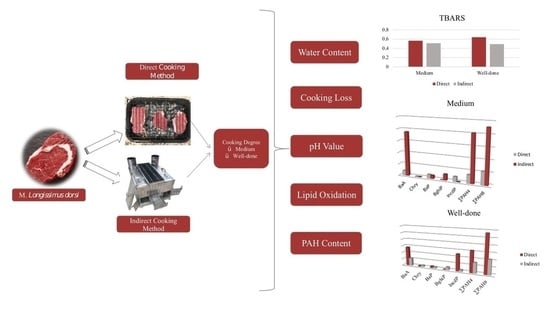
The Effect of Direct and Indirect Barbecue Cooking on Polycyclic Aromatic Hydrocarbon Formation and Beef Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Standards
2.3. Methods
2.3.1. Cooking Process
2.3.2. Water Content
2.3.3. Cooking Loss
2.3.4. pH Value
2.3.5. Determination of Lipid Oxidation Level (Thiobarbituric Acid Reactive Substances, TBARS)
2.3.6. Determination of Polycyclic Aromatic Hydrocarbon Content
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Analysis Results of Raw Material
3.2. Water Content Results of Cooked Meat Samples
3.3. Cooking Loss Results of Meat Samples
3.4. pH Value Results of Cooked Meat Samples
3.5. TBARS Results of Meat Samples
3.6. Polycyclic Aromatic Hydrocarbon Content Results of Cooked Meat Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oz, F.; Kaban, G.; Kaya, M. Effects of cooking methods and levels on formation of heterocyclic aromatic amines in chicken and fish with Oasis extraction method. LWT-Food Sci. Technol. 2010, 43, 1345–1350. [Google Scholar] [CrossRef]
- Öz, F.; Kızıl, M.; Çakmak, İ.; Aksu, M.İ. The effect of direct addition of conjugated linoleic acid on the formation of heterocyclic aromatic amines in beef chops. J. Food Process. Preserv. 2015, 39, 2820–2833. [Google Scholar] [CrossRef]
- Ledesma, E.; Rendueles, M.; Díaz, M.J.F.C. Contamination of meat products during smoking by polycyclic aromatic hydrocarbons: Processes and prevention. Food Control 2016, 60, 64–87. [Google Scholar] [CrossRef]
- Unal, K.; Karakaya, M.; Oz, F. The effects of different spices and fat types on the formation of heterocyclic aromatic amines in barbecued sucuk. J. Sci. Food Agric. 2018, 98, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Oz, E.; Oz, F. Mutagenic and/or carcinogenic compounds in meat and meat products: Heterocyclic aromatic amines perspective. Theory Pract. Meat Process. 2022, 7, 112–117. [Google Scholar] [CrossRef]
- Biesalski, H.K. Meat as a component of a healthy diet—Are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef]
- Haskaraca, G.; Demirok Soncu, E.; Kolsarıcı, N.; Öz, F.; Juneja, V.K. Heterocyclic aromatic amines content in chicken burgers and chicken nuggets sold in fast food restaurants and effects of green tea extract and microwave thawing on their formation. J. Food Process. Preserv. 2017, 41, e13240. [Google Scholar] [CrossRef]
- Oz, F. Effects of water extract of Urtica dioica L. on the quality of meatballs. J. Food Process. Preserv. 2014, 38, 1356–1363. [Google Scholar] [CrossRef]
- Nuray, M.; Oz, F. The effect of using different types and rates of onion-water extract in meatball production on the formation of heterocyclic aromatic amines. J. Sci. Food Agric. 2019, 99, 3538–3547. [Google Scholar] [CrossRef]
- Cordeiro, T.; Viegas, O.; Silva, M.; Martins, Z.E.; Fernandes, I.; Ferreira, I.M.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef]
