Formation and Characterization of Self-Assembled Rice Protein Hydrolysate Nanoparticles as Soy Isoflavone Delivery Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rice Protein Hydrolysates (RPHs)
2.3. Characterization of RPHs
2.3.1. Determination of the Molecular Weight Distribution
2.3.2. Measurement of the Surface Hydrophobicity (H0)
2.3.3. Amino Acid Composition Analysis
2.4. Preparation of RPH-SIF Nanoparticles
2.5. Characterization of RPH-SIF Nanoparticles
2.5.1. Determinations of Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.5.2. Measurements of Particle Size, Polydispersity Index (PDI), and Zeta Potential
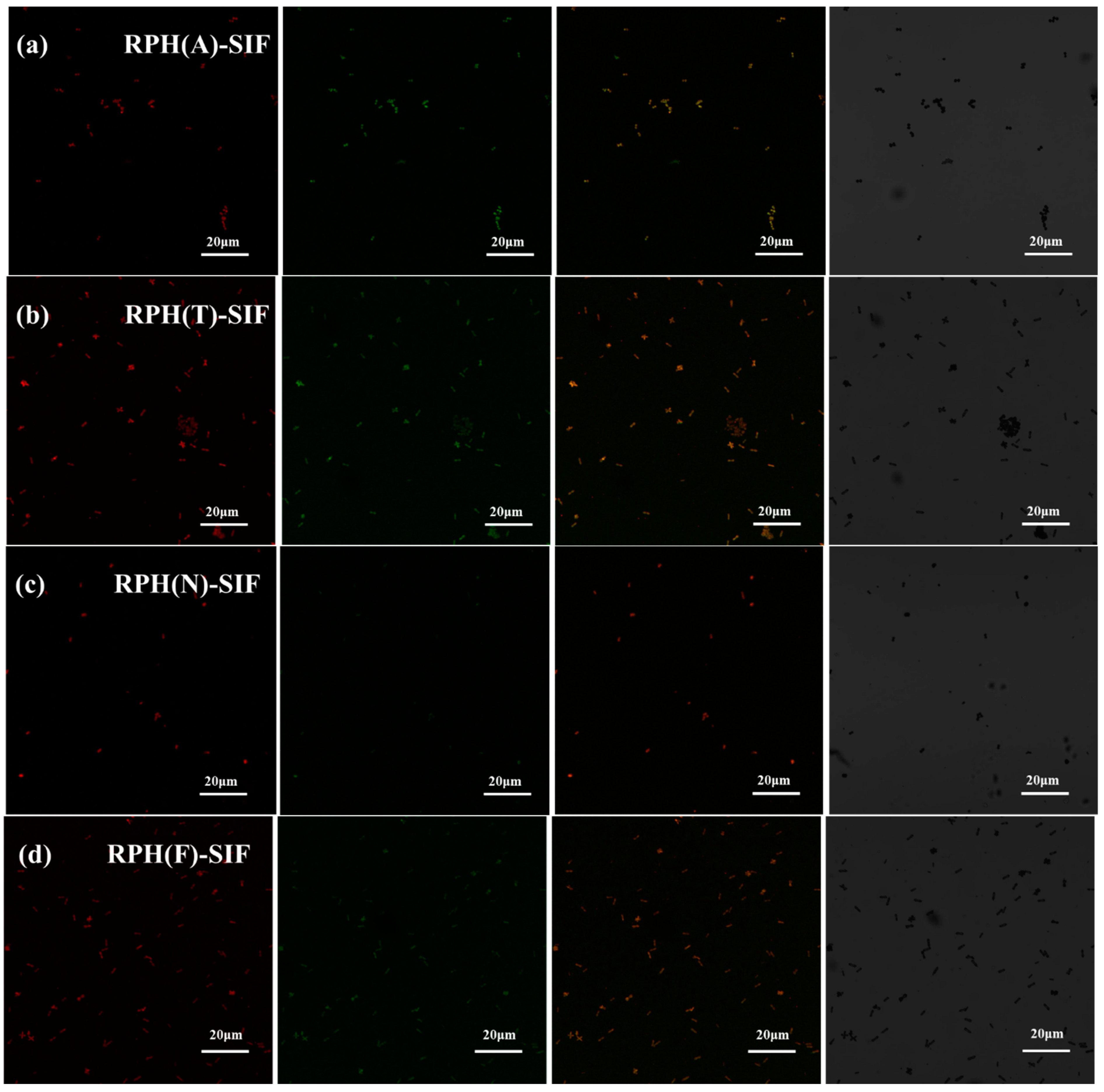
2.5.3. Confocal Laser Scanning Microscopy (CLSM)
2.5.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.5. X-ray Diffraction (XRD)
2.5.6. Fluorescence Spectroscopy
2.6. Antioxidant Activity of RPH-SIF Nanoparticles
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS Radical Scavenging Activity
2.7. Stability of RPH-SIF Nanoparticles
2.7.1. Thermal Stability
2.7.2. Ionic Stability
2.7.3. pH Stability
2.8. In Vitro Simulated Digestion
2.9. Statistical Analysis
3. Results and Discussion
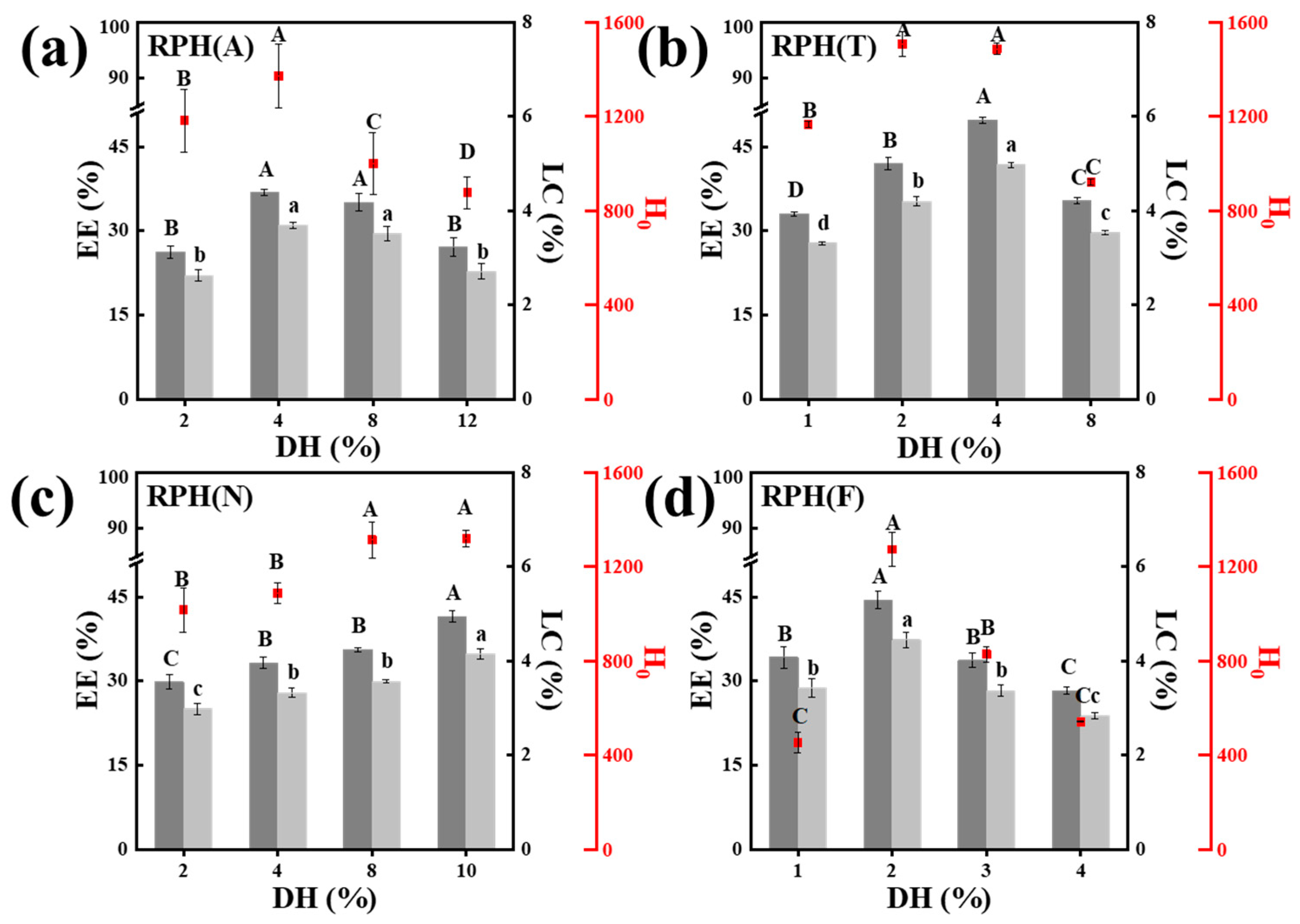
3.1. Effects of the DH and Molecular Weight of RPH on the Fabrication of Nanoparticles
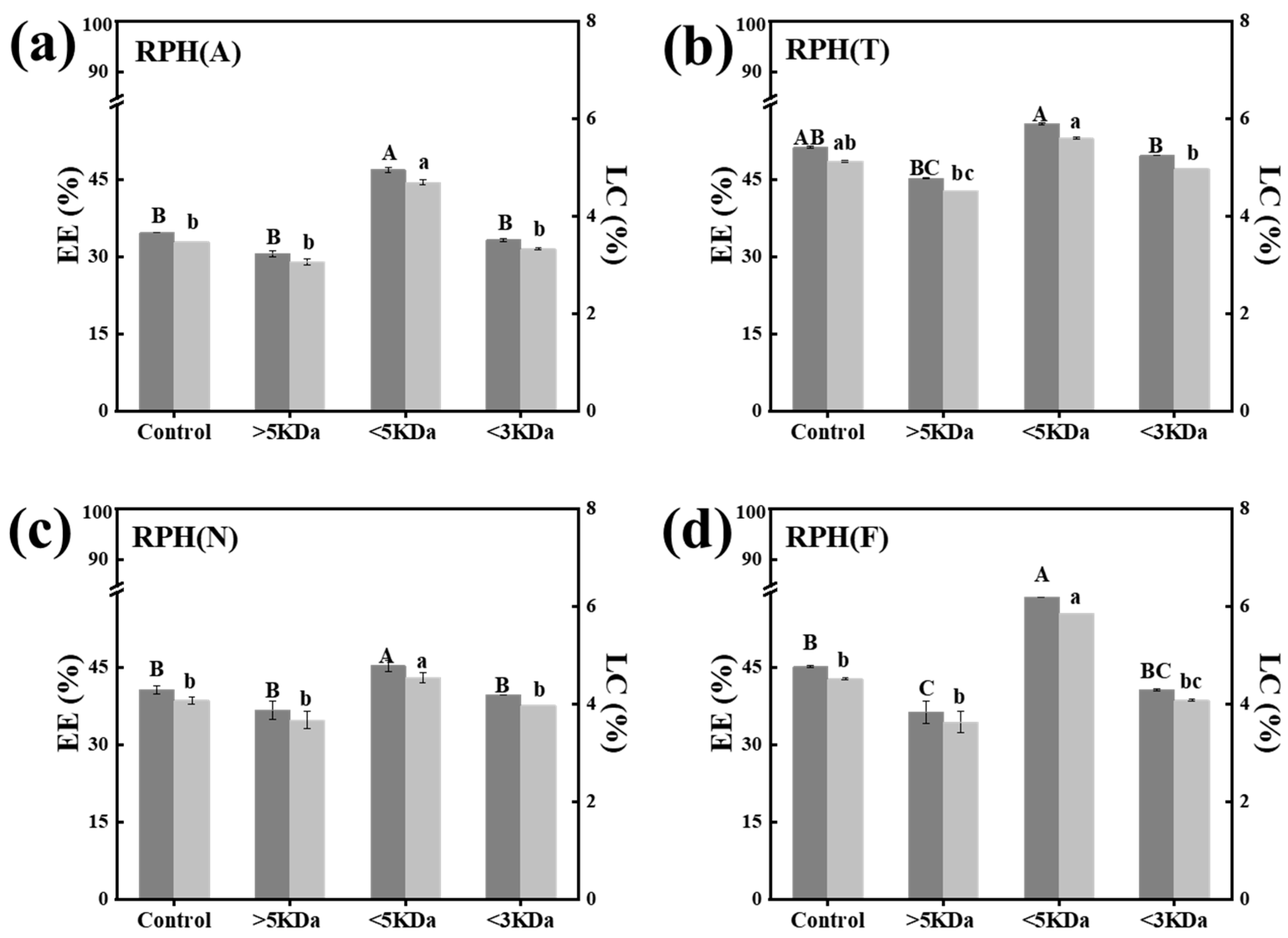
3.2. Optimization of the Preparation Conditions of RPH-SIF Nanoparticles
3.3. Interaction of RPH-SIF Nanoparticles
3.3.1. FTIR and XRD Analysis
3.3.2. Fluorescence Spectroscopy Analysis
3.3.3. Amino Acid Profile Analysis
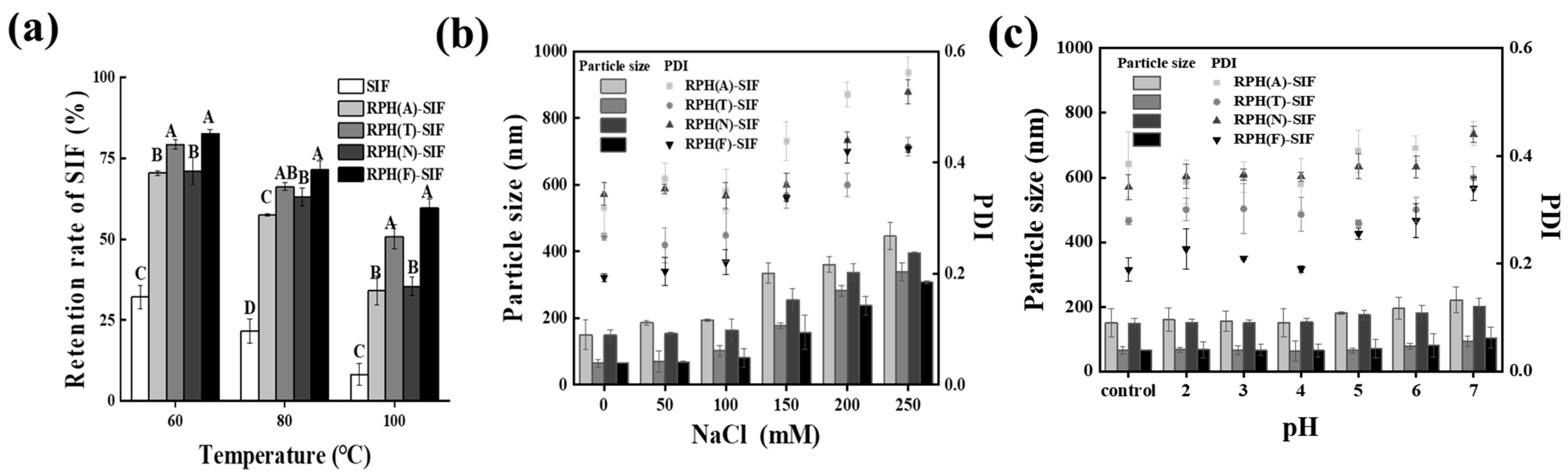
3.4. Evaluation of the Stability of RPH-SIF Nanoparticles
