Abstract
Bioaccumulation is the process by which living organisms accumulate substances, such as pesticides, heavy metals, and other pollutants, from their environment. These substances can accumulate in the organism’s tissues over time, leading to potential health risks. Bioaccumulation can occur in both aquatic and terrestrial ecosystems, and can have a significant impact on the health of both humans and wildlife. The objective of this study is to find out if the concentrations of metals in the tuna species of the Canary Islands are suitable for human consumption and if they pose a health risk. Fifteen samples of Acanthocybium solandri, Katsuwonus pelamis, Thunnus albacares, Thunnus obesus and Thunnus thynnus present in canaries were analyzed. Ten grams of muscle were taken from each specimen and the metals Al, Cd, Cr, Cu, Fe, Li, Ni, Pb and Zn were determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). The tuna species that presented more metals with a higher concentration compared to the others was T. thynnus, reaching up to 100 times more than the other studied species in Fe content with 137.8 ± 100.9 mg/Kg, which may be due to the fact that it is the largest species that reaches ages of more than fifteen years. The species Thunnus thynnus should not be suitable for commercialization according to the current legislation on the concentrations of Cd in blue fish, since 75% of the specimens studied exceeded the concentration legislated for Cd. A total of 40% of the studied specimens of this this species exceeded the legislated values for the concentration of Pb in oily fish meat, so this species must be monitored to ensure that it does not pose a risk to human health.
1. Introduction
Anthropogenic activities are the main cause of marine pollution. Residual and industrial discharges, overexploitation of fishery resources, and oil spills cause serious damage to coastlines and marine fauna. Among the different contaminants that can be found in the marine environment, metals are one of the most interesting due to their great persistence and their biomagnification through the food chain [1,2,3]. The toxic heavy metals present in the marine biota have different origins. The contribution of drainage from emerged continental and insular areas, the direct dumping of urban and industrial waste into the sea, the contribution from the atmosphere, and the underwater geological footprint itself are the main factors to be taken into account [4,5,6,7]. Biotic factors, such as the presence of organic matter in suspension, the presence of microorganisms, and the texture of sediments can also affect the biological assimilability and amplification of marine food webs. Organic matter in suspension can provide a source of nutrients for organisms, while microorganisms can break down organic matter and release nutrients into the environment. The texture of sediments can also affect the availability of nutrients and the growth of organisms [8,9,10,11]. Anthropogenic activities, such as port or industrial activities, or natural phenomena such as rock erosion, leaching or volcanic emissions, can release large amounts of metals into the marine environment. Most of the metals tend to accumulate in the bottoms, forming part of the sediments or remaining in suspension [12,13,14,15].
Multiple sources of water pollution have devastating consequences for marine life. Fish and marine mammals that are higher in the food chain are exposed to higher levels of toxins from their exposure to both contaminated water and from feeding on fish that are also exposed to them. Cadmium, mercury and lead are considered the most toxic metals for animal species and the environment [16,17,18,19,20]. These metals produce adverse biological effects in organisms, these being lethal or sub-lethal. The toxic effects of heavy metals are manifested by affecting the growth rate, physiological functions, reproduction and mortality in fish [21,22,23]. Metals can enter fish through three pathways: the gills, the digestive tract, and the skin surface. The gills are the route through which the highest rate of metal entry from the water occurs and the skin surface accounts for the lowest percentage. In the Canary Islands we have different modes of pollution: either natural, such as dust from the Sahara Desert, submarine volcanic eruptions and upwelling; or anthropogenic, such as discharges into the sea, submarine outfalls, and tourist pressure being the most important ones [24,25,26].
Tuna are found at a high trophic level in the ocean, preying on many types of large organisms such as anchovies and sardines, but also mackerel, flying fish, squid, shrimp and eels, as well as smaller tuna. The Canary archipelago is a transition zone for large tuna, so the monitoring of these species can explain the state of the ecosystem [27,28,29,30,31]. In addition, they are species of fishing interest in these islands. Environmental monitoring should also include risk monitoring, which is the determination of adverse effects on the health of consumers that may occur as a result of their exposure to food-borne hazards. The species studied in our work are pelagic fish that belong to the group of oily or fatty fish. The intake of fish from this group is generally recommended due to its content of polyunsaturated fatty acids, especially those known as omega-3 (eicosapentaenoic acid and docosahexaenoic acid) acids, whose consumption has been associated with the prevention of cardiovascular risk and the improvement of the lipid profile [32,33]. In addition, its ingestion provides proteins of high biological value, as well as a remarkable amount of vitamins (both soluble and fat-soluble) and minerals. However, consuming fish may also involve the ingestion of toxic substances that have accumulated in their tissues. There are numerous studies that show the presence of heavy metals in different species consumed by the population [34,35,36,37] in this manner. Among other toxic metal contaminants that do not fulfill any physiological function in the body, Hg, Pb and Cd can be found, among others. The legislation regulating tuna and cadmium levels are an important step in protecting consumers from the harmful effects of lead. Furthermore, it is a step towards sustainable fishing and a more responsible fishing industry. This legislation will help ensure that tuna caught are safe to eat and will help protect the oceans and their biodiversity [38].
To achieve this objective, the study analyzed the concentrations of metals in the tuna species of the Canary Islands, as well as the relationship between the metal content patterns of tuna by trophic level with ecological characteristics. The ecological characteristics of the tuna species will also be studied, including their feeding habits and habitat selection. Finally, the results of the study were used to determine the suitability of the metal concentrations for human consumption and the potential health risks associated with them.
2. Material and Methods
Seventy-five specimens of tuna found in the Canary archipelago (Figure 1) were caught by the Canarian fishing fleet in 2021 for this study. The species of the study included Acanthocybium solandri, Katsuwonus pelamis, Thunnus albacares, Thunnus obesus, and Thunnus thynnus, with fifteen specimens of each species being used in the study. The total length of each specimen was taken, and the species were identified by marine fishery biologists of the Canary Islands.
Figure 1.
Map showing the location of the study specimens.
2.1. Sample Processing
Ten grams of muscle were taken from each specimen and homogenized. The samples were dried in an oven at a temperature of 70 °C for 24 h. They were then incinerated in a muffle oven for 48 h at 450 °C ± 25 °C until white ash was obtained. If after this time the total mineralization of the samples was not achieved (white or greyish-white ashes), 65% HNO3 was added to them in the fume hood, and they were subsequently evaporated on a heating plate at 70–90 °C. Once treated, they were re-incinerated in a muffle oven at 450 °C ± 25 °C until white ashes were obtained. The determination of the metal content was determined by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES). Table 1 shows the detection limits for each metal with the wavelengths for the measurement of each one [39].

Table 1.
Limits of detection and quantification of each metal measured by ICP-OES.