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.R.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem. Toxicol. 2015, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Poltorak, A. Determination of polycyclic aromatic hydrocarbons using different extraction methods and HPLC-FLD detection in smoked and grilled meat products. Food Chem. 2022, 373, 131506. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Yettella, R.R.; Kim, J.S.; Kwon, K.; Kim, M.C.; Min, D.B. Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Babaoglu, A.S.; Karakaya, M.; Öz, F. Formation of polycyclic aromatic hydrocarbons in beef and lamb kokorec: Effects of different animal fats. Int. J. Food Prop. 2017, 20, 1960–1970. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, S.; Gong, G. Trends of research on polycyclic aromatic hydrocarbons in food: A 20-year perspective from 1997 to 2017. Trends Food Sci. Technol. 2019, 83, 86–98. [Google Scholar] [CrossRef]
- Alver, E.; Demirci, A.; Özcimder, M. Polisiklik aromatik hidrokarbonlar ve sağlığa etkileri. Fen Bil. Enst. Derg. 2012, 3, 45–52. [Google Scholar]
- Günç Ergönül, P.; Kaya, D. Polisiklik aromatik hidrokarbonlar ve gıdalarda önemi. Celal Bayar Üniversitesi Fen Bilim. Derg. 2015, 11, 143–153. [Google Scholar] [CrossRef]
- Kılıç, Ö.; Dinçer, E.A.; Erbaş, M. Gıdalarda polisiklik aromatik hidrokarbon bileşiklerinin bulunuşu ve sağlık üzerine etkileri. Gıda 2017, 42, 127–135. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Faridah, A.; Zaidul, I.S.M. Effects of marinating on the formation of polycyclic aromatic hydrocarbons (benzo [a] pyrene, benzo [b] fluoranthene and fluoranthene) in grilled beef meat. Food Control 2012, 28, 420–425. [Google Scholar] [CrossRef]
- Aydın, Ö.Ş.; Şahan, Y. Bazı et türlerinde polisiklik aromatik hidrokarbon oluşumuna farklı pişirme yöntemlerinin etkisi. Akademik Gıda 2018, 16, 387–394. [Google Scholar] [CrossRef]
- Stołyhwo, A.; Sikorski, Z.E. Polycyclic aromatic hydrocarbons in smoked fish—A critical review. Food Chem. 2005, 91, 303–311. [Google Scholar] [CrossRef]
- Oz, F.; Yuzer, M.O. The effects of cooking on wire and stone barbecue at different cooking levels on the formation of heterocyclic aromatic amines and polycyclic aromatic hydrocarbons in beef steak. Food Chem. 2016, 203, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Wang, Q.; Tokumura, M.; Miyake, Y.; Amagai, T. Simultaneous determination of polycyclic aromatic hydrocarbons and their chlorinated derivatives in grilled foods. Ecotoxicol. Environ. Safe. 2019, 178, 188–194. [Google Scholar] [CrossRef]
- Malarut, J.A.; Vangnai, K. Influence of wood types on quality and carcinogenic polycyclic aromatic hydrocarbons (PAHs) of smoked sausages. Food Control 2018, 85, 98–106. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S. Removal of polycyclic aromatic hydrocarbons by low density polyethylene from liquid model and roasted meat. Food Chem. 2005, 90, 461–469. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Aaslyng, M.; Meinert, L.; Christensen, T.; Jensen, A.H.; Binderup, M.L. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control 2015, 57, 169–176. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.; Choi, S.; Lee, I.; Moon, M.K.; Choi, K.; Park, J. Association of exposure to polycyclic aromatic hydrocarbons and heavy metals with thyroid hormones in general adult population and potential mechanisms. Sci. Total Environ. 2021, 762, 144227. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Abas, F.; Sakar, Z.I. Determination of polycyclic aromatic hydrocarbons in grilled meat. Food Control 2010, 21, 606–610. [Google Scholar] [CrossRef]