3.4.1. Thermal Stability
3.4.2. Ionic Stability
3.4.3. pH Stability
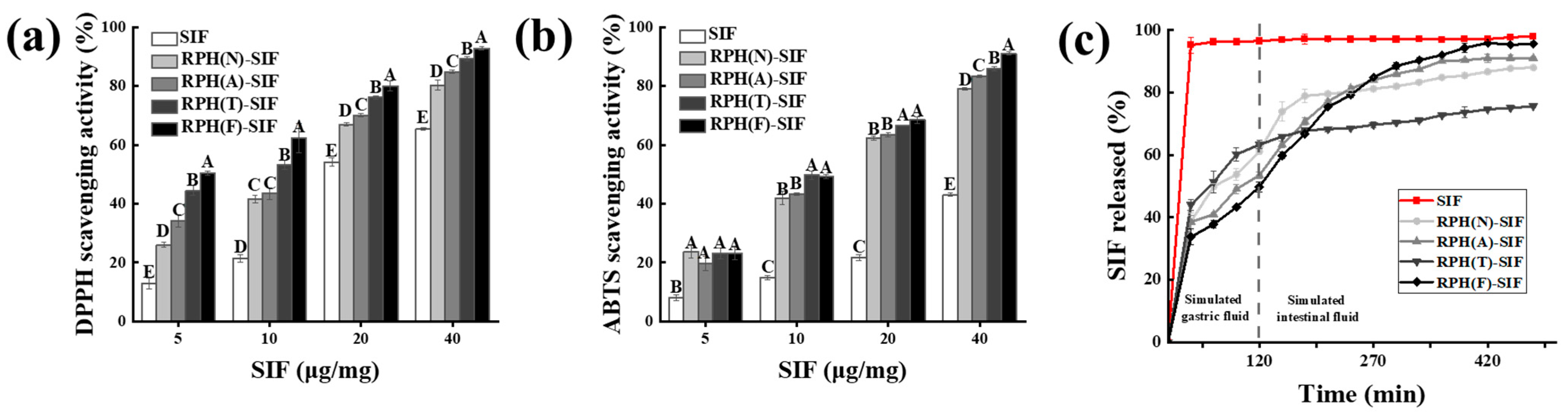
3.5. Antioxidant Activity of RPH-SIF Nanoparticles
3.6. In Vitro Release of RPH-SIF Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moras, B.; Rey, S.; Vilarem, G.; Pontalier, P.-Y. Pressurized water extraction of isoflavones by experimental design from soybean flour and Soybean Protein Isolate. Food Chem. 2017, 214, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, G.; Wójciak-Kosior, M.; Sowa, I.; Zapała, K.; Strzemski, M.; Kocjan, R. Evaluation of isoflavone content and antioxidant activity of selected soy taxa. J. Food Compos. Anal. 2017, 57, 40–48. [Google Scholar] [CrossRef]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Thatcher, N.J.; Dargham, S.R.; Kilpatrick, E.S.; Atkin, S.L. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 691–697. [Google Scholar] [CrossRef]
- Yan, L.; Spitznagel, E.L. Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am. J. Clin. Nutr. 2009, 89, 1155–1163. [Google Scholar] [CrossRef]
- Di Gioia, D.; Strahsburger, E.; de Lacey, A.M.L.; Bregola, V.; Marotti, I.; Aloisio, I.; Biavati, B.; Dinelli, G. Flavonoid bioconversion in Bifidobacterium pseudocatenulatum B7003: A potential probiotic strain for functional food development. J. Funct. Foods 2014, 7, 671–679. [Google Scholar] [CrossRef]
- Wuttke, W.; Jarry, H.; Seidlova-Wuttke, D. Isoflavones--safe food additives or dangerous drugs? Ageing Res. Rev. 2007, 6, 150–188. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Hoffmann, M.; Carle, R. Thermal degradation kinetics of isoflavone aglycones from soy and red clover. Mol. Nutr. Food Res. 2006, 50, 373–377. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Ruiz-Cruz, S.; Juárez, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; López-Ahumada, G.A.; Rodríguez-Félix, F. Gallic Acid-Loaded Zein Nanoparticles by Electrospraying Process. J. Food Sci. 2019, 84, 818–831. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.; Wang, S.; Sun, W.; Zhou, Q. Enzymatic hydrolysis of rice dreg protein: Effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012, 134, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D.J.; Wu, L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016, 61, 251–260. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Xu, P.; Qian, Y.; Wang, R.; Chen, Z.; Wang, T. Entrapping curcumin in the hydrophobic reservoir of rice proteins toward stable antioxidant nanoparticles. Food Chem. 2022, 387, 132906. [Google Scholar] [CrossRef] [PubMed]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Screening of whey protein isolate hydrolysates for their dual functionality: Influence of heat pre-treatment and enzyme specificity. Food Chem. 2013, 136, 1435–1443. [Google Scholar] [CrossRef]
- Chen, W.; Ju, X.; Aluko, R.E.; Zou, Y.; Wang, Z.; Liu, M.; He, R. Rice bran protein-based nanoemulsion carrier for improving stability and bioavailability of quercetin. Food Hydrocoll. 2020, 108, 106042. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Chen, X.-X.; Ma, M.-Y.; Xu, Y.; Wu, X.-M.; Mu, G.-Q.; Zhu, X.-M. Lutein transport systems loaded with rice protein-based self-assembled nanoparticles. Food Biosci. 2021, 42, 101061. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the Degree of Hydrolysis of Food Protein Hydrolysates by Trinitrobenzenesulfonic Acid. Am. Chem. Soc. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determind by a flourescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Kuang, L.; Jing, Z.; Wang, J.; Ma, L.; Liu, X.; Yang, J. Quantitative determination of epsilon-N-carboxymethyl-L-lysine in human plasma by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Xia, X.; Pu, N.; Yu, Z.; Nabih, M.; Zhu, Y.; Zhang, S.; Jiang, L. Preparation and physicochemical characterization of soy isoflavone (SIF) nanoparticles by a liquid antisolvent precipitation method. Adv. Powder Technol. 2019, 30, 1522–1530. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Cheng, J.; Guo, M. Development of whey protein nanoparticles as carriers to deliver soy isoflavones. LWT 2022, 155, 112953. [Google Scholar] [CrossRef]
- Liu, F.; Ma, D.; Luo, X.; Zhang, Z.; He, L.; Gao, Y.; McClements, D.J. Fabrication and characterization of protein-phenolic conjugate nanoparticles for co-delivery of curcumin and resveratrol. Food Hydrocoll. 2018, 79, 450–461. [Google Scholar] [CrossRef]
- Sakamoto, S.; Uchiyama, H.; Yusakul, G.; Kyokong, N.; Pongkitwitoon, B.; Putalun, W.; Tanaka, H.; Morimoto, S. Open sandwich fluorescence-linked immunosorbent assay for detection of soy isoflavone glycosides. Food Chem. 2021, 361, 129829. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Zhang, W.; Kou, G.; Zhou, Z. Physical stability and antioxidant activity of citrus flavonoids inarabic gum-stabilized microcapsules: Modulation of whey protein concentrate. Food Hydrocoll. 