Combined Effect of Acid Whey Addition and Ultrasonic Treatment on the Chemical and Microbiological Stability of Lamb Stuffing
Abstract
1. Introduction
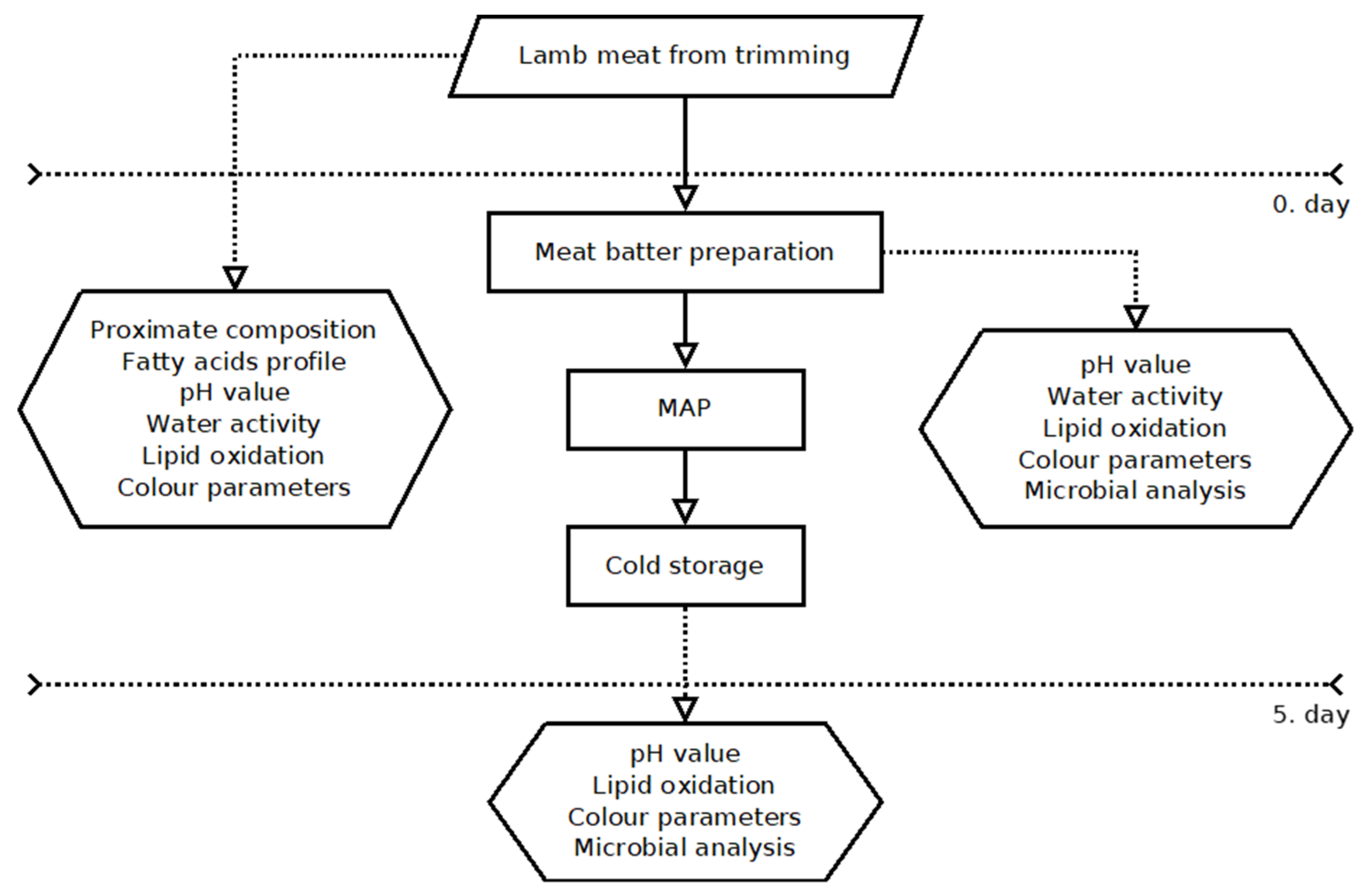
2. Materials and Methods
2.1. Preparation of Meat
2.2. Preparation of Acid Whey
2.3. Model Meat Stuffing
2.4. Proximate Composition of Meat
2.5. Fatty Acids Profile
2.6. pH Value
2.7. Water Activity
2.8. Lipid Oxidation
2.9. Color Parameters
2.10. Microbial Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of Lamb
3.2. Effect of Acid Whey Addition and Ultrasonic Treatment on Chemical Stability of Lamb Stuffing
3.3. Effect of Acid Whey Addition and Ultrasonic Treatment on Microbiological Stability of Lamb Stuffing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 25 January 2023).
