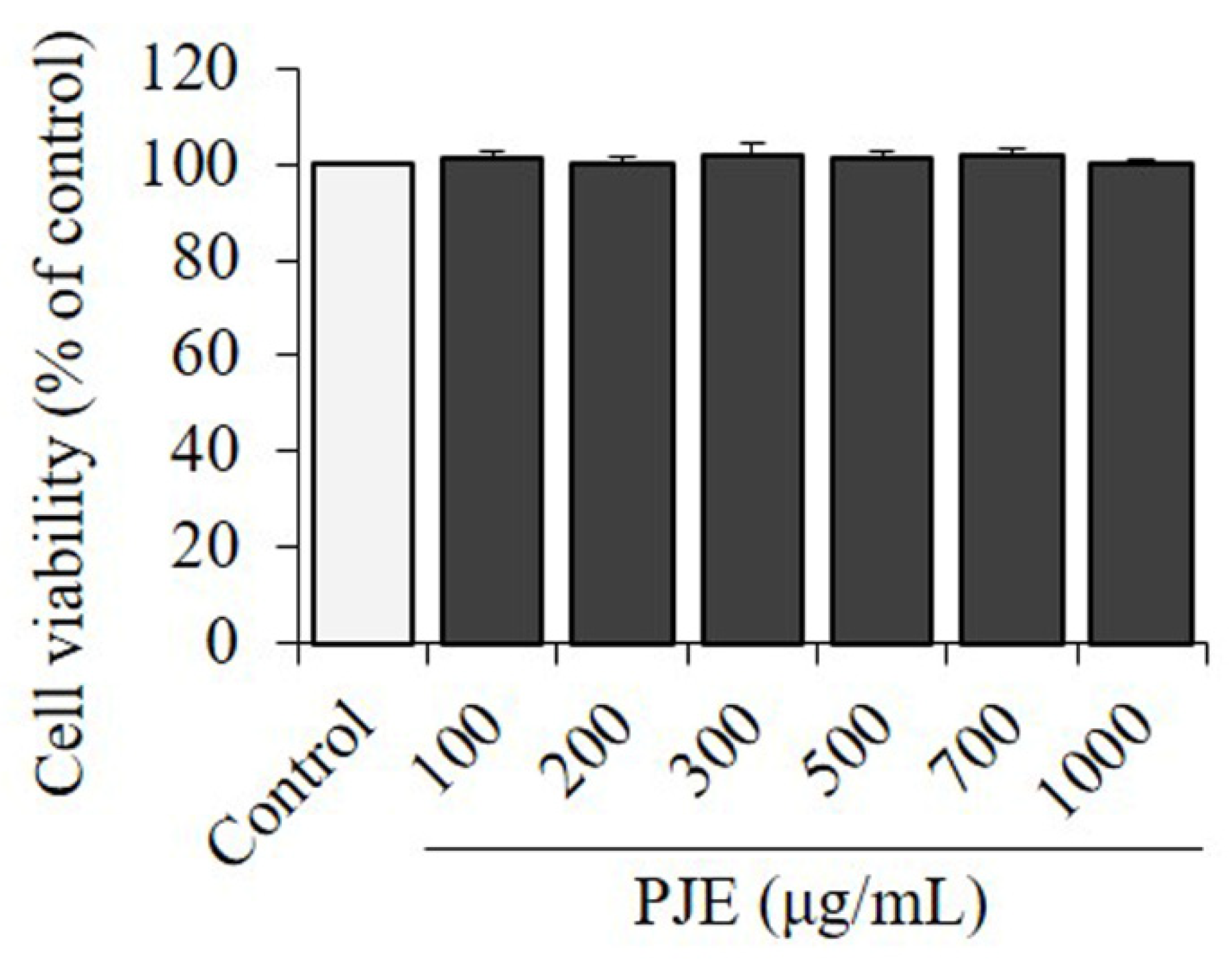
3.2. Effect of PJE on Cell Viability in 3T3-L1
3.3. Effect of PJE on Body Weight Gain and Feed Efficiency of DIO Mice Model
3.4. Effect of PJE on the Weight of Liver, Kidney, Spleen, and Fat Tissues
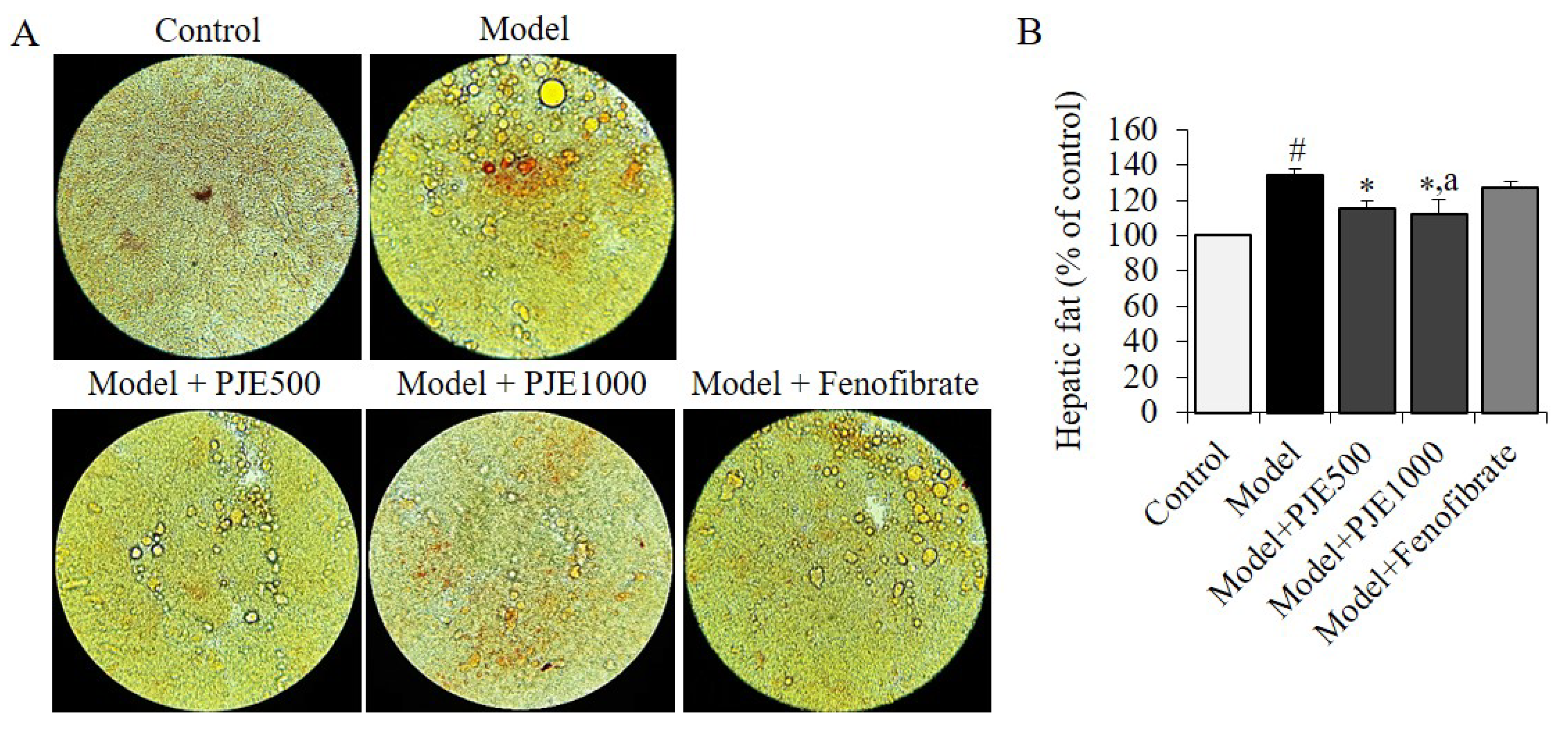
3.5. Effect of PJE on Hepatic Fat in DIO Mice Model
3.6. Effect of PJE on Serum Biochemical Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Štimac, D.; Klobučar Majanović, S.; Belančić, A. Endoscopic treatment of obesity: From past to future. Dig. Dis. 2020, 6, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Chae, H. Relationships between obesity, body image perception, and weight control in adult women. Korean J. Women Health Nurs. 2019, 25, 129–142. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.J.; Shin, S.R.; Hong, J.Y. Study on antioxidant and physiological activities of extract from Ligularia fischeri by extraction methods. Korean J. Food Preserv. 2017, 24, 1113–1121. [Google Scholar] [CrossRef]
- Choi, E.M.; Ding, V.; Nguyen, H.T.; Park, S.H.; Kim, Y.H. Antioxidant activity of Gomchi (Ligularia fischeri) leaves. Food Sci. Biotechnol. 2007, 16, 710–714. [Google Scholar]
- Park, J.H.; Ahn, E.K.; Kim, J.K.; Oh, J.S. Antihyperlipidemic activity of Ligularia fischeri extract in mice fed a high-carbohydrate diet. J. Med. Food 2019, 22, 374–383. [Google Scholar] [CrossRef]
- Choi, H.J.; Chung, M.J.; Ham, S.S. Antiobese and hypocholesterol aemic effects of an Adenophora triphylla extract in HepG2 cells and high fat diet-induced obese mice and high fat diet-induced obese mice. Food Chem. 2010, 119, 437–444. [Google Scholar] [CrossRef]
- Randy, A.; Kim, M.; Nho, C.W. Ligularia fischeri and its constituent 3,4-dicaffeoylquinic acid improve obesity-induced nonalcoholic fatty liver disease by regulating lipid metabolism and activating AMPK. J. Funct. Foods 2016, 27, 1–16. [Google Scholar] [CrossRef]
- Yu, K.H.; Lee, S.Y.; Yang, H.M.; Ham, Y.A.; Lee, S.U.; Chae, S.W.; Lee, Y.J. Effects of fermented water extracts from Ligularia fischeri on hepatotoxicity in ethanol-induced rats. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1431–1438. [Google Scholar] [CrossRef]
- Eom, H.J.; Shin, H.Y.; Jeong, Y.Y.; Kwon, N.R.; Kim, K.H.; Kim, I.J.; Yu, K.W. Nutritional components and physiological activities of Petasites japonicus solvent extracts. Korean J. Food Preserv. 2021, 28, 915–925. [Google Scholar] [CrossRef]