A quality control solution was used to evaluate the accuracy of the determinations in every ten samples. The precision of the analytical procedure was evaluated by analyzing the international standard reference materials DORM-1 and DORM-5 (National Research Council of Canada). All data is presented in milligrams per kilogram, wet weight, and the metals analyzed were Al, Cd, Cr, Cu, Fe, Li, Pb and Zn. The blanks and standard reference materials were analyzed together with the samples (Table 1) [40]. As there was little sample tissue, only these metals were analyzed, leaving the analysis of Hg and isotopes for later studies.
2.2. Statistical Analysis
In order to verify whether there was variation in the metal and trace element content and composition, a permutational multivariate analysis of distance (PERMANOVA) was performed with Euclidean distances [41]. A unidirectional design with the fixed factor “species” with five levels of variation was used, according to of the Acanthocybium solandri, Katsuwonus pelamis, Thunnus albacares, Thunnus obesus and Thunnus thynnus species.
An analysis of principal coordinates (PCA) was used, taking as a factor the five species analyzed (Acanthocybium solandri, Katsuwonus pelamis, Thunnus albacares, Thunnus obesus and Thunnus thynnus); these being the concentrations of metals and trace elements of each sample, indicating which variable is best explained by univariate evaluations. A total of 9999 permutations underwent analysis for pairwise comparison, and thus the determination of whether or not there are significant references (p value < 0.05) [42]. The isotope data used were collected from published studies of the same species in the Atlantic Ocean.
2.3. Risk Assessment
The risk assessment, the Acceptable Daily Intake (ADI), was determined according to the formulas given in [43]. The toxic assessment was carried out by following the recommendations of AESAN (the Spanish Agency for Food Safety and Nutrition) [44] on the consumption of fish throughout the week (three servings of 250 g) in adults with a mean weight of 70 kg and taking into account the reference values of Recommended Daily Intakes (RDIs) given by FESNAD, Estimated Daily Intake (EDIs) and Acceptable Daily Intakes (ADIs) [45] in metal (Al, Cd, Pb). For the calculations, an average weight of 70 kg was used. NOAEL is the toxicity index determined in the “toxicological evaluation” process, and F is a factor which we take as 100 since the interspecific [10] and intraspecific [10] variables are taken into account, and EDI characterizes the estimated daily intake of metal through the consumption of aquatic organism for an adult (μg Kg−1/day); Cmetal is the concentration of metal in organism (μg Kg−1) wet weight; Cons signifies the day-to-day consumption of seafood (g/day) in wet weight; and Bw is the body weight (Kg) of an adult [46].
ADI = NOAEL/F
EDI = (C metal × Cons)/Bw
MoS = IDE/IDA
EDI = (C metal × Cons)/Bw
MoS = IDE/IDA
CDI is the chronic daily intake dose of carcinogenic elements (mg/kg/day), and carcinogenic risk (CR) is quantified by the chemical element cancer slope factor (SF). The human health risk of heavy metal intake was evaluated based on the chronic daily intake dose (CDI) for a chemical contaminant in the tuna fish over the exposure period and the fish intake quantity. CDI (mg/kg/day) was calculated using the following equation:
CDI = (C × IR × EF × ED)/(BW × At)
CDI is the chronic daily dose of fish intake; C is the concentration of heavy metals present in the samples (mg/kg); IR is the intake rate (104.7 g/day); EF is the frequency of exposure (three times per week = 156 days/year); ED is the duration of exposure (lifetime exposure = 30 years); BW is the body weight (kg), which we assumed to be 70 kg for an adult. AT is the mean time (AT = SD × 365 days/year). The mean daily fish consumption was set at 130 g/day, which is close to the recommended amount [47].
3. Results and Discussion
The length of Acanthocybium solandri was 105 ± 12 cm, that of Katsuwonus pelamis was 76 ± 0.13 cm, that of Thunnus albacares was 175 ±19 cm, that of Thunnus obesus was 192 ± 20 cm, and for that of Thunnus thynnus, which was 235 ± 41 cm, biometric data was only available for half of the specimens, since many samples were chosen by local fishermen.
The results obtained in Table S1 are contrasted in Figure 2. This figure shows that the metal in which the species differ least significantly is Zn. These results were clearly visible in the PCA analysis, which accounted for about 95.5% of the total variability of the data (Figure 2). The ordination of the samples showed a clear difference in the heavy metal content of the Thunnus thynnus species with respect to the other four species. These groups appeared to be separated at a shorter distance than T. thynnus. Table S1 shows significant differences between the species in their metal content in most of them, which is what is reflected in the PCA with the variability with the vectors (metals and trace elements used). The metals that best explained the variability found in the data are represented as vectors in the PCA, which showed a clear increasing pattern for Zn, Cr, Cu and Fe in T. thynnus compared to the other species, despite the large variability between samples. However, A. solandri and T. albacares do not differ significantly in the content of Al, Cr, Li, Pb and Zn. The same occurs between A. solandri and T. obesus, although in this case, there are no significant differences in the content of Cd, Cr, Pb and Zn. The last pair of species that showed less significant differences in metal content is that of T. albacares and T. obesus, with no significant differences in Cu, Fe and Pb.
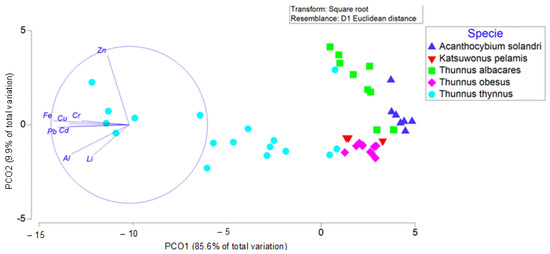
Figure 2.
Principal component analysis (PCoA) based on Euclidean distances of square root transformed data of heavy metals and metal element content in the study species.
On the other hand, those that differ most significantly are Al and Cd. In the case of Al, there is only no significant difference in the content between A. solandri and T. albacares, and in the case of Cd, there is no significant difference between A. solandri and T. obesus. It was also observed that the species that differs most significantly in metal content is T. thynnus, which was found to have higher values than those found in the other four species (Figure 2 and Table 2).

Table 2.
Metal concentrations in mg/kg for the five species analyzed (mean and standard deviation).