- Fowler, S.M.; Morris, S.; Hopkins, D.L. Nutritional composition of lamb retail cuts from the carcases of extensively finished lambs. Meat Sci. 2019, 154, 126–132. [Google Scholar] [CrossRef]
- Prache, S.; Schreurs, N.; Guillier, L. Review: Factors affecting sheep carcass and meat quality attributes. Animal 2021, 16, 100330. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Kaur, M.; Pillidge, C.J.; Torley, P.J. Microbial biopreservatives for controlling the spoilage of beef and lamb meat: Their application and effects on meat quality. Crit. Rev. Food Sci. Nutr. 2021, 62, 4571–4592. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products—Invited review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/nitrites in food—Risk for nitrosative stress and benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Laranjo, M.; Elias, M.; Patarata, L. Microbiological hazards associated with salt and nitrite reduction in cured meat products: Control strategies based on antimicrobial effect of natural ingredients and protective microbiota. Curr. Opin. Food Sci. 2021, 38, 32–39. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A.D.; Borrajo, P.; Lorenzo, J.M. Comparative studies on the fatty acid profile and volatile compounds of fallow deer and beef fermented sausages without nitrite produced with the addition of acid whey. Appl. Sci. 2021, 11, 1320. [Google Scholar] [CrossRef]
- Okoń, A.; Szymański, P.; Zielińska, D.; Szydłowska, A.; Siekierko, U.; Kołożyn-Krajewska, D.; Dolatowski, Z.J. The influence of acid whey on the lipid composition and oxidative stability of organic uncured fermented bacon after production and during chilling storage. Antioxidants 2021, 10, 1711. [Google Scholar] [CrossRef]
- Szymański, P.; Łaszkiewicz, B.; Kern-Jędrychowska, A.; Siekierko, U.; Kołożyn-Krajewska, D. The effect of the use of Limosilactobacillus fermentum S8 isolated from organic acid whey on nitrosyl pigment concentration and the colour formation of uncured cooked meat products. Meat Sci. 2023, 196, 109031. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochemistry 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Demirok, N.T.; Yıkmış, S. Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens. Processes 2022, 10, 2100. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Kumar, S.; Bhat, H.F.; Aadil, R.M.; Bekhit, A.E.D.A.; Brennan, C.S. Ultrasonication as an emerging technology for processing of animal derived foods: A focus on in vitro protein digestibility. Trends Food Sci. Technol. 2022, 124, 309–322. [Google Scholar] [CrossRef]
- Turantaş, F.; Kiliç, G.B.; Kiliç, B. Ultrasound in the meat industry: General applications and decontamination efficiency. Int. J. Food Microbiol. 2015, 198, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Piñon, M.; Alarcon-Rojo, A.; Paniwnyk, L.; Mason, T.; Luna, L.; Renteria, A. Ultrasound for improving the preservation of chicken meat. Food Sci. Technol. 2019, 39, 129–135. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Yang, C.-h. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochemistry 2020, 63, 104953. [Google Scholar] [CrossRef]
- ISO 15214; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 1998.