- Cho, B.S.; Lee, J.J.; Lee, M.Y. Effects of ethanol extracts from Petasites japonicus S. et Z. Max. on hepatic antioxidative systems in alcohol treated rats. J. Korean Soc. Food Sci. Nutr. 2007, 36, 298–300. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, H.; Kim, H.; Park, S. Anti-inflammatory activities of hot water extracts of Petasites japonicus leaves in LPS-induced RAW264.7 cells. J. Korean Soc. Food Sci. Nutr. 2020, 49, 289–294. [Google Scholar] [CrossRef]
- Hiemori-Kondo, M.; Nii, M. In vitro and in vivo evaluation of antioxidant activity of Petasites japonicus Maxim. flower buds extracts. Biosci. Biotechnol. Biochem. 2020, 84, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.M.; Asamenew, G.; Kim, H.W.; Lee, S.H.; Yoo, S.M.; Cho, S.M.; Cha, Y.S.; Kang, M.S. Anti-obesity effects of Petasites japonicus (Meowi) ethanol extract on RAW 264.7 macrophages and 3T3-L1 adipocytes and its characterization of polyphenolic compounds. Nutrients 2020, 12, 1261. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, S.; Hakozaki, M.; Kanno, Y.; Shiraishi, A.; Suzuki, M.; Kimura, K.I.; Shiono, Y.; Yano, A. Petasin is the main component responsible for the anti-adipogenic effect of Petasites japonicus. Fitoterapia 2022, 157, 105130. [Google Scholar] [CrossRef]
- Watanabe, T.; Hata, K.; Hiwatashi, K.; Hori, K.; Suzuki, N.; Itoh, H. Suppression of murine preadipocyte differentiation and reduction of visceral fat accumulation by a Petasites japonicus ethanol extract in mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010, 74, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, M.K.; Yeo, S.H.; Kim, S. Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters. Sci. Rep. 2020, 10, 21102. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Ikewuchi, C.J.; Ikewuchi, C.C. Alteration of plasma lipid profiles and atherogenic indices by Stachytarpheta jamaicensis L. (Vahl). Biokemistri 2009, 21, 71–77. [Google Scholar] [CrossRef]
- Vogeser, M.; König, D.; Frey, I.; Predel, H.G.; Parhofer, K.G.; Berg, A. Fasting serum insulin and the homeostasis model of insulin resistance (HOMA-IR) in the monitoring of lifestyle interventions in obese persons. Clin. Biochem. 2007, 40, 964–968. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.W.; Kim, S.; Kim, S.J. In vitro antioxidant and in vivo hypolipidemic effects of the king oyster culinary-medicinal mushroom, Pleurotus eryngii var. ferulae DDL01 (Agaricomycetes), in rats with high-fat diet-induced fatty liver and hyperlipidemia. Int. J. Med. Mushrooms 2017, 19, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.; Asaf, S.; Asif, S.; Kim, K.M. Bioactivity and therapeutic potential of kaempferol and quercetin: New insights for plant and human health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Park, S.E.; Kim, S.J.; Kim, S. Kaempferol inhibits thrombosis and platelet activation. Biochimie 2015, 115, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S. In vitro antithrombotic, hematological toxicity, and inhibitor studies of protocatechuic, isovanillic, and p-hydroxybenzoic acids from Maclura tricuspidata (Carr.) Bur. Molecules 2022, 27, 3496. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, cardio-protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Molecules 2022, 27, 7286. [Google Scholar]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, H.J.; Kim, Y.S.; Yeo, S.H.; Kim, S. Effects of Maclura tricuspidata (Carr.) Bur fruits and its phytophenolics on obesity-related enzymes. J. Food Biochem. 2020, 44, e13110. [Google Scholar] [CrossRef]
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and cardiovascular disease: Effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef]
- Kamarauskaite, J.; Baniene, R.; Raudone, L.; Vilkickyte, G.; Vainoriene, R.; Motiekaityte, V.; Trumbeckaite, S. Antioxidant and mitochondria-targeted activity of caffeoylquinic-acid-rich fractions of wormwood (Artemisia absinthium L.) and silver wormwood (Artemisia ludoviciana Nutt.). Antioxidants 2021, 10, 1405. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: Anti-allergic, anti-inflammatory, and skin-protective activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant activity of 1, 3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar] [CrossRef]
- Naya, K.; Takagi, I. The structure of petasitin, a new sesquiterpene from petasites japonicus maxim. Tetrahedron Letters 1968, 9, 629–632. [Google Scholar] [CrossRef]
- Hiemori-Kondo, M. Antioxidant compounds of Petasites japonicus and their preventive effects in chronic diseases: A review. J. Clin. Biochem. Nutr. 2020, 67, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar]
- Guo, L.; Kang, J.S.; Park, Y.H.; Je, B.I.; Lee, Y.J.; Kang, N.J.; Park, S.Y.; Hwang, D.Y.; Choi, Y.W. S-petasin inhibits lipid accumulation in oleic acid-induced HepG2 cells through activation of the AMPK signaling pathway. Food Funct. 2020, 11, 5664–5673. [Google Scholar] [CrossRef]