Table 2 shows the concentrations of the metals obtained in each tuna species, noting that T. thynnus presents a higher concentration of all metals compared to the other species. As regards Al, T. thynnus is the tuna species with the highest concentration of Al, with 19.56 ± 11.33 mg/kg (Table 2). Al concentrations in oceanic waters are low, in the order of nM, despite the fact that it is the third most abundant element in the earth’s crust and that it undergoes active transport to the oceans. The low concentrations of Al in the ocean are the result of the high reactivity of the element and its short amount of time spent in oceanic waters. Since Al bioaccumulates over the years, it is normal for the species that grows to the greatest size and age to be the one with the highest content of this metal [48,49,50,51]. One of the tissues in which Al accumulates the most is in the muscle, only being surpassed by the skin. In the present study, A. solandri was the species with the lowest concentration of Al, with a value of 0.515 ± 0.214 mg/kg. This may be due to the fact that, together with K. pelamis, it is the smallest species [52,53]. Regarding Cd, T. thynnus is the species with the highest concentration of Cd, with 0.051 ± 0.039 mg/kg. Rivers transport significant amounts of Cd to the oceans, from erosion processes, forming in the same large reservoirs where it is estimated that it can remain for 15,000 years. The Cd content in the oceans is around 0.1 µg/kg [54,55,56]. Cd accumulates mostly in the liver, so the high value obtained in the muscle tissue of T. thynnus should be monitored because the level of this metal is above the maximum content allowed (according to [57]) that sets the maximum content of different contaminants in food products, with the maximum legal limit of 0.05 mg of Cd/kg of fresh weight for fish meat, meaning that it would not be suitable for human consumption. [58] found higher concentrations of Cd in the muscle of K. pelamis compared to other larger tuna species, such as T. obesus. This has also been observed in the present study, since K. pelamis is the species with the second highest content of this heavy metal (Table 2). This may be due to the opportunistic diet based on invertebrates followed by K. pelamis [59,60], since crustaceans and mollusks are capable of accumulating higher levels of Cd than other organisms of a higher trophic level [18,61,62]. In addition, Table 2 shows the high concentrations of Zn in the different species compared to low concentrations of Cd, and this can be explained by the antagonism between these two elements, since Cd competes for the active site of Zn [63,64]. In the case of Cr, T. thynnus is the species with the highest Cr concentration compared to the other species, with 0.913 ± 1.263 mg/kg. Cr is one of the elements that can be found in wastewater from a wide variety of industrial processes. Its toxicity depends on the oxidation state and concentration in which it is found, and is of special importance the elimination of hexavalent chromium present in aqueous systems [65]. The concentration of Cr and its effects on teleost fish can be significantly modified by biological and abiotic variables such as water temperature and pH, the presence of other contaminating metals, and sex and tissue specificity [66,67]. K. pelamis has a lower concentration of this metal with 0.049 ± 0.011 mg/kg. In this case, the bioaccumulation effect is observed, since T. thynnus is the largest species and K. pelamis is the smallest. T. thynnus is also the species with the highest concentration of Cu, with 1526 ± 1114 mg/kg. In the sea, Cu is found at approximately 2.5 × 10−4 mg/L. Its presence is notably lower in the ocean the further one moves from the coast [68]. This explains why K. pelamis is the smallest species and is the species with the second highest concentration of Cu, as it is a species that comes closer to the coast and feeds on a greater number of crustaceans than the other species in the study. Crustaceans are rich in Cu, so K. pelamis can accumulate a higher concentration of this metal by ingesting them [69,70]. On the other hand, Fe is an important metal present in fish that migrate long distances, since they require strong swimming muscle tissue that requires high concentrations of blood that is rich in Fe. An Fe concentration of 137.8 ± 100.9 mg/kg has been found in T. thynnus, with this concentration being 100 times higher than in the other species, which can be explained by the large size that this species can grow to [71,72,73]. Regarding Pb, according to Regulation (EC) No. 1881/2006 [74,75,76], none of the species exceeded the maximum permissible concentration of 0.30 mg/kg of fresh weight for fish meat.
The combination of contaminants and stable isotopes of carbon and nitrogen can be a powerful tool to analyze the trophic transfer of contaminants through foods [77]. This is because the isotopic composition of an animal’s tissue mirrors that of its prey, with a slight trophic enrichment of δ13C and δ15N of the order of 1‰ and 3.4‰, respectively [78,79]. δ15N is used to estimate trophic position [80], while δ13C is used to indicate relative dietary contributions from different primary sources in a food web [81]. δ13C values are typically higher (less negative) in coastal areas or benthic food webs than in pelagic food webs [81]. There are studies that have correlated stable nitrogen isotopes with concentrations of pollutants, such as Hg in fish tissues [82]. Therefore, the metal content found in the present study in the analyzed specimens is related to the values of δ13C and δ15N found in the same species in other studies (Table 3). Figure 3 shows that the species with the highest trophic level is T. thynnus, and this indicates that it is the species with the highest metal content due to the biomagnification process, which is the successive propagation of bioconcentration between the links in a food web [83]. The same occurs in T. obesus, as it has both a high metal content and a high trophic level. It is noteworthy that in the case of T. albacares which, despite having a high content of metals (as Table 3 shows), its trophic level is lower than that of other species of the same genus. [84] conclude that the trophic level of T. albacares has decreased in recent years, and that this species has high trophic plasticity related to a flexible and opportunistic diet throughout the year [30]. On the other hand, there is no relationship between the high trophic level of A. solandri, as can be seen in Figure 3, and the low metal concentration found in the present study. This is because A. solandri has a high degree of parasitism, as reported in the study by [85], and nutritional stress generated by poor diet quality or starvation can affect δ15N enrichment [86,87]. In the last species studied, K. pelamis, the relationship between the metal content of individuals and their trophic level was confirmed. It can be concluded that, as in other studies such as the one by [88], it has been proven that associating the transfer of trace metals with the stable isotopes of carbon and nitrogen is useful in defining the degree of the trophic transfer of pollutants.

Table 3.
Values of δ13C and δ15N found in Acanthocybium solandri, Katsuwonus pelamis, Thunnus albacares, Thunnus obesus and Thunnus thynnus collected in the Atlantic Ocean. Results are expressed as mean ± standard deviation or as ranges.
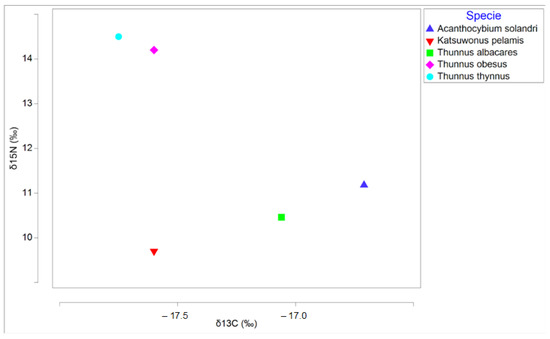
Figure 3.
Graph showing trophic relationships taken from studies in the Atlantic.
On the other hand, the results of the metal concentrations found in the study species have been compared with the results of other studies. In the Canary Islands, this type of study on the metal concentration in marine species is important because, in addition to the contribution arising from anthropogenic contamination, the soils of the volcanic regions act as metal reservoirs [92].