- Stasiak, D.M.; Dolatowski, Z.J.; Kordowska-Wiater, M. Total number of bacteria and Salmonella on the skin of broiler chicken carcasses after sonication. Med. Weter. 2007, 63, 1230–1233. [Google Scholar]
- Medeiros, E.; Queiroga, R.; Oliveira, M.; Medeiros, A.; Sabedot, M.; Bomfim, M.; Madruga, M. Fatty acid profile of cheese from dairy goats fed a diet enriched with castor, sesame and faveleira vegetable oils. Molecules 2014, 19, 992–1003. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Ivanova, A.; Hadzhinikolova, L. Evaluation of nutritional quality of common carp (Cyprinus carpio L.) lipids through fatty acid ratios and lipid indices. Bulg. J. Agric. Sci. 2015, 21 (Suppl. 1), 180–185. [Google Scholar]
- ISO 4833-2; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21528-2; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 16649-2; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 7937; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Clostridium perfringens—Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 11290-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Corazzin, M.; Del Bianco, S.; Bovolenta, S.; Piasentier, E. Carcass characteristics and meat quality of sheep and goat. In More than Beef, Pork and Chicken—The Production, Processing, and Quality Traits of Other Sources of Meat for Human Diet; Lorenzo, J.M., Munekata, P.E.S., Barba, F.J., Toldrá, F., Eds.; Springer: Cham, Switzerland, 2019; pp. 119–163. [Google Scholar] [CrossRef]
- Mazinani, M.; Rude, B. Population, world production and quality of sheep and goat products. Am. J. Anim. Vet. Sci. 2020, 15, 291–299. [Google Scholar] [CrossRef]
- Yagoubi, Y.; Joy, M.; Ripoll, G.; Mahouachi, M.; Bertolín, J.R.; Atti, N. Rosemary distillation residues reduce lipid oxidation, increase alpha-tocopherol content and improve fatty acid profile of lamb meat. Meat Sci. 2018, 136, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Woods, V.B.; Fearon, A.M. Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livest. Sci. 2009, 126, 1–20. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, J.; Urrutia, O.; Lobón, S.; Ripoll, G.; Bertolín, J.R.; Joy, M. Insights into the role of major bioactive dietary nutrients in lamb meat quality: A review. J. Anim. Sci. Biotechnol. 2022, 13, 20. [Google Scholar] [CrossRef]
- Stancheva, M.; Merdzhanova, A.; Dobreva, D.A.; Makedonski, L. Common carp (Cyprinus carpio) and European catfish (Silurus glanis) from Danube River as sources of fat soluble vitamins and fatty acids. Czech J. Food Sci. 2014, 32, 16–24. [Google Scholar] [CrossRef]
