Capsaicin Rich Low-Fat Salad Dressing: Improvement of Rheological and Sensory Properties and Emulsion and Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Characterization of Hot Pepper Seed Oil By-Products (HPOB)
2.3. Salad Dressing Preparation
2.4. Analysis of Salad Dressing
2.4.1. Rheological Analysis
2.4.2. Zeta Potential (ζ) and Particle Size Measurement
2.4.3. Optical Microscope Observation
2.4.4. Oxidative Stability
2.4.5. Statistical Analysis
3. Results and Discussions
3.1. Characterization of HPOB
3.2. Rheological Analyzes
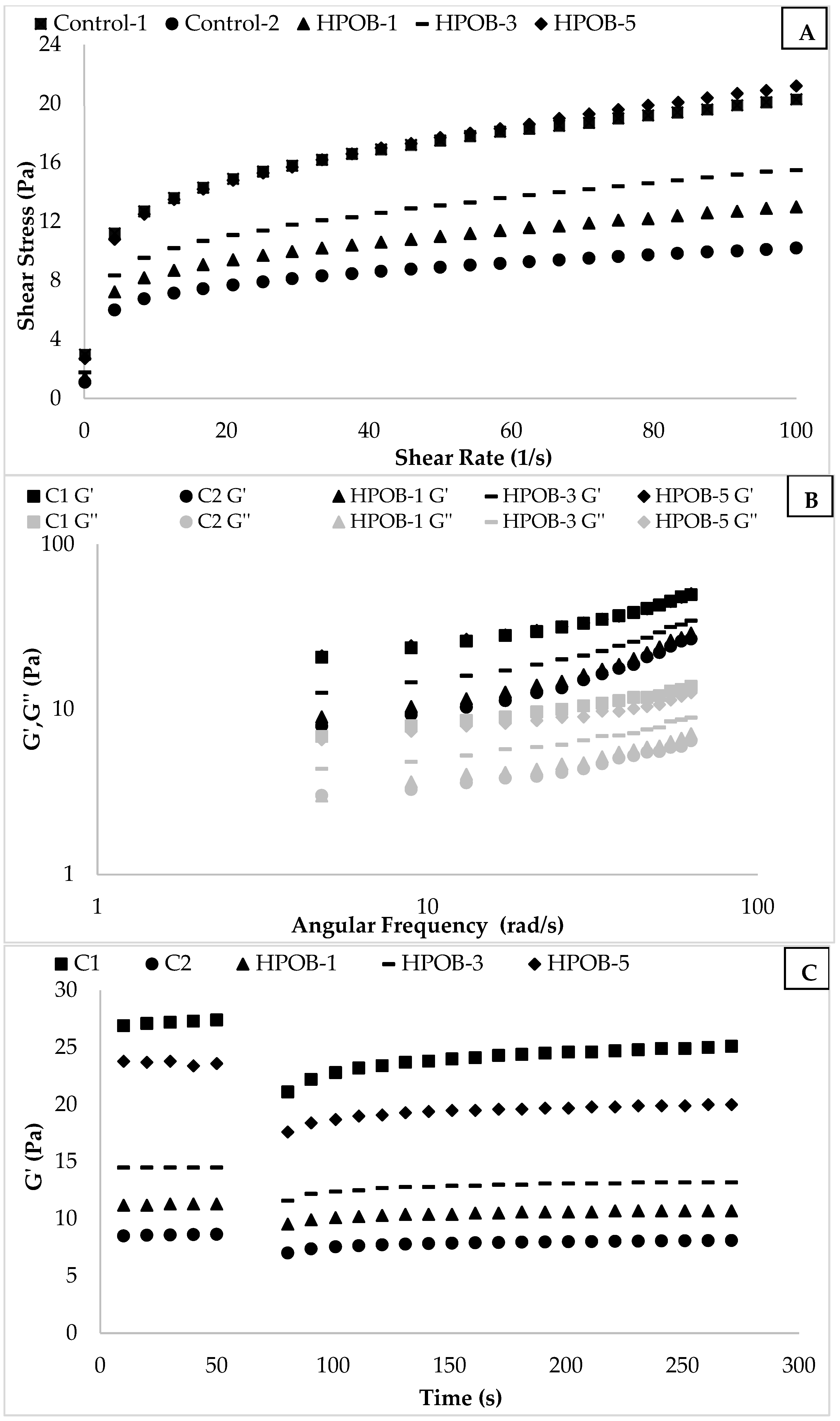
3.2.1. Flow Behavior Rheological Properties
3.2.2. Dynamical Rheological Properties
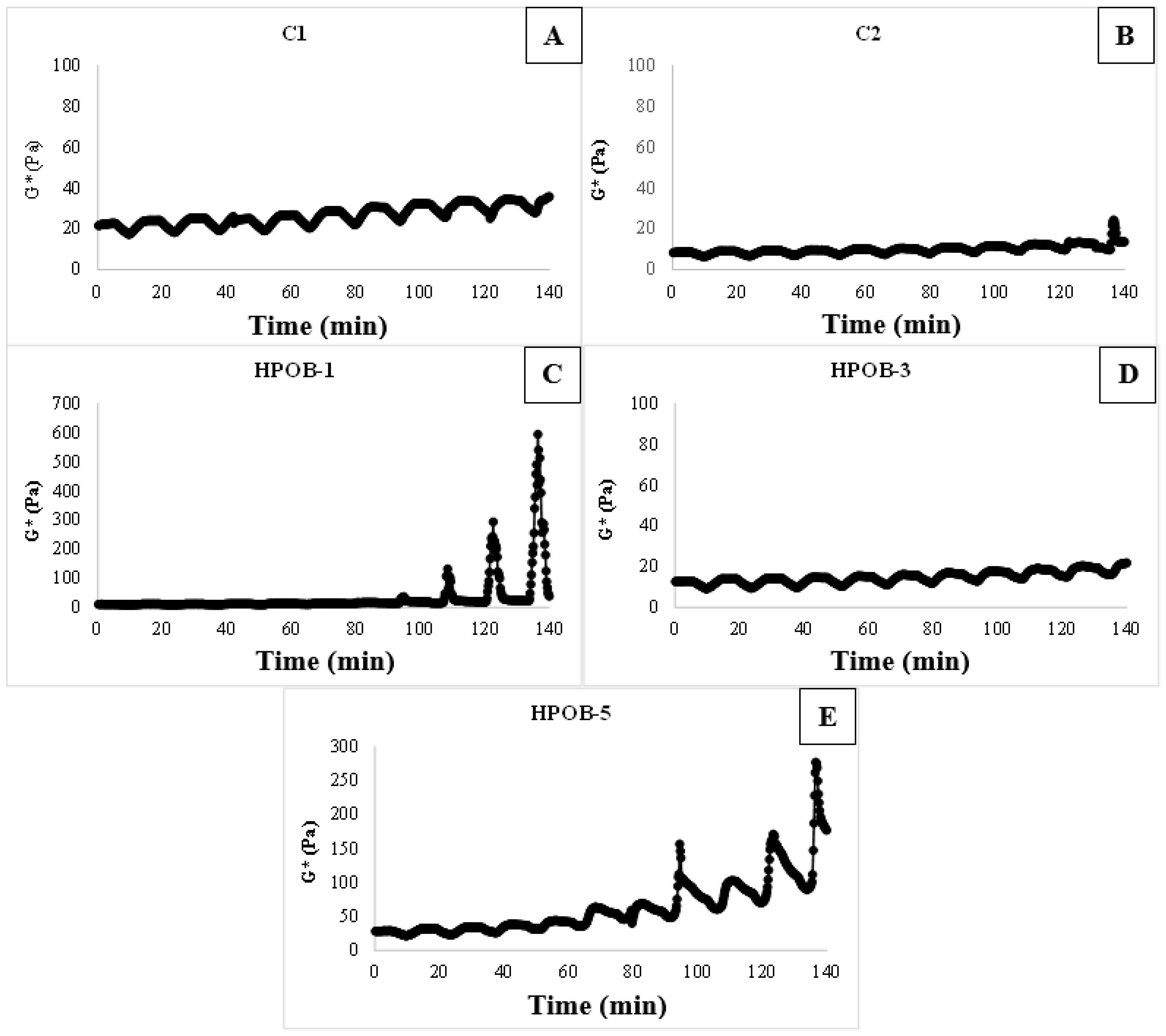
3.2.3. The 3-ITT Properties
3.2.4. Emulsion Stability
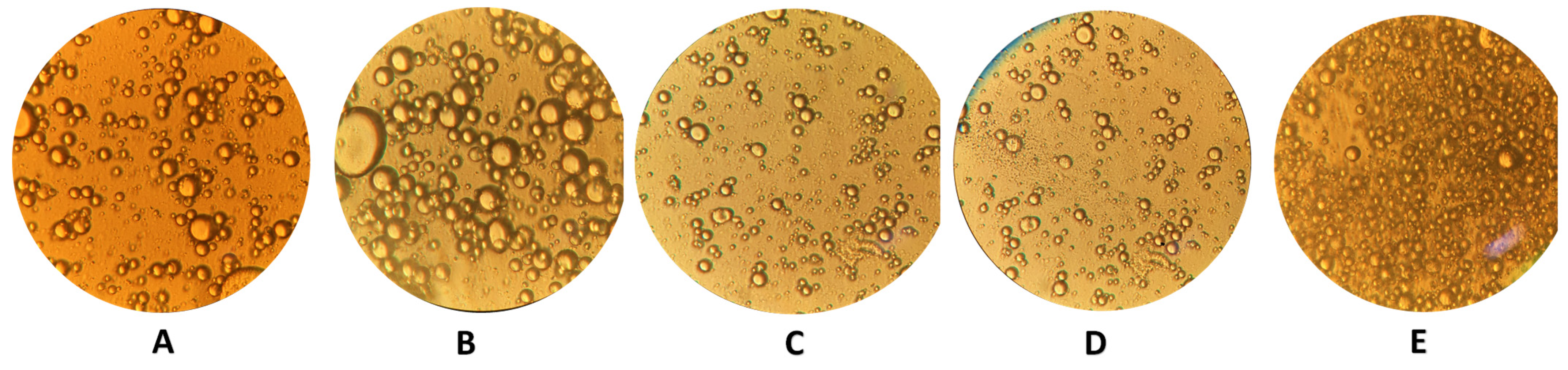
3.3. Zeta (ζ) Potential, Particle Size, and Light Microscope Images
3.4. IP Value and Oxidative Volatile Formation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mozafari, H.; Hosseini, E.; Hojjatoleslamy, M.; Mohebbi, G.H.; Jannati, N. Optimization low-fat and low cholesterol mayonnaise production by central composite design. J. Food Sci. Technol. 2017, 54, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. High fat diet and its effects on cognitive health: Alterations of neuronal and vascular components of brain. Physiol. Behav. 2021, 240, 113528. [Google Scholar] [CrossRef] [PubMed]
- Klaochanpong, N.; Puncha-arnon, S.; Uttapap, D.; Puttanlek, C.; Rungsardthong, V. Octenyl succinylation of granular and debranched waxy starches and their application in low-fat salad dressing. Food Hydrocoll. 2017, 66, 296–306. [Google Scholar] [CrossRef]
- Tekin-Cakmak, Z.H.; Karasu, S.; Kayacan-Cakmakoglu, S.; Akman, P.K. Investigation of potential use of by-products from cold-press industry as natural fat replacers and functional ingredients in a low-fat salad dressing. J. Food Process. Preserv. 2021, 45, e15388. [Google Scholar] [CrossRef]
- Peng, X.; Yao, Y. Carbohydrates as fat replacers. Annu. Rev. Food Sci. Technol. 2017, 8, 331–351. [Google Scholar] [CrossRef]
- Akbari, M.; Eskandari, M.H.; Davoudi, Z. Application and functions of fat replacers in low-fat ice cream: A review. Trends Food Sci. Technol. 2019, 86, 34–40. [Google Scholar] [CrossRef]