Table 4 shows that the values obtained in A. solandri in the present study are similar to those reported in Ghana by [93], with the exception of Pb. The concentration of Pb found in Ghana exceeds the maximum content allowed (according to [94]) which sets the maximum content of various contaminants in food products, setting the maximum legal limit at 0.30 mg of Pb/kg of fresh weight for fish meat. In the case of K. pelamis, one can see that the concentrations obtained in the present study are the lowest, with only the Pb value not exceeding the maximum allowed in [94]. In addition, it is worth mentioning the difference between the Cd and Pb content between this study and one undertaken in the Azores [58], despite both being located in the eastern Atlantic Ocean and the islands being of volcanic origin. The study by [58] confirmed that there is an important volcanic source of Cd in the waters of the mid-Atlantic region, so commercial marine species for human consumption need to be carefully monitored. Similarly, the aforementioned study confirmed that volcanic activity accounts for an important contribution of Pb in the Azores but that, due to the low content of Pb found in the species in the study, it seems that volcanic activity does not have a measurable effect on Pb in K. pelamis in the Azores archipelago. It is noteworthy that the specimens of T. albacares in the present study have lower values in Cd and Pb than those found in Mexico and Ecuador, as can be seen in Table 5. The lowest metal concentrations are also observed in T. obesus in this study, compared to the results of [58,95]. The same did not occur with T. thynnus, where the values of the present study are higher than those found in Canada, Italy and Turkey [96,97,98]. The exceptions to this is Cd, which was surpassed by the concentration in Italy (0.051 mg/kg versus 0.26 mg/kg), and Zn, which was found in the lowest concentration in the present study. Using the tuna species studied here as bioindicators, it can generally be concluded that the Atlantic Ocean is one of the least polluted oceans in the world.

Table 4.
Metal comparison of the species K. pelamis and A. sonlandri with other studies in mg/kg.

Table 5.
Metal comparison of the species T. albacares, T. obesus and T. thynnus with other studies in mg/kg.
Risk Assessment
Table 6 shows the consumption risk assessment for humans of the species studied for Al, Cd and Pb. To assess the toxic risk, three servings of 250 g each throughout the week have been estimated to be an adequate intake of the five species of the study. Table 6 shows the estimated daily intake of each metal for the study species (EDI), in addition to the calculation of the Margin of Safety. Metals are those with fixed ADI (Al, Cd and Pb). No metal exceeds or comes close to the MoS, so, considering the proposed intake scenario, there is no toxic risk to the health of consumers, since the reference values established by the European Food Safety Authority were not exceeded. The exception to this is Pb for T. thynnus, whose value is 0.85 and can present serious health risks in terms of the consumption of these species with regard to the recommended consumption guidelines. With the data obtained with regard to the EFSA, the ration of fish meat is 250 g three times a week, so none of the species except T. thynnus exceed the limits. For the three metals studied, the consumption of the established 750 g per week would exceed the IDE, so continued consumption throughout the life of this tuna meat can be dangerous.

Table 6.
Heavy metal risk assessment for the species studied in adults.
Cadmium levels in muscle are below the maximum content allowed according to Regulation (CE) No 420/2011 (2011), which sets the maximum content of various contaminants in food products, establishing the maximum legal limit at 0.05 mg of Cd/kg of fresh weight for fish meat, except for T. thynnus, whose average concentration was 0.051 ± 0.039 mg/kg. According to these results, for the specimens of this species that exceed the maximum admissible quantities, which are 75% of the specimens studied, T. thynnus should be declared as a species not suitable for commercialization, since it does not comply with the regulations.
The current European legislation on cadmium in tuna is Directive 2006/66/EC, which establishes that the total cadmium in tuna must not exceed 0.3 mg/kg. This directive applies to all species of tuna, both bluefin tuna and albacore tuna. [57]. T. thynnus with 0.280 ± 0.188 mg/kg is close to the value 40% of the studied specimens of this species that exceeded the legislated value. Taking into account the concentrations obtained in our work, a more exhaustive study should be carried out for the species T. thynnus, since many of the analyzed specimens exceed the legislated value as fit for sale. The Tuna Lead Legislation is a provision of the Food and Agriculture Organization of the United Nations (FAO) that limits the use of lead in tuna weighing. This legislation was created with the goal of protecting consumers from the negative effects that lead can have on human health. This legislation is one of the main measures of the FAO to promote the sustainability of tuna fishing. For the chronic daily dose obtained, all values in all species were low, so there would be no problem, except for Al for T. thynnus, with a value of 1.4. Therefore, special surveillance and the continuous monitoring of the metal content of this species should be carried out to ensure that it does not pose a risk to the population if the AE-SAN recommendations are surpassed.
4. Conclusions
Thunnus thynnus has the highest number of metals in higher concentrations compared to the other tuna species due to the large size of the species. T. thynnus is the species with the highest trophic level, and this is because it has the highest metal content as a result of the biomagnification process, which is the successive propagation of bioconcentration between the links in a food web. Furthermore, this species differs most significantly with regard to the content of metals from the other species studied.
A. solandri was found to have the lowest concentration of the heavy metals in the present study. This may be because A. solandri and K. pelamis are the smallest of the study species. A. solandri and T. albacares do not differ significantly in the content of Al, Cr, Li, Pb, and Zn. The same is true between A. solandri and T. obesus, although in this case there are no significant differences in the content of Cd, Cr, Pb and Zn. The last pair of species that show less significant differences in metal content are T. albacares and T. obesus, without significant differences found in Cu, Fe and Pb.
Furthermore, this study shows that associating the transfer of trace metals with the stable isotopes of carbon and nitrogen is useful in defining the degree of trophic transfer of pollutants. Finally, using the species studied here (Acanthocybium solandri, Katsuwonus pelamis, Thunnus albacares, Thunnus obesus and Thunnus thynnus) as bioindicators and comparing metal concentrations with studies by other authors, one can conclude that the Atlantic Ocean is one of the least polluted oceans in the world.
The specimens of Thunnus thynnus captured in the Canary Islands present a risk to human health, since the concentrations of Cd and Pb present very high values. The EFSA recommends eating 750 g of fish meat a week, but one were to eat 750 g of T. Thynnus throughout their life they would have serious health problems.
The species Thunnus thynnus should not be suitable for commercialization according to the current legislation on the concentrations of Cd in blue fish, since 75% of the specimens studied exceeded the concentration legislated for Cd. A total of 40% of the specimens of this species exceeded the legislated values for the concentration of Pb in oily fish meat, so this species must be monitored with great effort so that it does not pose a risk to human health. Taking into account the concentrations obtained in our work, a more exhaustive study should be carried out for the species T. thynnus, since many of the analyzed specimens exceed recommended amounts; in addition, analyses should be carried out to verify the levels of organic pesticides and persistents that may be present in the tuna.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12071438/s1, Figure S1. Box plot of metals concentrations in tuna species of the Canary Islands in mg/kg. Table S1. Results of the pairwise test examining the significant factor "species" obtained in ANOVA for each metal.
Author Contributions
Conceptualization, C.R.; Methodology, Á.J.G.; Validation, S.P.-M.; Formal analysis, E.L.-B. and D.G.W.; Investigation, I.D.-S., S.P.-M., J.J.P.-F., C.R. and Á.J.G.; Data curation, A.H. and D.G.W.; Writing—original draft, E.L.-B. and I.D.-S.; Writing—review & editing, S.P.-M., A.H. and Á.J.G.; Supervision, J.J.P.-F.; Funding acquisition, J.J.P.-F. and Á.J.G. All authors have read and agreed to the published version of the manuscript.