- Chen, J.P.; Liu, H.B. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Popova, T.; Piedra, R.B.; Tolsdorf, A.; Geß, A.; Pires, J.; Domínguez, R.; Chiesa, F.; Brugiapaglia, A.; Viola, I.; et al. Fatty acid composition of lamb meat from Italian and German local breeds. Small Rumin. Res. 2021, 200, 106384. [Google Scholar] [CrossRef]
- Janiszewski, P.; Grześkowiak, E.; Lisiak, D.; Borys, B.; Borzuta, K.; Lisiak, B. Evaluation of the meat traits of lambs of Polish native breeds. Ann. Anim. Sci. 2021, 21, 347–360. [Google Scholar] [CrossRef]
- Jia, W.; Wu, X.; Li, R.; Liu, S.; Shi, L. Effect of nisin and potassium sorbate additions on lipids and nutritional quality of Tan sheep meat. Food Chem. 2021, 365, 130535. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A.D.; Stasiak, D.M.; Patkowski, K. Fatty acid profile and antioxidative properties of peptides isolated from fermented lamb loin treated with fermented milk. Antioxidants 2020, 9, 1094. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Hopkins, D.L. The use of conventional laboratory-based methods to predict consumer acceptance of beef and sheep meat: A review. Meat Sci 2021, 181, 108586. [Google Scholar] [CrossRef]
- Karwowska, M.; Munekata, P.E.S.; Lorenzo, J.M.; Tomasevic, I. Functional and clean label dry fermented meat products: Phytochemicals, bioactive peptides, and conjugated linoleic acid. Appl. Sci. 2022, 12, 5559. [Google Scholar] [CrossRef]
- Ripoll, G.; Joy, M.; Muñoz, F. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Sci. 2011, 87, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Corlett, M.T.; Pethick, D.W.; Kelman, K.R.; Jacob, R.H.; Gardner, G.E. Consumer perceptions of meat redness were strongly influenced by storage and display times. Foods 2021, 10, 540. [Google Scholar] [CrossRef]
- Ruedt, C.; Gibis, M.; Weiss, J. Effect of varying salt concentration on iridescence in precooked pork meat. Eur. Food Res. Technol. 2022, 248, 57–68. [Google Scholar] [CrossRef]
- Barcenilla, C.; Álvarez-Ordóñez, A.; Lópe, M.; Alvseike, O.; Prieto, M. Microbiological safety and shelf-life of low-salt meat products—A Review. Foods 2022, 11, 2331. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalasi, S.; Mahgoub, O. Carcass and meat quality characteristics of omani sheep fed diets based on raw or processed mesquite (Prosopis Juliflora) pods. J. Vet. Sci. Anim. Husb. 2018, 6, 206. [Google Scholar] [CrossRef]
- Hussain, Z.; Li, X.; Ijaz, M.; Xiao, X.; Hou, C.; Zheng, X.; Ren, C.; Zhang, D. Effect of chinese cinnamon powder on the quality and storage properties of ground lamb meat during refrigerated storage. Food Sci. Anim. Resour. 2020, 40, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, F.; Helbert, C.B.; Romdhane, M.B.; Koubaa, M.; Bhiri, F.; Kallel, F.; Chaari, F.; Driss, D.; Buon, L.; Chaabouni, S.E. Structural data and biological properties of almond gum oligosaccharide: Application to beef meat preservation. Int. J. Biol. Macromol. 2015, 72, 472–479. [Google Scholar] [CrossRef]