- Akcicek, A.; Karasu, S. Utilization of cold pressed chia seed oil waste in a low-fat salad dressing as natural fat replacer. J. Food Process Eng. 2018, 41, e12694. [Google Scholar] [CrossRef]
- Aksoy, F.S.; Tekin-Cakmak, Z.H.; Karasu, S.; Aksoy, A.S. Oxidative stability of the salad dressing enriched by microencapsulated phenolic extracts from cold-pressed grape and pomegranate seed oil by-products evaluated using OXITEST. Food Sci. Technol. 2022, 42, 57220. [Google Scholar] [CrossRef]
- Tekin, Z.H.; Karasu, S. Cold-pressed flaxseed oil by-product as a new source of fat replacers in low-fat salad dressing formulation: Steady, dynamic and 3-ITT rheological properties. J. Food Process. Preserv. 2020, 44, e14650. [Google Scholar] [CrossRef]
- Firatligil-Durmus, E.; Evranuz, O. Response surface methodology for protein extraction optimization of red pepper seed (Capsicum frutescens). LWT-Food Sci. Technol. 2010, 43, 226–231. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind. Crops Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- Yılmaz, E.; Sevgi Arsunar, E.; Aydeniz, B.; Güneşer, O. Cold pressed capia pepperseed (Capsicum annuum L.) oils: Composition, aroma, and sensory properties. Eur. J. Lipid Sci. Technol. 2015, 117, 1016–1026. [Google Scholar] [CrossRef]
- Ozyildiz, F.; Karagonlu, S.; Basal, G.; Uzel, A.; Bayraktar, O. Micro-encapsulation of ozonated red pepper seed oil with antimicrobial activity and application to nonwoven fabric. Lett. Appl. Microbiol. 2013, 56, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, T.; Ranilović, J.; Jokić, S. Quality of pepper seed by-products: A review. Foods 2022, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Pub AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Karaman, S.; Karasu, S.; Tornuk, F.; Toker, O.S.; Gecgel, U.; Sagdic, O.; Ozcan, N.; Gul, O. Recovery potential of cold press byproducts obtained from the edible oil industry: Physicochemical, bioactive, and antimicrobial properties. J. Agric. Food Chem. 2015, 63, 2305–2313. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Zhu, K.; Yang, W.; Ye, J.; Chai, L.; Xu, Q.; Deng, X. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. Plant Physiol. 2018, 176, 2657–2676. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Alvarado, L.A.; Vera-Avila, L.E. Analysis of capsaicin and dihydrocapsaicin in hot peppers by ultrasound assisted extraction followed by gas chromatography–mass spectrometry. Instrum. Sci. Technol. 2012, 40, 429–440. [Google Scholar] [CrossRef]
- Kayacan, S.; Karasu, S.; Akman, P.K.; Goktas, H.; Doymaz, I.; Sagdic, O. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and HMF formation of persimmon. LWT 2020, 118, 108830. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Karousioti, A.; Kiosseoglou, V. Formulation optimization of a potentially prebiotic low-in-oil oat-based salad dressing to improve Lactobacillus paracasei subsp. paracasei survival and physicochemical characteristics. LWT-Food Sci. Technol. 2013, 53, 560–568. [Google Scholar] [CrossRef]
- Akcicek, A.; Yildirim, R.M.; Tekin-Cakmak, Z.H.; Karasu, S. Low-Fat Salad Dressing as a Potential Probiotic Food Carrier Enriched by Cold-Pressed Tomato Seed Oil By-Product: Rheological Properties, Emulsion Stability, and Oxidative Stability. ACS Omega 2022, 7, 48520–48530. [Google Scholar] [CrossRef]
- Toker, O.S.; Karasu, S.; Yilmaz, M.T.; Karaman, S. Three interval thixotropy test (3ITT) in food applications: A novel technique to determine structural regeneration of mayonnaise under different shear conditions. Food Res. Int. 2015, 70, 125–133. [Google Scholar] [CrossRef]
- Tekin, Z.H.; Avci, E.; Karasu, S.; Toker, O.S. Rapid determination of emulsion stability by rheology-based thermal loop test. LWT 2020, 122, 109037. [Google Scholar] [CrossRef]
- Shi, L.; Bucknall, M.P.; Young, T.L.; Zhang, M.; Hu, L.; Bing, J.; Lee, D.S.; Kim, J.; Wu, T.; Takamure, N. Gas chromatography–mass spectrometry analyses of encapsulated stable perovskite solar cells. Science 2020, 368, eaba2412. [Google Scholar] [CrossRef]
- Yılmaz, E.; Hüriyet, Z.; Arifoğlu, N.; Emir, D.D. Functional properties of the capia pepper seed defatted press cakes. Waste Biomass Valorization 2017, 8, 783–791. [Google Scholar] [CrossRef]