Funding
FoodE, Food Systems in European Cities: Grant Agreement 862663 — H2020; Tuna Analysis Research Contract with the Government of the Canary Islands, Fishing General Direction, number A21100059.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
The authors would like to thank all the crew who participated on the Tuna Analysis Research Contract (A21100059) and FoodE, Food Systems in European Cities (Grant agreement number 862663—H2020), financed by the European Union.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dalman, Ö.; Demirak, A.; Balcı, A. Determination of heavy metals (Cd, Pb) and trace elements (Cu, Zn) in sediments and fish of the Southeastern Aegean Sea (Turkey) by atomic absorption spectrometry. Food Chem. 2006, 95, 157–162. [Google Scholar] [CrossRef]
- Topcuo, S. Heavy metal monitoring of marine algae from the Turkish Coast of the Black Sea, 1998–2000. Chemosphere 2003, 52, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.-G.; Paz, S.; Gutiérrez, Á.J. Seasonal and ontogenic variations of metal content in the European pilchard (Sardina pilchardus) in northwestern African waters. Environ. Pollut. 2020, 266, 115113. [Google Scholar] [CrossRef] [PubMed]
- Anbuselvan, N.; Sridharan, M. Heavy metal assessment in surface sediments off Coromandel Coast of India: Implication on marine pollution. Mar. Pollut. Bull. 2018, 131, 712–726. [Google Scholar]
- Liu, X.; Xu, L.; Chen, Q.; Sun, L.; Wang, Y.; Yan, H.; Liu, Y. Chemosphere Historical change of mercury pollution in remote Yongle archipelago, South China Sea. Chemosphere 2012, 87, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tong, Y.; Chen, C.; Liu, X.; Lu, Y.; Zhang, W.; He, W.; Wang, X.; Zhao, S.; Lin, Y. Ecological risk assessment to marine organisms induced by heavy metals in China’s coastal waters. Mar. Pollut. Bull. 2018, 126, 349–356. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, X.; Zeng, J.; Shi, X.; Liao, Y.; Du, P.; Tang, Y.; Huang, W.; Chen, Q.; Shou, L. Heavy metal concentrations in commercial marine organisms from Xiangshan Bay, China, and the potential health risks. Mar. Pollut. Bull. 2019, 141, 215–226. [Google Scholar] [CrossRef]
- Dehn, L.-A.; Follmann, E.H.; Thomas, D.L.; Sheffield, G.G.; Rosa, C.; Duffy, L.K.; O’Hara, T.M. Trophic relationships in an Arctic food web and implications for trace metal transfer. Sci. Total Environ. 2006, 362, 103–123. [Google Scholar] [CrossRef]
- Kuijper, L.D.J.; Kooi, B.W.; Zonneveld, C.; Kooijman, S.A.L.M. Omnivory and food web dynamics. Ecol. Modell. 2003, 163, 19–32. [Google Scholar] [CrossRef]
- Pimm, S.L.; Lawton, J.H.; Cohen, J.E. Food web patterns and their consequences. Nature 1991, 350, 669–674. [Google Scholar] [CrossRef]
- Pringle, R.M.; Hutchinson, M.C. Resolving food-web structure. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 55–80. [Google Scholar] [CrossRef]
- Almeida, C.; Vaz, S.; Cabral, H.; Ziegler, F. Environmental assessment of sardine (Sardina pilchardus) purse seine fishery in Portugal with LCA methodology including biological impact categories. Int. J. Life Cycle Assess. 2014, 19, 297–306. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; García-Gómez, J.C. Assessing pollution levels in sediments of a harbour with two opposing entrances. Environmental implications. J. Environ. Manag. 2005, 77, 1–11. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Alcázar-Treviño, J.; Alduán, M.; Lozano, G.; Hardisson, A.; Rubio, C.; González-Weller, D.; Paz, S.; Carrillo, M.; Gutiérrez, Á.J. Metal content in stranded pelagic vs deep-diving cetaceans in the Canary Islands. Chemosphere 2021, 285, 131441. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, C.; Obenat, S.; Velazquez, S.N.; Gancedo, B.; Spivak, E. Seasonal variation of peracarid assemblages in natural and artificial marine environments of the Southwestern Atlantic Ocean. Mar. Biodivers 2018, 48, 1743–1754. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, T.; Yasunaga, G.; Iwata, H.; Subramanian, A.; Ismail, A.; Tanabe, S. Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Mar. Pollut. Bull. 2005, 51, 896–911. [Google Scholar] [CrossRef]
- Capelli, R.; Das, K.; De Pellegrini, R.; Drava, G.; Lepoint, G.; Miglio, C.; Minganti, V.; Poggi, R. Distribution of trace elements in organs of six species of cetaceans from the Ligurian Sea (Mediterranean), and the relationship with stable carbon and nitrogen ratios. Sci. Total Environ. 2008, 390, 569–578. [Google Scholar] [CrossRef]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Ontogenic and seasonal variations of metal content in a small pelagic fish (Trachurus picturatus) in northwestern African waters. Mar. Pollut. Bull. 2020, 156, 111251. [Google Scholar] [CrossRef]
- Copat, C.; Grasso, A.; Fiore, M.; Cristaldi, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. Trace elements in seafood from the Mediterranean sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 13–19. [Google Scholar] [CrossRef]
- Garcia-Garin, O.; Vighi, M.; Aguilar, A.; Tsangaris, C.; Digka, N.; Kaberi, H.; Borrell, A. Boops boops as a bioindicator of microplastic pollution along the Spanish Catalan coast. Mar. Pollut. Bull. 2019, 149, 110648. [Google Scholar] [CrossRef]
- Özden, Ö. Monitoring Programme on Toxic Metal in Bluefish (Pomatomus saltatrix), Anchovy (Engraulis encrasicolus) and Sardine (Sardina pilchardus) from Istanbul, Turkey: Levels and Estimated Weekly Intake. Bull. Environ. Contam. Toxicol. 2013, 90, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Özden, Ö.; Erkan, N.; Ulusoy, S. Determination of mineral composition in three commercial fish species (Solea solea, Mullus surmuletus, and Merlangius merlangus). Environ. Monit. Assess. 2010, 170, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Playle, R.C.; Dixon, D.G.; Burnison, K. Copper and cadmium binding to fish gills: Estimates of metal–gill stability constants and modelling of metal accumulation. Can. J. Fish. Aquat. Sci. 1993, 50, 2678–2687. [Google Scholar] [CrossRef]
- Playle, R.C. Modelling metal interactions at fish gills. Sci. Total Environ. 1998, 219, 147–163. [Google Scholar] [CrossRef]
- Uysal, K.; Emre, Y.; Köse, E. The determination of heavy metal accumulation ratios in muscle, skin and gills of some migratory fish species by inductively coupled plasma-optical emission spectrometry (ICP-OES) in Beymelek Lagoon (Antalya/Turkey). Microchem. J. 2008, 90, 67–70. [Google Scholar] [CrossRef]