- Guo, L.; Li, K.; Cui, Z.W.; Kang, J.S.; Son, B.G.; Choi, Y.W. S-Petasin isolated from Petasites japonicus exerts anti-adipogenic activity in the 3T3-L1 cell line by inhibiting PPAR-γ pathway signaling. Food Funct. 2019, 10, 4396–4406. [Google Scholar] [CrossRef]
- Vinola, S.M.J.; Karthikeyan, K.; Sharma, A.; Sudheshna, S.; Sekar, M. Anti-inflammatory efficacy of petasin-incorporated zinc oxide eugenol sealer—An in vivo zebrafish study. J. Conserv. Dent. 2021, 24, 539–543. [Google Scholar] [CrossRef]
- Cardel, M.I.; Atkinson, M.A.; Taveras, E.M.; Holm, J.C.; Kelly, A.S. Obesity treatment among adolescents: A review of current evidence and future directions. JAMA Pediatr. 2020, 174, 609–617. [Google Scholar] [CrossRef]
- Williams, D.M.; Nawaz, A.; Evans, M. Drug therapy in obesity: A review of current and emerging treatments. Diabetes Ther. 2020, 11, 1199–1216. [Google Scholar] [CrossRef]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: From molecular to clinical studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Sharma, M.; Li, Y.; Stoll, M.L.; Tollefsbol, T.O. The epigenetic connection between the gut microbiome in obesity and diabetes. Front. Genet. 2020, 10, 1329. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An overview on dietary polyphenols and their biopharmaceutical classification system (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Hu, Z.; Li, M.; Cao, Y.; Akan, O.D.; Guo, T.; Luo, F. Targeting AMPK signaling by dietary polyphenols in cancer prevention. Mol. Nutr. Food Res. 2022, 66, e2100732. [Google Scholar] [CrossRef]
- Sotoudeheian, M.; Hoseini, S.A. Therapeutic properties of polyphenols affect AMPK molecular pathway in hyperlipidemia. Preprints 2023, 2023010528. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Debelo, H.; Li, M.; Ferruzzi, M.G. Processing influences on food polyphenol profiles and biological activity. Cur. Opi. Food Sci. 2020, 32, 90–102. [Google Scholar] [CrossRef]
- Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological properties of polyphenols: Bioavailability, mechanisms of action, and biological effects in in vitro studies, animal models, and humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef]

| Composition | Control | Model | PJE500 | PJE1000 | Fenofibrate |
|---|---|---|---|---|---|
| % (w/w) | |||||