- Azabou, S.; Taheur, F.B.; Jridi, M.; Bouaziz, M.; Nasri, M. Discarded seeds from red pepper (Capsicum annum) processing industry as a sustainable source of high added-value compounds and edible oil. Environ. Sci. Pollut. Res. 2017, 24, 22196–22203. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Mandal, P.K.; Han, K.-H.; Fukushima, M.; Choi, K.; Kim, C.-J.; Lee, C.-H. Capsaicin and tocopherol in red pepper seed oil enhances the thermal oxidative stability during frying. J. Food Sci. Technol. 2010, 47, 162–165. [Google Scholar] [CrossRef]
- Della Valle, A.; Dimmito, M.P.; Zengin, G.; Pieretti, S.; Mollica, A.; Locatelli, M.; Cichelli, A.; Novellino, E.; Ak, G.; Yerlikaya, S. Exploring the nutraceutical potential of dried pepper Capsicum annuum L. on market from altino in abruzzo region. Antioxidants 2020, 9, 400. [Google Scholar] [CrossRef]
- Bortnowska, G.; Balejko, J.; Schube, V.; Tokarczyk, G.; Krzemińska, N.; Mojka, K. Stability and physicochemical properties of model salad dressings prepared with pregelatinized potato starch. Carbohydr. Polym. 2014, 111, 624–632. [Google Scholar] [CrossRef]
- Fernandez, V.E.; Palazolo, G.G.; Bosisio, N.A.; Martínez, L.M.; Wagner, J.R. Rheological properties and stability of low-in-fat dressings prepared with high-pressure homogenized yeast. J. Food Eng. 2012, 111, 57–65. [Google Scholar] [CrossRef]
- Kumar, Y.; Roy, S.; Devra, A.; Dhiman, A.; Prabhakar, P.K. Ultrasonication of mayonnaise formulated with xanthan and guar gums: Rheological modeling, effects on optical properties and emulsion stability. LWT 2021, 149, 111632. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Advances in the design and production of reduced-fat and reduced-cholesterol salad dressing and mayonnaise: A review. Food Bioprocess Technol. 2013, 6, 648–670. [Google Scholar] [CrossRef]
- Sikora, M.; Badrie, N.; Deisingh, A.K.; Kowalski, S. Sauces and dressings: A review of properties and applications. Crit. Rev. Food Sci. Nutr. 2008, 48, 50–77. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Canet, W. Time-independent and time-dependent rheological characterization of vegetable-based infant purees. J. Food Eng. 2013, 114, 449–464. [Google Scholar] [CrossRef]