- Gleiber, M.R.; Sponaugle, S.; Cowen, R.K. Some like it hot, hungry tunas do not! Implications of temperature and plankton food web dynamics on growth and diet of tropical tuna larvae. ICES J. Mar. Sci. 2020, 77, 3058–3073. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Clemente, S.; Espinosa, J.M.; Jurado-Ruzafa, A.; Lozano, G.; Raimundo, J.; Hardisson, A.; Rubio, C.; González-Weller, D.; Jiménez, S.; et al. Inferring trophic groups of fish in the central-east Atlantic from eco-toxicological characterization. Chemosphere 2019, 229, 247–255. [Google Scholar] [CrossRef]
- Madigan, D.J.; Carlisle, A.B.; Dewar, H.; Snodgrass, O.E.; Litvin, S.Y.; Micheli, F.; Block, B.A. Stable isotope analysis challenges wasp-waist food web assumptions in an upwelling pelagic ecosystem. Sci. Rep. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Olson, R.J.; Popp, B.N.; Graham, B.S.; López-Ibarra, G.A.; Galván-Magaña, F.; Lennert-Cody, C.E.; Bocanegra-Castillo, N.; Wallsgrove, N.J.; Gier, E.; Alatorre-Ramírez, V. Food-web inferences of stable isotope spatial patterns in copepods and yellowfin tuna in the pelagic eastern Pacific Ocean. Prog. Oceanogr. 2010, 86, 124–138. [Google Scholar] [CrossRef]
- Sutton, S.G.; Ditton, R.B. Understanding catch-and-release behavior among US Atlantic bluefin tuna anglers. Hum. Dimens. Wildl. 2001, 6, 49–66. [Google Scholar] [CrossRef]
- Dewey, A.; Baughan, C.; Dean, T.P.; Higgins, B.; Johnson, I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef] [PubMed]
- Rambjør, G.S.; Windsor, S.L.; Harris, W.S. Eicosapentaenoic acid is primarily responsible for hypotriglyceridemic effect of fish oil in humans. Lipids 1996, 31, S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.; Gutiérrez, Á.J.; Lozano, G.; González-Weller, D.; Lozano-Bilbao, E.; Rubio, C.; Caballero, J.M.; Revert, C.; Hardisson, A. Metals in Diplodus sargus cadenati and Sparisoma cretense—A risk assessment for consumers. Environ. Sci. Pollut. Res. 2018, 25, 2630–2642. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Bilbao, E.; Jurado-Ruzafa, A.; Lozano, G.; Jiménez, S.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Development stage and season influence in the metal content of small pelagic fish in the North-West Africa. Chemosphere 2020, 261, 127692. [Google Scholar] [CrossRef]
- Has-Schön, E.; Bogut, I.; Strelec, I. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006, 50, 545–551. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Hao, Q.; Xu, X.-R.; Luo, X.-J.; Wang, S.-L.; Zhang, Z.-W.; Mai, B.-X. Persistent organic pollutants in marine fish from Yongxing Island, South China Sea: Levels, composition profiles and human dietary exposure assessment. Chemosphere 2014, 98, 84–90. [Google Scholar] [CrossRef]
- Li, N.; Hou, Y.; Ma, D.; Jing, W.; Dahms, H.-U.; Wang, L. Lead accumulation, oxidative damage and histopathological alteration in testes and accessory glands of freshwater crab, Sinopotamon henanense, induced by acute lead exposure. Ecotoxicol. Environ. Saf. 2015, 117, 20–27. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Espinosa, J.M.; Lozano, G.; Hardisson, A.; Rubio, C.; González-Weller, D.; Gutiérrez, Á.J. Determination of metals in Anemonia sulcata (Pennant, 1777) as a pollution bioindicator. Environ. Sci. Pollut. Res. 2020, 27, 21621–21627. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Adern, N.; Hardisson, A.; González-Weller, D.; Rubio, C.; Paz, S.; Pérez, J.A.; Zupa, R.; Gutiérrez, Á.J. Differences in macroelements, trace elements and toxic metals between wild and captive-reared greater amberjack (Seriola dumerili) from the Mediterranean Sea. Mar. Pollut. Bull. 2021, 170, 112637. [Google Scholar] [CrossRef]
- Anderson, M.; Braak, C. Ter Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Anderson, M.R. The Resource for the Power Industry Professional. Proc. ASME Power 2004, 32, 35–40. [Google Scholar] [CrossRef]
- Renwick, A.G. Safety factors and establishment of acceptable daily intakes. Food Addit. Contam. 1991, 8, 135–149. [Google Scholar] [CrossRef] [PubMed]
- AESAN. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) Regarding Criteria for the Estimation of Concentrations for the Discussion Proposals for Migration Limits of Certain Heavy Metals and Other Elements from Ceramic; AESAN: Madrid, Spain, 2012. [Google Scholar]
- FESNAD. Ingestas Dietéticas de Referencia (IDR) para la Población Española. Act. Diet 2010, 14, 196–197. [Google Scholar]
- Lozano-Bilbao, E.; Domínguez, D.; González, J.A.; Lorenzo, J.M.; Lozano, G.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Risk assessment and study of trace/heavy metals in three species of fish of commercial interest on the island of El Hierro (Canary Islands, eastern-central Atlantic). J. Food Compos. Anal. 2021, 99, 103855. [Google Scholar] [CrossRef]
- de Lima, N.V.; Granja Arakaki, D.; Melo, E.S.d.P.; Machate, D.J.; do Nascimento, V.A. Assessment of trace elements supply in canned tuna fish commercialized for human consumption in Brazil. Int. J. Environ. Res. Public Health 2021, 18, 12002. [Google Scholar] [CrossRef]
- Measures, C.I.; Vink, S. On the use of dissolved aluminum in surface waters to estimate dust deposition to the ocean. Global Biogeochem. Cycles 2000, 14, 317–327. [Google Scholar] [CrossRef]
- Orians, K.J.; Bruland, K.W. The biogeochemistry of aluminum in the Pacific Ocean. Earth Planet. Sci. Lett. 1986, 78, 397–410. [Google Scholar] [CrossRef]
- Sackett, W.; Arrhenius, G. Distribution of aluminum species in the hydrosphere—I Aluminum in the ocean. Geochim. Cosmochim. Acta 1962, 26, 955–968. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Influence of Biometric and Seasonal Parameters on the Metal Content of Scomber colias in Northwestern African Waters. Biol. Trace Elem. Res. 2021, 199, 3886–3897. [Google Scholar] [CrossRef]
- Berg, D.J.; Burns, T.A. The distribution of aluminum in the tissues of three fish species. J. Freshw. Ecol. 1985, 3, 113–120. [Google Scholar] [CrossRef]
- Sparling, D.W.; Lowe, T.P.; Campbell, P.G.C. Ecotoxicology of aluminum to fish and wildlife. In Research Issues in Aluminum Toxicity; CRC: Boca Raton, FL, USA, 1997. [Google Scholar]