| Nitrogen-free extract | 59.5 | 37.5 | 37.5 | 37.5 | 37.5 |
| Fat | 4.5 | 34.9 | 34.9 | 34.9 | 34.9 |
| Protein | 20.1 | 23.6 | 23.6 | 23.6 | 23.6 |
| Fiber | 4.6 | − | − | − | − |
| Ash | 5.8 | − | − | − | − |
| Mineral mixture | 3.5 | 3 | 3 | 3 | 3 |
| Vitamin mixture | 1 | 1 | 1 | 1 | 1 |
| PJE500 | − | − | 0.5 | − | − |
| PJE1000 | − | − | − | 1 | − |
| Fenofibrate | − | − | − | − | 0.2 |
| Protein calories (%) | 21.0 | 18.1 | 18.1 | 18.1 | 18.1 |
| Fat calories (%) | 13.7 | 61.6 | 61.6 | 61.6 | 61.6 |
| Carbohydrates calories (%) | 65.3 | 20.3 | 20.3 | 20.3 | 20.3 |
| Energy (kcal/g) | 4.04 | 4.65 | 4.79 | 4.92 | 4.65 |
| Peak no. | RT (min) | Compounds | PJE |
|---|---|---|---|
| Concentration (μg/g, dw) | |||
| 1 | 3.762 | Gallic acid | 34.05 ± 0.61 |
| 2 | 4.797 | Neochlorogenic acid | − |
| 3 | 5.849 | Protocatechuic acid | 4.31 ± 0.05 |
| 4 | 6.981 | Chlorogenic acid | 20.14 ± 0.16 |
| 5 | 9.119 | p-Hydroxybenzoic acid | 60.32 ± 0.81 |
| 6 | 10.114 | Caffeic acid | 15.84 ± 0.12 |
| 7 | 11.104 | 1,3-Dicaffeoylquinic acid | 0.57 ± 0.01 |
| 8 | 14.595 | Rutin | 9.10 ± 0.33 |
| 9 | 15.049 | p-Coumaric acid | 2.27 ± 0.04 |
| 10 | 15.594 | Quercetin 3-β-galactoside | − |
| 11 | 16.531 | Ferulic acid | 34.43 ± 0.53 |
| 12 | 17.859 | Taxifolin | − |
| 13 | 18.141 | 3,4-Dicaffeoylquinic acid | 18.18 ± 0.37 |
| 14 | 18.322 | trans-m-Coumaricacid | − |
| 15 | 19.495 | Quercetin 3-α-L-rhamnoside | − |
| 16 | 19.762 | 3,5-Dicaffeoylquinic acid | 4.19 ± 0.072 |
| 17 | 21.381 | 4,5-Dicaffeoylquinic acid | 3.95 ± 0.02 |
| 18 | 22.511 | Rosmarinic acid | − |
| 19 | 23.096 | Myrcetin | − |
| 20 | 29.969 | Luteolin | − |
| 21 | 30.404 | Quercetin | 15.23 ± 0.11 |
| 22 | 31.119 | trans-Cinnamic acid | − |
| 23 | 35.751 | Apigenin | 1.59 ± 0.02 |
| 24 | 37.437 | Kaempferol | 83.88 ± 1.04 |
| Parameters | Control | Model | PJE500 | PJE1000 | Fenofibrate |
|---|---|---|---|---|---|
| Liver | 2.43 ± 0.04 | 3.41 ± 0.19 # | 2.32 ± 0.12 ** | 2.29 ± 0.75 ** | 2.10 ± 0.15 ** |
| Kidney | 0.43 ± 0.03 | 0.49 ± 0.05 | 0.45 ± 0.02 | 0.44 ± 0.03 | 0.41 ± 0.04 |
| Spleen | 0.123 ± 0.006 | 0.143 ± 0.009 | 0.129 ± 0.014 | 0.131 ± 0.016 | 0.121 ± 0.012 |
| Epididymal fat | 0.58 ± 0.09 | 1.85 ± 0.15 # | 1.35 ± 0.17 * | 1.28 ± 0.25 * | 1.52 ± 0.11 |
| Perirenal fat | 0.06 ± 0.01 | 0.20 ± 0.02 # | 0.16 ± 0.01 * | 0.14 ± 0.02 ** | 0.18 ± 0.01 |
| Mesenteric fat | 0.12 ± 0.01 | 0.43 ± 0.04 # | 0.31 ± 0.01 * | 0.30 ± 0.02 * | 0.35 ± 0.02 ** |