- Raad, A.I. The correlation between pH and microbial spoilage of minced meat during refrigeration. Int. J. Adv. Biol. Res. 2017, 7, 728–732. Available online: http://www.scienceandnature.org/IJABR/IJABR_Vol7(4)2017/IJABR_V7(4)17-18.pdf (accessed on 1 March 2022).
- Vergara, H.; Cózar, A.; Rubio, N. Lamb meat burgers shelf life: Effect of the addition of different forms of rosemary (Rosmarinus officinalis, L.). CyTA—J. Food 2021, 19, 606–613. [Google Scholar] [CrossRef]
- Siekmann, L.; Plötz, M.; Krischek, C. Alternative Curing Methods. Curr. Clin. Micro. Rpt. 2021, 8, 40–48. [Google Scholar] [CrossRef]
- Honikel, K.O. Chemical analysis of specific components curing agents. In Encyclopedia of Meat Sciences; Dikeman, M., Devine, C., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 200–205. [Google Scholar]
- Kononiuk, A.D.; Karwowska, M. Comparison of the effect of freeze-dried acid whey on physicochemical properties of organic fermented sausages made from beef and fallow deer meat. J. Food Sci. Technol. 2020, 57, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.M.; Sasaki, S.; McClements, D.J.; Decker, E.A. Mechanisms of the antioxidant activity of a high molecular weight fraction of whey. J. Agric. Food Chem. 2000, 48, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Tunieva, E.K.; Semenova, A.A.; Ivankin, A.N.; Nasonova, V.V.; Nikolaeva, A.N. Effect of sodium chloride on fat oxidation in the presence of heme pigments. Carpathian J. Food Sci. Technol. 2019, 11, 116–125. [Google Scholar] [CrossRef]
- Hernández, P.; Park, D.; Rhee, K.S. Chloride salt type/ionic strength, muscle site and refrigeration effects on antioxidant enzymes and lipid oxidation in pork. Meat Sci. 2002, 61, 405–410. [Google Scholar] [CrossRef]
- Kauser-Ul-Alam, M.; Hayakawa, T.; Kumura, H.; Wakamatsu, J. High ZnPP-forming food-grade lactic acid bacteria as a potential substitute for nitrite/nitrate to improve the color of meat products. Meat Sci. 2021, 176, 108467. [Google Scholar] [CrossRef]
- Petit, G.; Jury, V.; Lamballerie, M.; Duranton, F.; Pottier, L.; Martin, J. Salt intake from processed meat products: Benefits, risks and evolving practices. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1453–1473. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef]
- Wang, T.; Guo, H.; Zhang, H.; Ren, F.; Zhang, M.; Ge, S.; Luo, H.; Zhao, L. Dynamics of bacterial communities of lamb meat packaged in air and vacuum pouch during chilled storage. Food Sci. Anim. Resour. 2019, 39, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis; Advanced Evaluation Methods, Techniques, and Technologies; Biswas, A.K., Mandal, P.K., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 307–334. [Google Scholar] [CrossRef]