- Abouchami, W.; Galer, S.J.G.; de Baar, H.J.W.; Alderkamp, A.C.; Middag, R.; Laan, P.; Feldmann, H.; Andreae, M.O. Modulation of the Southern Ocean cadmium isotope signature by ocean circulation and primary productivity. Earth Planet. Sci. Lett. 2011, 305, 83–91. [Google Scholar] [CrossRef]
- Conway, T.M.; John, S.G. Biogeochemical cycling of cadmium isotopes along a high-resolution section through the North Atlantic Ocean. Geochim. Cosmochim. Acta 2015, 148, 269–283. [Google Scholar] [CrossRef]
- de Baar, H.J.W.; Saager, P.M.; Nolting, R.F.; van der Meer, J. Cadmium versus phosphate in the world ocean. Mar. Chem. 1994, 46, 261–281. [Google Scholar] [CrossRef]
- Reglamento (CE) No 420/2011. Reglamento (CE) No 420/2011 de la Comisión de 29 de abril de 2011 que modifica el Reglamento (CE) no 1881/2006, por el que se fija el contenido máximo de determinados contaminantes en los productos alimenticios. Diario Oficial de la Unión Europea 2011, L111, 3–6. [Google Scholar]
- Torres, P.; Rodrigues, A.; Soares, L.; Garcia, P. Metal Concentrations in Two Commercial Tuna Species from an Active Volcanic Region in the Mid-Atlantic Ocean. Arch. Environ. Contam. Toxicol. 2016, 70, 341–347. [Google Scholar] [CrossRef]
- Mugo, R.; SAITOH, S.; Nihira, A.; Kuroyama, T. Habitat characteristics of skipjack tuna (Katsuwonus pelamis) in the western North Pacific: A remote sensing perspective. Fish. Oceanogr. 2010, 19, 382–396. [Google Scholar] [CrossRef]
- Di, Y.U.; Chang-Feng, C.H.I.; Bin, W.; Guo-Fang, D.; Zhong-Rui, L.I. Characterization of acid-and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis). Chin. J. Nat. Med. 2014, 12, 712–720. [Google Scholar]
- Chanto-García, D.A.; Saber, S.; Macías, D.; Sureda, A.; Hernández-Urcera, J.; Cabanellas-Reboredo, M. Species-specific heavy metal concentrations of tuna species: The case of Thunnus alalunga and Katsuwonus pelamis in the Western Mediterranean. Environ. Sci. Pollut. Res. 2022, 29, 1278–1288. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Espinosa, J.M.; Jurado-Ruzafa, A.; Lozano, G.; Hardisson, A.; Rubio, C.; Weller, D.G.; Gutiérrez, Á.J.; González Weller, D.; Gutiérrez, Á.J. Inferring Class of organisms in the Central-East Atlantic from eco-toxicological characterization. Reg. Stud. Mar. Sci. 2020, 35, 101190. [Google Scholar] [CrossRef]
- Jakimska, A.; Konieczka, P.; Skóra, K.; Namieśnik, J. Bioaccumulation of Metals in Tissues of Marine Animals, Part I: The Role and Impact of Heavy Metals on Organisms. Polish J. Environ. Stud. 2011, 20, 1117–1125. [Google Scholar]
- Stillman, M.J. Metallothioneins. Coord. Chem. Rev. 1995, 144, 461–511. [Google Scholar] [CrossRef]
- Jeandel, C. Cycles Biogéochimiques Océaniques du Chrome et du Vanadium; Doctoral Dissertation: Paris, France, 1987. [Google Scholar]
- Duraisamy, R.; Shamena, S.; Berekete, A.K. A review of bio-tanning materials for processing of fish skin into leather. Int. J. Eng. Trends Technol. 2016, 39, 10–20. [Google Scholar]
- Ozuni, E.; Dhaskali, L.; Beqiraj, D.; Abeshi, J.; Latifi, F.; Zogaj, M.; Haziri, I. Mercury, lead, cadmium and chrome concentration levels in fish for public consumption. Albanian J. Agric. Sci. 2011, 10, 1–5. [Google Scholar]
- Blossom, N. Copper in the ocean environment. Am. Chemet Corp 2007, 6, 1–8. [Google Scholar]
- Matsumoto, W.M.; Skillman, R.A.; Dizon, A.E. Synopsis of Biological Data on Skipjack Tuna, Katsuwonus Pelamis; National Oceanic and Atmospheric Administration, National Marine Fisheries: Washington, DC, USA, 1984. [Google Scholar]
- Taylor, H.H.; Anstiss, J.M. Copper and haemocyanin dynamics in aquatic invertebrates. Mar. Freshw. Res. 1999, 50, 907–931. [Google Scholar] [CrossRef]
- Bury, N.; Grosell, M. Iron acquisition by teleost fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 97–105. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Raimundo, J.; Jurado-Ruzafa, A.; Lozano, G.; Jiménez, S.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Comparing Element Content in Small Pelagic Fish Species from Different Fishing Grounds in the Central-East Atlantic Ocean. Risk Assessment. Thalass. Int. J. Mar. Sci. 2021, 37, 861–869. [Google Scholar] [CrossRef]
- Rooker, J.R.; Alvarado Bremer, J.R.; Block, B.A.; Dewar, H.; De Metrio, G.; Corriero, A.; Kraus, R.T.; Prince, E.D.; Rodríguez-Marín, E.; Secor, D.H. Life history and stock structure of Atlantic bluefin tuna (Thunnus thynnus). Rev. Fish. Sci. 2007, 15, 265–310. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar]
- EFSA (European Food Safety Authority). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- EFSA (European Food Safety Authority). Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2010, 8, 1570. [Google Scholar]
- Tittlemier, S.A.; Fisk, A.T.; Hobson, K.A.; Norstrom, R.J. Examination of the bioaccumulation of halogenated dimethyl bipyrroles in an Arctic marine food web using stable nitrogen isotope analysis. Environ. Pollut. 2002, 116, 85–93. [Google Scholar] [CrossRef] [PubMed]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Domi, N.; Bouquegneau, J.-M.; Das, K. Feeding ecology of five commercial shark species of the Celtic Sea through stable isotope and trace metal analysis. Mar. Environ. Res. 2005, 60, 551–569. [Google Scholar] [CrossRef]
- Zhu, D.; Ding, J.; Wang, Y.-F.; Zhu, Y.-G. Effects of Trophic Level and Land Use on the Variation of Animal Antibiotic Resistome in the Soil Food Web. Environ. Sci. Technol. 2022, 56, 14937–14947. [Google Scholar] [CrossRef]
- Dauby, P.; Khomsi, A.; Bouquegneau, J.-M. Trophic relationships within intertidal communities of the Brittany coasts: A stable carbon isotope analysis. J. Coast. Res. 1998, 14, 1202–1212. [Google Scholar]
- Jardine, T.D.; McGeachy, S.A.; Paton, C.M.; Savoie, M.; Cunjak, R.A. Stable Isotopes in Aquatic Systems: Sample Preparation, Analysis and Interpretation; Citeseer: Princeton, NJ, USA, 2003; Volume 2656. [Google Scholar]
- Gupta, S.K.; Singh, J. Evaluation of mollusc as sensitive indicator of heavy metal pollution in aquatic system: A review. IIOAB J. 2011, 2, 49–57. [Google Scholar]
- Olson, R.J.; Young, J.W.; Ménard, F.; Potier, M.; Allain, V.; Goñi, N.; Logan, J.M.; Galván-Magaña, F. Chapter Four-Bioenergetics, Trophic Ecology, and Niche Separation of Tunas. In Advances in Marine Biology; Curry, B.E., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 74, pp. 199–344. ISBN 0065-2881. Available online: https://www.sciencedirect.com/science/article/pii/S0065288116300049 (accessed on 15 December 2022).