- Oz, E. The presence of polycyclic aromatic hydrocarbons and heterocyclic aromatic amines in barbecued meatballs formulated with different animal fats. Food Chem. 2021, 352, 129378. [Google Scholar] [CrossRef]
- Jaegerstad, M.; Skog, K. Genotoxicity of heat-processed foods. Mutat. Rese/Fundam. Mol. Mech. Mutagen. 2005, 574, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, A.; Jinap, S.; Hanifah, H.N.; Zaidul, I.S. Effects of meat preheating and wrapping on the levels of polycyclic aromatic hydrocarbons in charcoal-grilled meat. Food Chem. 2011, 124, 141–146. [Google Scholar] [CrossRef]
- Chen, B.H.; Lin, Y.S. Formation of polycyclic aromatic hydrocarbons during processing of duck meat. J. Agric. Food Chem. 1997, 45, 1394–1403. [Google Scholar] [CrossRef]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo [a] pyrene and estimation of its intake in an epidemiologic study. Food and Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Duedahl-Olesen, L.; Jensen, K.; Meinert, L. Content of heterocyclic amines and polycyclic aromatic hydrocarbons in pork, beef and chicken barbecued at home by Danish consumers. Meat Sci. 2013, 93, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Erhunmwunse, N.O.; Ainerua, M.O.; Ogboghodo, I.B.; Ekene, B. Effects of barbecuing on the levels of polycyclic aromatic hydrocarbons in fish (Pseudotolitus Elongatus and Clarias Gariepinus). J. Nat. Sci. Res. 2016, 6, 23–27. [Google Scholar]
- Kafouris, D.; Koukkidou, A.; Christou, E.; Hadjigeorgiou, M.; Yiannopoulos, S. Determination of polycyclic aromatic hydrocarbons in traditionally smoked meat products and charcoal grilled meat in Cyprus. Meat Sci. 2020, 164, 108088. [Google Scholar] [CrossRef]
- Bostan, S. Farklı Pişirme Yöntemlerinin Akçaabat Köftesinde Polisiklik Aromatik Hidrokarbon (PAH) Oluşumuna Etkisi. Master’s Thesis, Gümüşhane Üniversitesi, Gümüşhane, Turkey, 2021. [Google Scholar]
- Gökalp, H.Y.; Kaya, M.; Tülek, Y.; Zorba, Ö. Et ve Ürünlerinde Kalite Kontrolü ve Laboratuvar Uygulama Klavuzu. (V. Baskı); Atatürk Üniversitesi Yayınları, Yayın No: 751, Ziraat Fakültesi, Yayın No: 318. Ders Kitapları Seri No: 69; Atatürk Üniversitesi: Erzurum, Turkey, 2010. [Google Scholar]
- Öz, F.; Kızıl, M. Determination of heterocyclic aromatic amines in cooked commercial frozen meat products by ultrafast liquid chromatography. Food Anal. Methods 2013, 6, 1370–1378. [Google Scholar] [CrossRef]
- Kılıç, B.; Richards, M. Lipid oxidation in poultry döner kebab: Pro-oxidative and anti-oxidative factors. J. Food Sci. 2003, 68, 686–689. [Google Scholar] [CrossRef]
- Yıldız, N.; Bircan, H. Uygulamalı İstatistik; Atatürk Üniversitesi Yayınları No: 704; Atatürk Üniversitesi: Erzurum, Turkey, 1991; pp. 308–360. [Google Scholar]
- Fencioglu, H.; Oz, E.; Turhan, S.; Proestos, C.; Oz, F. The Effects of the marination process with different vinegar varieties on various quality criteria and heterocyclic aromatic amine formation in beef steak. Foods 2022, 11, 3251. [Google Scholar] [CrossRef]
- Sánchez del Pulgar, J.; Gázquez, A.; Ruiz-Carrascal, J. Physico—Chemical, textural and structural characteristics of sous—Vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef]