- Ma, S.; Yu, S.-J.; Zheng, X.-L.; Wang, X.-X.; Bao, Q.-D.; Guo, X.-M. Extraction, characterization and spontaneous emulsifying properties of pectin from sugar beet pulp. Carbohydr. Polym. 2013, 98, 750–753. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.; Gutiérrez-López, G. Zeta potential of food matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Lee, C.-H.; Han, K.-H.; Kim, A.-Y.; Lee, S.-K.; Hong, G.-E.; Pyun, C.-W.; Choi, K.-D.; Yang, C.-Y. Effect of hot pepper seed oil, capsaicin, and alpha-tocopherol on thermal oxidative stability in lard and soy bean oil. Food Sci. Anim. Resour. 2008, 28, 660–666. [Google Scholar] [CrossRef]
| Water (%) | Oil (%) | By-Product (%) | Vinegar (%) | EYP (%) | Salt (%) | XG (%) | |
|---|---|---|---|---|---|---|---|
| C1 | 58.65 | 30 | - | 7 | 3 | 1 | 0.35 |
| C2 | 78.65 | 10 | - | 7 | 3 | 1 | 0.35 |
| HPOB-1 | 77.86 | 10 | 1 | 7 | 3 | 1 | 0.35 |
| HPOB-3 | 75.65 | 10 | 3 | 7 | 3 | 1 | 0.35 |
| HPOB-5 | 73.65 | 10 | 5 | 7 | 3 | 1 | 0.35 |
| HPOB | |
|---|---|
| Moisture (%) | 6.69 ± 0.03 |
| Protein (%) | 20.25 ± 0.18 |
| Oil (%) | 11.24 ± 0.13 |
| Carbonhydrate (%) | 57.72 ± 0.04 |
| Crude fiber (%) | 31.91 ± 0.28 |
| Ash (%) | 3.83 ± 0.07 |
| TPC (mg GA/100 g) | 317.5 ± 19.17 |
| IDPPH (%) | 81.87 ± 0.01 |
| CUPRAC (mg Trolox/100 g) | 6952.8 ± 170.01 |
| Capsaicin (µg/g) | 175.8 ± 1.65 |
| Dihidrocapsaisin (µg /g) | 71.00 ± 1.46 |
| Total carotenoids (µg/g) | 106.3 ± 2.31 |
| Fatty acid composition | |
| C12:0 | 0.006 ± 0.00 |
| C16:0 | 13.51 ± 0.36 |
| C18:0 | 2.542 ± 0.03 |
| C18:1 | 6.87 ± 0.06 |
| C18:2 | 73.29 ± 0.41 |
| C18:3 | 2.75 ± 0.03 |
| C20:0 | 0.045 ± 0.003 |
| Phenolic Compounds | μg/g of Dry Weight |
| Gallic acid | 66.02 ± 0.72 |
| Protocatechuic acid | 15.05 ± 0.35 |
| Catechin | 16.73 ± 0.51 |
| 4-Hydroxy-benzoic acid | nd |
| Syringic acid | 3.85 ± 0.50 |
| Ellagic acid | 41.85 ± 0.61 |
| m-Coumaric acid | nd |
| o-Coumaric acid | 8.03 ± 0.37 |
| Chrysin | 1.60 ± 0.03 |
| Caffeic acid | 17.19 ± 0.32 |
| p-Coumaric acid | 5.88 ± 0.02 |
| Ferulic acid | 6.56 ± 0.04 |
| Myricetin | 87.61 ± 0.43 |
| Quercetin | 48.09 ± 0.52 |
| Kaempferol | 30.57 ± 0.15 |
| Chlorogenic acid | 36.87 ± 0.62 |