- Martins, K.; Pelage, L.; Justino, A.K.S.; Frédou, F.L.; Júnior, T.V.; Le Loc’h, F.; Travassos, P. Assessing trophic interactions between pelagic predatory fish by gut content and stable isotopes analysis around Fernando de Noronha Archipelago (Brazil), Equatorial West Atlantic. J. Fish Biol. 2021, 99, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, N.N.; Knoff, M.; Torres, E.J.L.; Pimenta, E.G.; de Amorim, A.F.; Gomes, D.C. Hirudinella ventricosa (trematoda) parasitizing makaira nigricans and acanthocybium solandri from neotropical region, Brazil. Neotrop. Helminthol. 2013, 7, 75–82. [Google Scholar]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Ruelas-Inzunza, J.; Soto-Jimenez, M.F.; Ruiz-Fernández, A.C.; Ramos-Osuna, M.; Mones-Saucedo, J.; Paez-Osuna, F. 210Po, Cd and Pb distribution and biomagnification in the yellowfin tuna Thunnus albacares and skipjack tuna Katsuwonus pelamis from the Eastern Pacific. Mar. Pollut. Bull. 2014, 87, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Coletto, J.L.; Botta, S.; Fischer, L.G.; Newsome, S.D.; Madureira, L.S.P. Isotope-based inferences of skipjack tuna feeding ecology and movement in the southwestern Atlantic Ocean. Mar. Environ. Res. 2021, 165, 105246. [Google Scholar] [CrossRef] [PubMed]
- Sardenne, F.; Bodin, N.; Chassot, E.; Amiel, A.; Fouché, E.; Degroote, M.; Hollanda, S.; Pethybridge, H.; Lebreton, B.; Guillou, G. Trophic niches of sympatric tropical tuna in the Western Indian Ocean inferred by stable isotopes and neutral fatty acids. Prog. Oceanogr. 2016, 146, 75–88. [Google Scholar] [CrossRef]
- Estrada, J.A.; Lutcavage, M.; Thorrold, S.R. Diet and trophic position of Atlantic bluefin tuna (Thunnus thynnus) inferred from stable carbon and nitrogen isotope analysis. Mar. Biol. 2005, 147, 37–45. [Google Scholar] [CrossRef]
- Henry, F.; Amara, R.; Courcot, L.; Lacouture, D.; Bertho, M.-L. Heavy metals in four fish species from the French coast of the Eastern English Channel and Southern Bight of the North Sea. Environ. Int. 2004, 30, 675–683. [Google Scholar] [CrossRef]
- Kwaansa-Ansah, E.E.; Nti, S.O.; Opoku, F. Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo Market, Ghana. Food Sci. Biotechnol. 2019, 28, 569–579. [Google Scholar] [CrossRef] [PubMed]
- REGLAMENTO (CE) No 1881/2006. REGLAMENTO (CE) No 1881/2006 DE LA COMISIÓN de 19 de diciembre de 2006 por el que se fija el contenido máximo de determinados contaminantes en los productos alimenticios. 2006. [Google Scholar]
- Chen, C.-Y.; Chen, Y.-T.; Chen, K.-S.; Hsu, C.-C.; Liu, L.-L.; Chen, H.-S.; Chen, M.-H. Arsenic and five metal concentrations in the muscle tissue of bigeye tuna (Thunnus obesus) in the Atlantic and Indian Oceans. Mar. Pollut. Bull. 2018, 129, 186–193. [Google Scholar] [CrossRef]
- Hellou, J.; Fancey, L.L.; Payne, J.F. Concentrations of twenty-four elements in bluefin tuna, Thunnus thynnus from the Northwest Atlantic. Chemosphere 1992, 24, 211–218. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Caló, M.; Naccari, F. Heavy Metals in Liver and Muscle of Bluefin Tuna (Thunnus thynnus) Caught in the Straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef]
- Mol, S.; Karakulak, F.S.; Ulusoy, S. Potential health risks due to heavy metal uptake via consumption of Thunnus thynnus from the northern Levantine Sea. Toxin Rev. 2018, 37, 56–61. [Google Scholar] [CrossRef]
- Sadeghi, P.; Loghmani, M.; Frokhzad, S. Human health risk assessment of heavy metals via consumption of commercial marine fish (Thunnus albacares, Euthynnus affinis, and Katsuwonus pelamis) in Oman Sea. Environ. Sci. Pollut. Res. 2020, 27, 14944–14952. [Google Scholar] [CrossRef] [PubMed]
- Pragnya, M.; Dinesh Kumar, S.; Solomon Raju, A.J.; Murthy, L.N. Bioaccumulation of heavy metals in different organs of Labeo rohita, Pangasius hypophthalmus, and Katsuwonus pelamis from Visakhapatnam, India. Mar. Pollut. Bull. 2020, 157, 111326. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, B.K.K.K.; Mahaliyana, A.S.; Liyanage, N.P.P.; Jayasinghe, G.D.T.M. Trace metals in the muscle tissues of skipjack tuna (Katsuwonus pelamis) in Sri Lanka. Cogent Food Agric. 2015, 1, 1038975. [Google Scholar] [CrossRef]
- Ruelas-Inzunza, J.; Soto-Jiménez, M.F.; Ruiz-Fernández, A.C.; Bojórquez-Leyva, H.; Pérez-Bernal, H.; Páez-Osuna, F. 210Po Activity and Concentrations of Selected Trace Elements (As, Cd, Cu, Hg, Pb, Zn) in the Muscle Tissue of Tunas Thunnus albacares and Katsuwonus pelamis from the Eastern Pacific Ocean. Biol. Trace Elem. Res. 2012, 149, 371–376. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Cedeño-Macias, L.A. Heavy metals in yellowfin tuna (Thunnus albacares) and common dolphinfish (Coryphaena hippurus) landed on the Ecuadorian coast. Sci. Total Environ. 2016, 541, 149–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).