2018, 77, 588–597. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Liu, F.; Peng, F.; Li, F.; Lou, X.; Jin, Y.; Wang, J.; Xu, H. Fabrication and characterization of zein-tea polyphenols-pectin ternary complex nanoparticles as an effective hyperoside delivery system: Formation mechanism, physicochemical stability, and in vitro release property. Food Chem. 2021, 364, 130335. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, L.; Feng, S.; Liu, F.; Luo, Y. Mechanistic study on the nanocomplexation between curcumin and protein hydrolysates from Great Northern bean (Phaseolus vulgaris L.) for delivery applications in functional foods. LWT 2021, 139, 110572. [Google Scholar] [CrossRef]
- You, X.; Wang, L.; Zhang, J.; Tong, T.; Dai, C.; Chen, C.; Wu, J. Effects of Polymer Molecular Weight on In Vitro and In Vivo Performance of Nanoparticle Drug Carriers for Lymphoma Therapy. Chin. Chem. Lett. 2023, 34, 107720. [Google Scholar] [CrossRef]
- Li, X.; Xiong, H.; Yang, K.; Peng, D.; Peng, H.; Zhao, Q. Optimization of the biological processing of rice dregs into nutritional peptides with the aid of trypsin. J. Food Sci. Technol. 2012, 49, 537–546. [Google Scholar] [CrossRef]
- Kadam, D.; Palamthodi, S.; Lele, S.S. Complexation of curcumin with Lepidium sativum protein hydrolysate as a novel curcumin delivery system. Food Chem. 2019, 298, 125091. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Fu, Y.; Dai, H.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Encapsulation of β-carotene by self-assembly of rapeseed meal-derived peptides: Factor optimization and structural characterization. LWT 2021, 138, 110456. [Google Scholar] [CrossRef]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.; Wu, X. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for β-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Wang, X.; Fei, P.; Liu, F.; Xiao, Y.; Li, F.; Lei, H.; Wang, J.; Li, M.; Xu, H. Zein-pectin composite nanoparticles as an efficient hyperoside delivery system: Fabrication, characterization, and in vitro release property. LWT 2020, 133, 109869. [Google Scholar] [CrossRef]
- Tian, M.; Wang, C.; Cheng, J.; Wang, H.; Jiang, S.; Guo, M. Preparation and Characterization of Soy Isoflavones Nanoparticles Using Polymerized Goat Milk Whey Protein as Wall Material. Foods 2020, 9, 1198. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Han, Z.; Chen, S. Constructing Selenium Nanoparticles with Enhanced Storage Stability and Antioxidant Activities via Conformational Transition of Curdlan. Foods 2023, 12, 563. [Google Scholar] [CrossRef]
- Xie, H.; Xiang, C.; Li, Y.; Wang, L.; Zhang, Y.; Song, Z.; Ma, X.; Lu, X.; Lei, Q.; Fang, W. Fabrication of ovalbumin/κ-carrageenan complex nanoparticles as a novel carrier for curcumin delivery. Food Hydrocoll. 2019, 89, 111–121. [Google Scholar] [CrossRef]
- Li, L.; Yao, P. High dispersity, stability and bioaccessibility of curcumin by assembling with deamidated zein peptide. Food Chem. 2020, 319, 126577. [Google Scholar] [CrossRef]