| Rutin | 29.52 ± 0.18 |
| Sinapic acid | 3.30 ± 0.02 |
| C1 | C2 | HPOB-1 | HPOB-3 | HPOB-5 | |
|---|---|---|---|---|---|
| Steady Shear Rheological Parameters | |||||
| K (Pasn) | 7.79 ± 0.21 a | 4.10 ± 0.07 e | 4.82 ± 0.15 d | 5.79 ± 0.06 b | 7.45 ± 0.17 a |
| n | 0.208 | 0.200 | 0.214 | 0.221 | 0.231 |
| R2 | 0.993 | 0.982 | 0.987 | 0.991 | 0.995 |
| Dynamic Rheological Parameters | |||||
| K′ (Pasn) | 10.47 ± 0.06 a | 2.21 ± 0.08 e | 2.72 ± 0.15 d | 5.18 ± 0.12 b | 10.47 ± 0.02 a |
| n′ | 0.359 | 0.589 | 0.553 | 0.440 | 0.359 |
| R2 | 0.978 | 0.978 | 0.972 | 0.974 | 0.978 |
| K″ (Pasn) | 4.40 ± 0.03 b | 1.71 ± 0.04 f | 1.81 ± 0.02 e | 2.74 ± 0.03 c | 4.76 ± 0.05 a |
| n″ | 0.263 | 0.300 | 0.311 | 0.269 | 0.209 |
| R2 | 0.991 | 0.967 | 0.972 | 0.972 | 0.946 |
| 3-ITT Rheological Parameters | |||||
| G0′ | 19.80 | 6.39 | 8.99 | 10.69 | 15.73 |
| Ge′ | 25.81 | 8.24 | 10.91 | 13.44 | 20.31 |
| Ge′/G0′ | 1.303 | 1.290 | 1.213 | 1.257 | 1.291 |
| K × 1000 | 60.61 | 53.52 | 43.02 | 52.73 | 57.39 |
| R2 | 0.997 | 0.998 | 0.994 | 0.995 | 0.997 |
| ζ Potential and Particle Size Distribution | |||||
| ζ-potential (mV) | −40.17 ± 1.50 c | −28.97 ± 2.17 bc | −29.33 ± 0.68 a | −32.20 ± 0.59 a | −35.67 ± 0.55 b |
| d32 (µm) | 3.44 ± 0.16 a | 4.94 ± 0.24 a | 4.83 ± 0.15 bc | 4.44 ± 0.08 cd | 4.22 ± 0.13 b |
| PdI | 0.95 ± 0.04 a | 0.85 ± 0.22 a | 0.87 ± 0.17 bc | 0.92 ± 0.15 ab | 0.93 ± 0.26 c |
| OVC and IP Value | Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | HPOB1 | HPOB3 | HPOB5 | ||||||
| IP value (h) | 3.20 ± 0.04 d | 2.58 ± 0.05 e | 6.33 ± 0.03 c | 7.18 ± 0.12 b | 8:33 ± 0.06 a | |||||
| Storage time (days) Peak area (TIC units·10−6) | ||||||||||
| 0. | 7. | 0. | 7. | 0. | 7. | 0. | 7. | 0. | 7. | |
| Aldehydes | ||||||||||
| Pentanal | nd | 0.33 | 0.34 | nd | nd | 0.21 | nd | |||
| Hexanal | 2.77 | 6.42 | 1.52 | 0.85 | 0.75 | |||||
| 2-Heptenal | 0.45 | 0.43 | nd | nd | nd | |||||
| Octanal | 0.60 | 0.82 | nd | 0.28 | 1.57 | |||||
| 2-Octenal | 0.61 | 0.26 | nd | 0.38 | nd | |||||
| Nonanal | 0.78 | 1.17 | 0.29 | 0.86 | 0.74 | 0.27 | 0.69 | |||
| 2-Nonenal | nd | nd | 0.72 | nd | nd | |||||
| 2-Decenal | nd | nd | 0.81 | nd | nd | |||||
| Decanal | 0.17 | nd | nd | nd | nd | |||||