- Lamas, A.; Miranda, J.M.; Vazquez, B.; Cepeda, A.; Franco, C.M. An evaluation of alternatives to nitrites and sulfites to inhibit the growth of Salmonella enterica and Listeria monocytogenes in meat products. Foods 2016, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Lewus, C.B.; Kaiser, A.; Montville, T.J. Inhibition of food-borne bacterial pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl. Environ. Microbiol. 1991, 57, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Hospital, X.F.; Carballo, J.; Fernández, M.; Arnau, J.; Gratacós-Cubarsí, M.; Hierro, E. Technological implications of reducing nitrate and nitrite levels in dry-fermented sausages: Typical microbiota, residual nitrate and nitrite and volatile profile. Food Control 2015, 57, 275–281. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Zhou, T.; Li, J.; Yang, J.; Wenhua, C.; Xiong, Y.L. Two efficient nitrite-reducing Lactobacillus strains isolated from traditional fermented pork (Nanx Wudl) as competitive starter cultures for Chinese fermented dry sausage. Meat Sci. 2016, 121, 302–309. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg/2007/1441/oj (accessed on 11 February 2023).
| Variant | Brine (99.5% Salt, 0.5% Sodium Nitrite) [%] | Salt [%] | Water [%] | Liquid Acid Whey [%] | Sonication [Yes/No] |
|---|---|---|---|---|---|
| K | - | - | - | - | No |
| C | 2 | - | 10 | - | No |
| S | - | 2 | 10 | - | No |
| W | - | - | - | 10 | No |
| SW | - | 2 | - | 10 | No |
| UK | - | - | - | - | Yes |
| UC | 2 | - | 10 | - | Yes |
| US | - | 2 | 10 | - | Yes |
| UW | - | - | - | 10 | Yes |
| USW | - | 2 | - | 10 | Yes |
| Proximate Composition of Meat | ||
| Moisture (%) | 74.9 ± 3.80 | |
| Fat (%) | 7.36 ± 0.82 | |
| Proteins (%) | 18.1 ± 0.50 | |
| Collagen (%) | 2.30 | |
| Sodium (%) | 0.28 | |
| Fatty acid composition (more than 0.05% FAME) | ||
| SFA | C10:0 | 0.23 ± 0.02 |
| C12:0 | 0.18 ± 0.01 | |
| C14:0 | 2.36 ± 0.23 | |
| C15:0 | 0.61 ± 0.02 | |
| C16:0 | 20.47 ± 0.32 | |
| C17:0 | 1.40 ± 0.01 | |
| C18:0 | 24.08 ± 0.25 | |
| C20:0 | 0.23 ± 0.02 | |
| MUFA | C14:ln5 | 0.12 ± 0.01 |
| C16:ln7 | 1.77 ± 0.51 | |
| C17:ln7 | 0.81 ± 0.02 | |
| C18:ln9c + C18:ln9t | 43.65 ± 1.65 | |
| C20:ln9 | 0.11 ± 0.01 | |
| PUFA | C18:2n6c | 2.28 ± 0.32 |
| C18:3n3(alpha) | 0.66 ± 0.04 | |
| C20:2n6 | 0.11 ± 0.03 | |
| C20:4n6 | 0.18 ± 0.01 | |
| C20:5n3 | 0.07 ± 0.02 | |
| The nutritional indices of fat | ||
| SFA (%) | 49.77 | |
| MUFA (%) | 46.67 | |
| PUFA (%) | 3.56 | |
| PUFA n-3 (%) | 0.72 | |
| PUFA n-6 (%) | 2.64 | |
| PUFA n-9 (%) | 43.76 | |