- Babür, T.E.; Gürbüz, Ü. Geleneksel pişirme yöntemlerinin et kalitesine etkileri. J. Tour. Gastron. Stud. 2015, 3, 58–64. [Google Scholar]
- Şireli, H.D. Karkaslarda et kalitesinin belirlenmesinde kullanılan geleneksel yöntemler ve yeni teknikler. Dicle Üniversitesi Fen Bilim. Enstitüsü Derg. 2018, 7, 126–132. [Google Scholar]
- Sanwo, K.A.; Adegoke, A.V.; Akinola, O.S.; Njoku, C.P.; Okolo, S.O.; Oladipo, N.A.; Oladejo, A.S. Meat quality characteristics of improved indigenous chickens (FUNAAB-ALPHA) fed turmeric (Curcuma longa) or clove (Syzygium aromaticum) as feed additives. J. Agric. Sci. Environ. 2019, 19, 102–112. [Google Scholar] [CrossRef]
- Kiliç, S.; Öz, E.; Öz, F. Effect of turmeric on the reduction of heterocyclic aromatic amines and quality of chicken meatballs. Food Control 2021, 128, 108189. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kataoka, H.; Ishihara, J.; Takachi, R.; Hamada, G.S.; Sharma, S.; Tsugane, S. Heterocyclic amines content of meat and fish cooked by Brazilian methods. J. Food Compos. Anal. 2010, 23, 61–69. [Google Scholar] [CrossRef]
- Jinap, S.; Mohd-Mokhtar, M.S.; Farhadian, A.; Hasnol, N.D.S.; Jaafar, S.N.; Hajeb, P. Effects of varying degrees of doneness on the formation of heterocyclic aromatic amines in chicken and beef satay. Meat Sci. 2013, 94, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.S.; Kim, I.S.; Park, J.H.; Lee, S.H.; Park, D.C.; Yeum, D.M.; Kım, S.B. Effects of seasoning and heating device on mutagenicity and heterocyclic amines in cooked beef. Biosci. Biotechnol. Biochem. 2001, 65, 2284–2287. [Google Scholar] [CrossRef]
- Khan, I.A.; Liu, D.; Yao, M.; Memon, A.; Huang, J.; Huanga, M. Inhibitory effect of Chrysanthemum morifolium flower extract on the formation of heterocyclic amines in goat meat patties cooked by various cooking methods and temperatures. Meat Sci. 2019, 147, 70–81. [Google Scholar] [CrossRef]
- Girard, P.J. Cooking. In Technology of Meat and Meat Products; Ellis Horwood: Chichester, France, 1992; pp. 32–83. [Google Scholar]
- Öz, F.; Çakmak, İ.H. The effects of conjugated linoleic acid usage in meatball production on the formation of heterocyclic aromatic amines. LWT-Food Sci. Technol. 2016, 65, 1031–1037. [Google Scholar] [CrossRef]
- Öz, F.; Kızıl, M.; Zaman, A.; Turhan, S. The effects of direct addition of low and medium molecular weight chitosan on the formation of heterocyclic aromatic amines in beef chop. LWT-Food Sci. Technol. 2016, 65, 861–867. [Google Scholar] [CrossRef]
- Aşçioğlu, Ç. Farklı Pişirme Yöntemlerinin Sığır Bonfilelerinin (Longissimus dorsi) Besinsel ve Kalite Özellikleri Üzerine Etkisi. Master’s Thesis, Afyon Kocatepe Üniversitesi, Afyonkarahisar, Turkey, 2013. [Google Scholar]
- Estévez, M. What’s New İn Meat Oxidation. In New Aspects of Meat Quality, 1st ed.; Purslow, P.P., Ed.; Woodhead Publishing: Buenos Aires, Argentina, 2017; pp. 91–109. [Google Scholar]
- Sabuncular, G.; Akbulut, G.; Yaman, M. Ette Lipit oksidasyonu ve etkileyen faktörler. Avrupa Bilim Teknoloji Dergisi 2021, 27, 362–369. [Google Scholar] [CrossRef]
- Visessanguan, W.; Benjakul, S.; Riebroy, S.; Yarchai, M.; Wanaporn Tapingkae, W. Changes in lipid composition and fatty acid profile of nham, a thai fermented pork sausage, during fermentation. Food Chem. 2006, 94, 580–588. [Google Scholar] [CrossRef]