| Parameters | Control | Model | PJE500 | PJE1000 | Fenofibrate |
|---|---|---|---|---|---|
| TC | 98.3 ± 3.5 | 161.0 ± 10.2 # | 137.2 ± 3.4 ** | 131.9 ± 4.1 ** | 110.3 ± 6.7 ** |
| TG | 85.1 ± 2.2 | 557.3 ± 26.3 # | 273.6 ± 12.8 ** | 255.7 ± 37.3 ** | 162.8 ± 5.0 ** |
| HDL | 108.0 ± 4.4 | 149.2 ± 1.0 # | 143.8 ± 5.1 | 147.6 ± 4.6 * | 135.1 ± 6.5 ** |
| LDL | 7.3 ± 6.5 | 123.3 ± 10.2 # | 48.1 ± 7.4 ** | 35.4 ± 13.5 ** | 7.76 ± 2.5 ** |
| VLDL | 17.0 ± 0.4 | 111.5 ± 5.3 # | 54.7 ± 2.6 ** | 51.1 ± 7.5 ** | 32.6 ± 1.0 ** |
| Glucose | 204.4 ± 8.7 | 241.6 ± 6.1 # | 224.3 ± 5.9 | 216.1 ± 7.8 * | 190.3 ± 9.2 ** |
| Insulin | 0.045 ± 0.013 | 0.179 ± 0.022 # | 0.093 ± 0.08 ** | 0.055 ± 0.012 ** | 0.037 ± 0.011 ** |
| HOMA-IR | 0.41 ± 0.02 | 1.92 ± 0.05 # | 0.93 ± 0.01 ** | 0.53 ± 0.03 ** | 0.31 ± 0.02 ** |
| TP | 5.10 ± 0.25 | 5.23 ± 0.33 | 5.25 ± 0.38 | 5.32 ± 0.31 | 5.15 ± 0.25 |
| AI | 0.79 ± 0.02 | 3.74 ± 0.11 # | 1.90 ± 0.06 ** | 1.73 ± 0.05 ** | 1.21 ± 0.04 ** |
| AC | −0.090 ± −0.003 | 0.079 ± 0.002 # | −0.046 ± −0.001 ** | −0.106 ± −0.003 ** | −0.184 ± −0.006 ** |
| CRR | 0.91 ± 0.03 | 1.08 ± 0.03 # | 0.95 ± 0.03 ** | 0.89 ± 0.03 ** | 0.82 ± 0.02 ** |
| CAI | 0.068 ± 0.002 | 0.826 ± 0.025 # | 0.335 ± 0.01 ** | 0.240 ± 0.007 ** | 0.057 ± 0.002 ** |
| Leptin | 5.06 ± 0.41 | 11.97 ± 1.15 # | 9.84 ± 0.72 * | 9.11 ± 0.84 ** | 9.52 ± 0.59 * |
| Adiponectin | 5.87 ± 0.13 | 4.95 ± 0.30 # | 5.07 ± 0.25 | 5.52 ± 0.12 * | 5.59 ± 0.17 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Kim, K.-M.; Park, S.-E.; Kim, M.-K.; Kim, S. Short-Term Effects of PJE Administration on Metabolic Parameters in Diet-Induced Obesity Mice. Foods 2023, 12, 1675. https://doi.org/10.3390/foods12081675
Choi J-H, Kim K-M, Park S-E, Kim M-K, Kim S. Short-Term Effects of PJE Administration on Metabolic Parameters in Diet-Induced Obesity Mice. Foods. 2023; 12(8):1675. https://doi.org/10.3390/foods12081675
Chicago/Turabian StyleChoi, Jun-Hui, Ki-Man Kim, Se-Eun Park, Myung-Kon Kim, and Seung Kim. 2023. "Short-Term Effects of PJE Administration on Metabolic Parameters in Diet-Induced Obesity Mice" Foods 12, no. 8: 1675. https://doi.org/10.3390/foods12081675
APA StyleChoi, J.-H., Kim, K.-M., Park, S.-E., Kim, M.-K., & Kim, S. (2023). Short-Term Effects of PJE Administration on Metabolic Parameters in Diet-Induced Obesity Mice. Foods, 12(8), 1675. https://doi.org/10.3390/foods12081675