| PUFA n-6/n-3 | 3.66 | |
| DFA | 5.46 | |
| OFA | 1.70 | |
| AI | 0.60 | |
| TI | 0.78 | |
| h/H | 2.02 | |
| Parameter | Value |
|---|---|
| Acidity (pH24) | 5.56 ± 0.06 |
| Water activity | 0.972 ± 0.003 |
| Lipid oxidation (mg malonaldehyde kg−1) | 0.62 ± 0.10 |
| CIE Lightness L* | 52.27 ± 2.02 |
| CIE Redness a* | 23.68 ± 0.37 |
| CIE Yellowness b* | 16.60 ± 1.46 |
| Day | K | C | S | W | SW | UK | UC | US | UW | USW | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 0 | 5.62 Aa ± 0.04 | 5.72 Ba ± 0.01 | 5.71 Ba ± 0.04 | 5.56 Ca ± 0.01 | 5.63 Aa ± 0.04 | 5.68 Aa ± 0.02 | 5.73 Ba ± 0.03 | 5.66 Ba ± 0.02 | 5.55 Ca ± 0.00 | 5.65 Ba ± 0.01 |
| 5 | 5.83 Eb ± 0.01 | 5.65 Ba ± 0.03 | 5.32 Cb ± 0.02 | 5.01 Db ± 0.02 | 5.34 Cb ± 0.01 | 5.79 Eb ± 0.02 | 5.72 Ba ± 0.05 | 5.37 Cb ± 0.01 | 5.16 Ab ± 0.04 | 5.38 Cb ± 0.04 | |
| aw | 0 | 0.971 Aa ± 0.02 | 0.970 Aa ± 0.04 | 0.970 Aa ± 0.03 | 0.972 Aa ± 0.01 | 0.971 Aa ± 0.01 | 0.973 Aa ± 0.01 | 0.972 Aa ± 0.03 | 0.973 Aa ± 0.02 | 0.975 Aa ± 0.04 | 0.974 Aa ± 0.03 |
| 5 | 0.965 Ab ± 0.01 | 0.963 Ab ± 0.02 | 0.964 Aa ± 0.02 | 0.963 Ab ± 0.03 | 0.970 Ba ± 0.03 | 0.967 Ab ± 0.03 | 0.965 Ab ± 0.02 | 0.966 Aa ± 0.01 | 0.969 Aa ± 0.02 | 0.973 Ba ± 0.01 | |
| TBARS (mg MDA kg−1) | 0 | 0.24 Aa ± 0.13 | 0.13 Aa ± 0.00 | 0.40 Ba ± 0.08 | 0.27 Aa ± 0.03 | 0.57 Ca ± 0.21 | 0.17 Aa ± 0.02 | 0.24 Aa ± 0.00 | 0.44 Ba ± 0.00 | 0.26 Aa ± 0.02 | 0.52 Ca ± 0.08 |
| 5 | 0.31 Aa ± 0.02 | 0.27 Ab ± 0.04 | 0.35 Aa ± 0.01 | 0.39 Ab ± 0.04 | 0.43 Ab ± 0.01 | 0.28 Ab ± 0.04 | 0.29 Aa ± 0.04 | 0.31 Ab ± 0.04 | 0.33 Ab ± 0.02 | 0.39 Ab ± 0.03 | |
| L* | 0 | 50.41 Aa ± 4.69 | 50.41 Aa ± 6.49 | 47.90 Aa ± 3.25 | 51.17 Aa ± 2.82 | 48.89 Aa ± 3.67 | 50.64 Aa ± 2.81 | 46.63 Aa ± 3.70 | 48.65 Aa ± 4.61 | 52.38 Aa ± 3.11 | 48.73 Aa ± 3.84 |
| 5 | 52.16 Aa ± 3.32 | 48.68 Aa ± 3.81 | 48.40 Aa ± 2.49 | 53.19 Aa ± 2.25 | 51.14 Aa ± 4.72 | 52.91 Aa ± 2.53 | 50.64 Aa ± 2.81 | 49.09 Aa ± 2.61 | 53.92 Aa ± 2.53 | 49.24 Aa ± 3.06 | |
| a* | 0 | 9.05 Aa ± 1.68 | 14.04 Ba ± 3.43 | 8.39 Aa ± 2.24 | 8.33 Aa ± 1.28 | 7.70 Aa ± 1.33 | 8.34 Aa ± 1.32 | 15.56 Ba ± 1.89 | 8.41 Aa ± 1.58 | 7.92 Aa ± 1.40 | 8.38 Aa ± 1.54 |
| 5 | 9.07 Aa ± 1.46 | 16.39 Ba ± 2.06 | 9.46 Aa ± 1.10 | 8.45 Aa ± 0.93 | 8.13 Aa ± 1.85 | 8.41 Aa ± 1.09 | 14.34 Aa ± 1.32 | 10.17 Aa ± 2.15 | 8.45 Aa ± 1.37 | 8.11 Aa ± 1.06 | |
| b* | 0 | 11.44 Aa ± 1.22 | 11.73 Aa ± 3.35 | 10.24 Aa ± 0.75 | 11.79 Aa ± 0.93 | 9.81 Aa ± 1.29 | 11.60 Aa ± 0.99 | 12.11 Aa ± 1.09 | 10.68 Aa ± 1.69 | 12.0 Aa ± 0.84 | 10.51 Aa ± 1.21 |
| 5 | 11.80 Aa ± 1.26 | 13.54 Aa ± 1.01 | 10.70 Aa ± 1.12 | 11.95 Aa ± 0.61 | 11.10 Aa ± 1.23 | 11.85 Aa ± 1.04 | 11.60 Aa ± 0.99 | 11.39 Aa ± 1.04 | 12.30 Aa ± 1.19 | 10.18 Aa ± 1.11 |