- Lepper-Blilie, A.N.; Berg, E.P.; Buchanan, D.S.; Keller, W.L.; Maddock-Carlin, K.R.; Berg, P.T. Effectiveness of oxygen barrier oven bags in low temperature cooking on reduction of warmed– over flavor in beef roasts. Meat Sci. 2014, 96, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.R.; Morcuende, D.; Estevez, M.; Lopez, R.C. Fatty acid profiles of intramuscular fat from pork loin chops fried in different culinary fats following refrigerated storage. Food Chem. 2005, 92, 159–167. [Google Scholar] [CrossRef]
- Rojas, M.C.; Brewer, M.S. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J. Food Sci. 2007, 72, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Alfaia, C.M.; Alves, S.P.; Lopes, A.F.; Fernandes, M.J.; Costa, A.S.; Fontes, C.M.; Prates, J.A. Effect of cooking methods on fatty acids, conjugated isomers of linoleic acid and nutritional quality of beef intramuscular fat. Meat Sci. 2010, 84, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.; Failla, S.; Ficco, A.; Peña, F.; Avilés, C.; Polvillo, O. Buffalo meat composition as affected by different cooking methods. Food Bioprod. Process. 2010, 88, 145–148. [Google Scholar] [CrossRef]
- Zikirov, E. Sous-Vide Pişirme Yönteminin Sığır Etinde Heterosiklik Aromatik Amin Oluşumu ve Bazı Kalitatif Kriterler Üzerine Etkisi. Master’s Thesis, Atatürk Üniversitesi, Erzurum, Turkey, 2014. [Google Scholar]
- Korkmaz, A.; Oz, F. Effect of the use of dry breadcrumb in meatball production on the formation of heterocyclic aromatic amines. Br. Food J. 2020, 122, 2105–2119. [Google Scholar] [CrossRef]
- Uzun, I.; Oz, F. Effect of basil use in meatball production on heterocyclic aromatic amine formation. J. Food Sci. Technol. 2021, 58, 3001–3009. [Google Scholar] [CrossRef]
- Kılıç, S. Mühürleme işleminin etin Tekstür, Protein Profili ve Heterosiklik Aromatik amin Oluşumu Üzerine Etkisi. Master’s Thesis, Atatürk Üniversitesi, Fen Bilimleri Enstitüsü, Gıda Mühendisliği Anabilim Dalı, Erzurum, Turkey, 2021. [Google Scholar]
- Oz, E. Effects of smoke flavoring using different wood chips and barbecuing on the formation of polycyclic aromatic hydrocarbons and heterocyclic aromatic amines in salmon fillets. PLoS ONE 2020, 15, e0227508. [Google Scholar] [CrossRef]
- Babaoğlu, A.S. Dana ve Kuzu Kokoreçlerinde Polisiklik Aromatik Hidrokarbonların (PAH) Oluşum Düzeyi Üzerine Farklı Hayvansal Yağların Etkisi. Master’s Thesis, Selçuk Üniversitesi, Konya, Turkey, 2015. [Google Scholar]
- Gosetti, F.; Chiuminatto, U.; Mazzucco, E.; Robotti, E.; Calabrese, G.; Gennaro, M.C.; Marengo, E. Simultaneous determination of thirteen polycyclic aromatic hydrocarbons and twelve aldehydes in cooked food by an automated on-line solid phase extraction Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 6308–6318. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.A.; Lalah, J.O.; Wandiga, S.O. The effect of local cooking methods on polycyclic aromatic hydrocarbons (PAHs) contents in beef, goat meat, and pork as potential sources of human exposure in Kisumu City, Kenya. Polycyclic Aromat. Compd. 2012, 32, 656–668. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Polycyclic aromatic hydrocarbons in food-scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 6, 724. [Google Scholar] [CrossRef]