- He, A.; Guan, X.; Song, H.; Li, S.; Huang, K. Encapsulation of epigallocatechin-gallate (EGCG) in hordein nanoparticles. Food Biosci. 2020, 37, 100727. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of Protein Association Reactions: Forces Contributing to Stability. Am. Chem. Soc. 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Anamika, S.; Vasantha, T.; Khoiroh, I.; Devunuri, N.; Venkatesu, P. Preferential and competitive role of hydrophilic/hydrophobic interactions quantifying amino acid-based ILs for papain stabilization. J. Mol. Liq. 2022, 363, 119920. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Ouyang, X.-K.; Ling, J. Fabrication of soy protein isolate/cellulose nanocrystal composite nanoparticles for curcumin delivery. Int. J. Biol. Macromol. 2020, 165 Pt A, 1468–1474. [Google Scholar] [CrossRef]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Wu, H.; Chen, H.; Shiau, C. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]

| RPH(A)-SIF | RPH(T)-SIF | RPH(N)-SIF | RPH(F)-SIF | |
|---|---|---|---|---|
| Temperature (°C) | 40 | 55 | 40 | 55 |
| Time (min) | 60 | 60 | 60 | 60 |
| pH | 5 | 5 | 3 | 4 |
| SIF/RPH ratios | 1/10 | 1/10 | 1/10 | 1/10 |
| EE (%) | 61.16 ± 0.92 | 82.86 ± 1.32 | 77.62 ± 0.31 | 90.65 ± 0.19 |
| LC (%) | 6.12 ± 0.09 | 8.29 ± 0.13 | 7.62 ± 0.03 | 9.06 ± 0.02 |
| Particle size (nm) | 77.61 ± 28.31 | 64.84 ± 11.53 | 92.91 ± 4.53 | 64.77 ± 1.34 |
| Zeta potential (mV) | −21.35 ± 1.03 | −19.45 ± 0.88 | −20.45 ± 3.24 | −25.64 ± 0.63 |
| PDI | 0.3 ± 0.02 | 0.27 ± 0.01 | 0.34 ± 0.02 | 0.19 ± 0.02 |
| Parameters | RPH(A)-SIF | RPH(T)-SIF | RPH(N)-SIF | RPH(F)-SIF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 298 K | 304 K | 310 K | 298 K | 304 K | 310 K | 298 K | 304 K | 310 K | 298 K | 304 K | 310 K | |
| KSV (L/mol) | 8802 | 7599 | 7122.5 | 15,288 | 7473.5 | 6586.6 | 10,082 | 10,370 | 5994.7 | 9191.4 | 8348.3 | 4170.2 |