| Bacteria | Day | K | C | S | W | SW | UK | UC | US | UW | USW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LAB | 0 | 6.30 Ba ± 0.12 | 5.73 Aa ± 0.15 | 5.90 Aa ± 0.15 | 7.30 Ca ± 0.17 | 7.30 Ca ± 0.15 | 6.28 Ba ± 0.15 | 5.70 Aa ± 0.15 | 5.86 Aa ± 0.16 | 7.27 Ca ± 0.16 | 7.26 Ca ± 0.16 |
| 5 | 7.80 Bb ± 0.15 | 7.03 Ab ± 0.15 | 7.15 Ab ± 0.15 | 8.10 Bb ± 0.17 | 8.00 Bb ± 0.14 | 7.86 Bb ± 0.15 | 7.15 Ab ± 0.15 | 7.18 Ab ± 0.14 | 8.16 Bb ± 0.16 | 8.11 Bb ± 0.15 | |
| Cl. perfringens | 0 | <10 | |||||||||
| 5 | |||||||||||
| Enterobacteriaceae | 0 | 2.74 Aa ± 0.08 | 2.66 Aa ± 0.08 | 2.73 Aa ± 0.09 | 2.52 Aa ± 0.08 | 2.62 Aa ± 0.07 | 2.71 Aa ± 0.08 | 2.62 Aa ± 0.08 | 2.70 Aa ± 0.08 | 2.48 Aa ± 0.08 | 2.57 Aa ± 0.07 |
| 5 | 3.53 Db ± 0.08 | 2.30 Bb ± 0.15 | 2.66 Ca ± 0.08 | 2.26 Bb ± 0.08 | 2.28 Bb ± 0.09 | 3.39 Db ± 0.07 | 2.01 Ab ± 0.08 | 2.45 Bb ± 0.07 | 2.10 Ab ± 0.04 | 2.03 Ab ± 0.07 | |
| E. coli | 0 | 1.68 Ba ± 0.11 | 1.48 Aa ± 0.20 | 1.56 Ba ± 0.13 | 1.43 Aa ± 0.12 | 1.64 Ba ± 0.10 | 1.64 a ± 0.10 | 1.43 Aa ± 0.18 | 1.52 Aa ± 0.11 | 1.39 Aa ± 0.09 | 1.61 Ba ± 0.09 |
| 5 | 2.15 Ab ± 0.17 | 1.85 Ab ± 0.14 | 2.00 Ab ± 0.16 | 2.18 Ab ± 0.13 | 2.02 Ab ± 0.12 | 2.11 Ab ± 0.15 | 1.80 Ab ± 0.12 | 1.98 Ab ± 0.13 | 2.09 Ab ± 0.11 | 1.98 Ab ± 0.10 | |
| L. monocytogenes | 0 | not present | |||||||||
| 5 | |||||||||||
| TVC | 0 | 5.10 Aa ± 0.15 | 5.08 Aa ± 0.19 | 5.11 Aa ± 0.16 | 5.60 Ba ± 0.14 | 5.62 Ba ± 0.15 | 5.08 Aa ± 0.14 | 5.05 Aa ± 0.17 | 5.08 Aa ± 0.14 | 5.57 Ba ± 0.13 | 5.58 Ba ± 0.14 |
| 5 | 5.83 Bb ± 0.12 | 4.74 Aa ± 0.17 | 4.88 Aa ± 0.12 | 6.22 Bb ± 0.11 | 6.15 Bb ± 0.14 | 5.92 Bb ± 0.13 | 4.79 Aa ± 0.19 | 4.91 Aa ± 0.15 | 6.28 Bb ± 0.13 | 6.19 Bb ± 0.16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latoch, A.; Stasiak, D.M.; Junkuszew, A. Combined Effect of Acid Whey Addition and Ultrasonic Treatment on the Chemical and Microbiological Stability of Lamb Stuffing. Foods 2023, 12, 1379. https://doi.org/10.3390/foods12071379
Latoch A, Stasiak DM, Junkuszew A. Combined Effect of Acid Whey Addition and Ultrasonic Treatment on the Chemical and Microbiological Stability of Lamb Stuffing. Foods. 2023; 12(7):1379. https://doi.org/10.3390/foods12071379
Chicago/Turabian StyleLatoch, Agnieszka, Dariusz M. Stasiak, and Andrzej Junkuszew. 2023. "Combined Effect of Acid Whey Addition and Ultrasonic Treatment on the Chemical and Microbiological Stability of Lamb Stuffing" Foods 12, no. 7: 1379. https://doi.org/10.3390/foods12071379
APA StyleLatoch, A., Stasiak, D. M., & Junkuszew, A. (2023). Combined Effect of Acid Whey Addition and Ultrasonic Treatment on the Chemical and Microbiological Stability of Lamb Stuffing. Foods, 12(7), 1379. https://doi.org/10.3390/foods12071379