- Gorji, M.E.H.; Ahmadkhaniha, R.; Moazzen, M.; Yunesian, M.; Azari, A.; Rastkari, N. Polycyclic aromatic hydrocarbons in Iranian Kebabs. Food Control 2016, 60, 57–63. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Huang, T.; Yu, Y.; Bassey, A.P.; Huang, M. The contamination, formation, determination and control of polycyclic aromatic hydrocarbons in meat products. Food Control 2022, 141, 109194. [Google Scholar] [CrossRef]
- Min, S.; Patra, J.K.; Shin, H.S. Factors influencing inhibition of eight polycyclic aromatic hydrocarbons in heated meat model system. Food Chem. 2018, 239, 993–1000. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Ashaolu, T.J. Polycyclic aromatic hydrocarbons formation and mitigation in meat and meat products. Polycyclic Aromat. Compd. 2020, 42, 3401–3411. [Google Scholar] [CrossRef]
- European Commission, E.C. Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, 215, 1–5. [Google Scholar]

| n | Water (%) | Cooking Loss (%) | pH | TBARS (mg MDA/kg) | |
|---|---|---|---|---|---|
| Cooking method | |||||
| Direct | 6 | 62.26 ± 4.10a | 36.39 ± 7.30a | 5.99 ± 0.01a | 0.603 ± 0.05a |
| Indirect | 6 | 61.80 ± 3.74a | 35.91 ± 6.54a | 5.95 ± 0.07b | 0.500 ± 0.03b |
| Sign. | ns | ns | * | ** | |
| Cooking degree | |||||
| Medium | 6 | 64.61 ± 3.22a | 30.78 ± 4.22b | 5.95 ± 0.06b | 0.539 ± 0.05a |
| Well-done | 6 | 59.44 ± 2.13b | 41.53 ± 3.01a | 5.99 ± 0.02a | 0.565 ± 0.09a |
| Sign. | * | ** | * | ns | |
| Cooking Method | n | Cooking Degree | BaA | Chry | BbF | BkF | BaP | DahA | BghiP | IncdP | ∑PAH4 | ∑PAH8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct | 3 | Medium | 0.40 ± 0.18 | nd | nd | nd | 0.31 ± 0.13 | nd | nd | 0.41 ± 0.03 | 0.71 ± 0.08 | 1.12 ± 0.07 |
| 3 | Well | 5.62 ± 2.97 | 0.23 ± 0.15 | nq | nq | 0.49 ± 0.26 | nd | nd | 4.99 ± 3.29 | 6.35 ± 2.73 | 11.34 ± 3.59 | |
| Indirect | 3 | Medium | 3.78 ± 2.06 | nd | nd | nd | 0.27 ± 0.07 | nd | 0.51 ± 0.06 | nd | 4.06 ± 2.13 | 4.57 ± 2.12 |
| 3 | Well | 2.25 ± 1.76 | 0.43 ± 0.24 | nd | nd | 0.39 ± 0.25 | nd | 0.82 ± 0.66 | 0.48 ± 0.10 | 3.07 ± 1.78 | 4.37 ± 2.41 |
| N | ∑PAH4 | ∑PAH8 | |
|---|---|---|---|
| Cooking method | |||
| Direct | 6 | 3.53 ± 3.53a | 6.23 ± 6.04a |
| Indirect | 6 | 3.56 ± 1.83a | 4.47 ± 2.04a |
| Sign. | ns | ns | |
| Cooking degree | |||
| Medium | 6 | 2.39 ± 2.73a | 2.84 ± 2.32b |
| Well-done | 6 | 4.71 ± 2.73a | 7.86 ± 4.70a |
| Sign. | ns | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumer, G.; Oz, F. The Effect of Direct and Indirect Barbecue Cooking on Polycyclic Aromatic Hydrocarbon Formation and Beef Quality. Foods 2023, 12, 1374. https://doi.org/10.3390/foods12071374
Sumer G, Oz F. The Effect of Direct and Indirect Barbecue Cooking on Polycyclic Aromatic Hydrocarbon Formation and Beef Quality. Foods. 2023; 12(7):1374. https://doi.org/10.3390/foods12071374
Chicago/Turabian StyleSumer, Gulsah, and Fatih Oz. 2023. "The Effect of Direct and Indirect Barbecue Cooking on Polycyclic Aromatic Hydrocarbon Formation and Beef Quality" Foods 12, no. 7: 1374. https://doi.org/10.3390/foods12071374
APA StyleSumer, G., & Oz, F. (2023). The Effect of Direct and Indirect Barbecue Cooking on Polycyclic Aromatic Hydrocarbon Formation and Beef Quality. Foods, 12(7), 1374. https://doi.org/10.3390/foods12071374