| KA × 106 (L/mol) | 1.63 | 1.36 | 1.23 | 2.79 | 2.54 | 2.23 | 2.04 | 1.99 | 1.17 | 4.09 | 3.54 | 2.61 |
| n | 0.42 | 0.37 | 0.34 | 0.66 | 0.68 | 0.60 | 0.51 | 0.59 | 0.34 | 1.01 | 0.89 | 0.70 |
| △H (KJ/mol) | −18 | −14.34 | −35.12 | −28.79 | ||||||||
| △S (J/mol) | −56.42 | −39.51 | −110.78 | −84.66 | ||||||||
| △G (KJ/mol) | −1.18 | −0.84 | −0.51 | −2.56 | −2.32 | −2.09 | −2.11 | −1.44 | −0.78 | −3.57 | −3.06 | −2.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, H.; Chen, X.; Cui, B.; Chen, Y.; Chen, M.; Xu, Z.; Wen, L.; Cheng, Y.; Jiao, Y. Formation and Characterization of Self-Assembled Rice Protein Hydrolysate Nanoparticles as Soy Isoflavone Delivery Systems. Foods 2023, 12, 1523. https://doi.org/10.3390/foods12071523
Mo H, Chen X, Cui B, Chen Y, Chen M, Xu Z, Wen L, Cheng Y, Jiao Y. Formation and Characterization of Self-Assembled Rice Protein Hydrolysate Nanoparticles as Soy Isoflavone Delivery Systems. Foods. 2023; 12(7):1523. https://doi.org/10.3390/foods12071523
Chicago/Turabian StyleMo, Haoran, Xiuwen Chen, Bo Cui, Yangling Chen, Maolong Chen, Zhou Xu, Li Wen, Yunhui Cheng, and Ye Jiao. 2023. "Formation and Characterization of Self-Assembled Rice Protein Hydrolysate Nanoparticles as Soy Isoflavone Delivery Systems" Foods 12, no. 7: 1523. https://doi.org/10.3390/foods12071523
APA StyleMo, H., Chen, X., Cui, B., Chen, Y., Chen, M., Xu, Z., Wen, L., Cheng, Y., & Jiao, Y. (2023). Formation and Characterization of Self-Assembled Rice Protein Hydrolysate Nanoparticles as Soy Isoflavone Delivery Systems. Foods, 12(7), 1523. https://doi.org/10.3390/foods12071523