| 2,4-Decadienal | nd | nd | nd | 0.47 | nd | |||||
| Alcohol | nd | |||||||||
| 1-Pentanol | nd | 0.31 | nd | nd | nd | |||||
| 1-Heptanol | nd | 0.24 | 0.32 | 0.55 | 0.65 | |||||
| 1-Octene-3-ol | 0.99 | 1.52 | 0.26 | 0.88 | 0.31 | 0.71 | 0.64 | |||
| 1-Penten-3-ol | nd | nd | nd | nd | nd | |||||
| Ketone | ||||||||||
| 2-Heptanone | nd | 0.27 | 0.35 | nd | nd | |||||
| 3-Heptanone-5-methyle | nd | 0.23 | nd | nd | 0.99 | |||||
| Hydrocarbons | ||||||||||
| Pentane | nd | nd | nd | nd | 0.38 | |||||
| Heptane | nd | nd | nd | nd | nd | |||||
| Octane | nd | nd | 1.98 | nd | nd | |||||
| 1-3-Hexadiene | nd | 0.48 | nd | nd | nd | |||||
| Furan | ||||||||||
| 2-Pentylfuran | 1.41 | 1.51 | 0.91 | 0.83 | 0.89 | |||||
| 2-Ethylfuran | nd | 0.13 | nd | nd | nd | |||||
| Acids | ||||||||||
| Hexanoic acid | nd | nd | nd | nd | nd | |||||
| Propanoic acid | nd | nd | 0.38 | nd | nd | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avci, E.; Tekin-Cakmak, Z.H.; Ozgolet, M.; Karasu, S.; Kasapoglu, M.Z.; Ramadan, M.F.; Sagdic, O. Capsaicin Rich Low-Fat Salad Dressing: Improvement of Rheological and Sensory Properties and Emulsion and Oxidative Stability. Foods 2023, 12, 1529. https://doi.org/10.3390/foods12071529
Avci E, Tekin-Cakmak ZH, Ozgolet M, Karasu S, Kasapoglu MZ, Ramadan MF, Sagdic O. Capsaicin Rich Low-Fat Salad Dressing: Improvement of Rheological and Sensory Properties and Emulsion and Oxidative Stability. Foods. 2023; 12(7):1529. https://doi.org/10.3390/foods12071529
Chicago/Turabian StyleAvci, Esra, Zeynep Hazal Tekin-Cakmak, Muhammed Ozgolet, Salih Karasu, Muhammed Zahid Kasapoglu, Mohamed Fawzy Ramadan, and Osman Sagdic. 2023. "Capsaicin Rich Low-Fat Salad Dressing: Improvement of Rheological and Sensory Properties and Emulsion and Oxidative Stability" Foods 12, no. 7: 1529. https://doi.org/10.3390/foods12071529
APA StyleAvci, E., Tekin-Cakmak, Z. H., Ozgolet, M., Karasu, S., Kasapoglu, M. Z., Ramadan, M. F., & Sagdic, O. (2023). Capsaicin Rich Low-Fat Salad Dressing: Improvement of Rheological and Sensory Properties and Emulsion and Oxidative Stability. Foods, 12(7), 1529. https://doi.org/10.3390/foods12071529









