Hybrid Sausages Using Pork and Cricket Flour: Texture and Oxidative Storage Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functional Properties of CF
2.3. Proximate Composition
2.4. Sausage Preparation
2.5. Rheological Properties of Raw Sausage Batter
2.6. Color and Textural Properties of Cooked Sausages
2.7. Lipid Oxidation of Cooked Sausages
2.8. Protein Oxidation of Cooked Sausages
2.9. Statistical Analysis
3. Results and Discussion
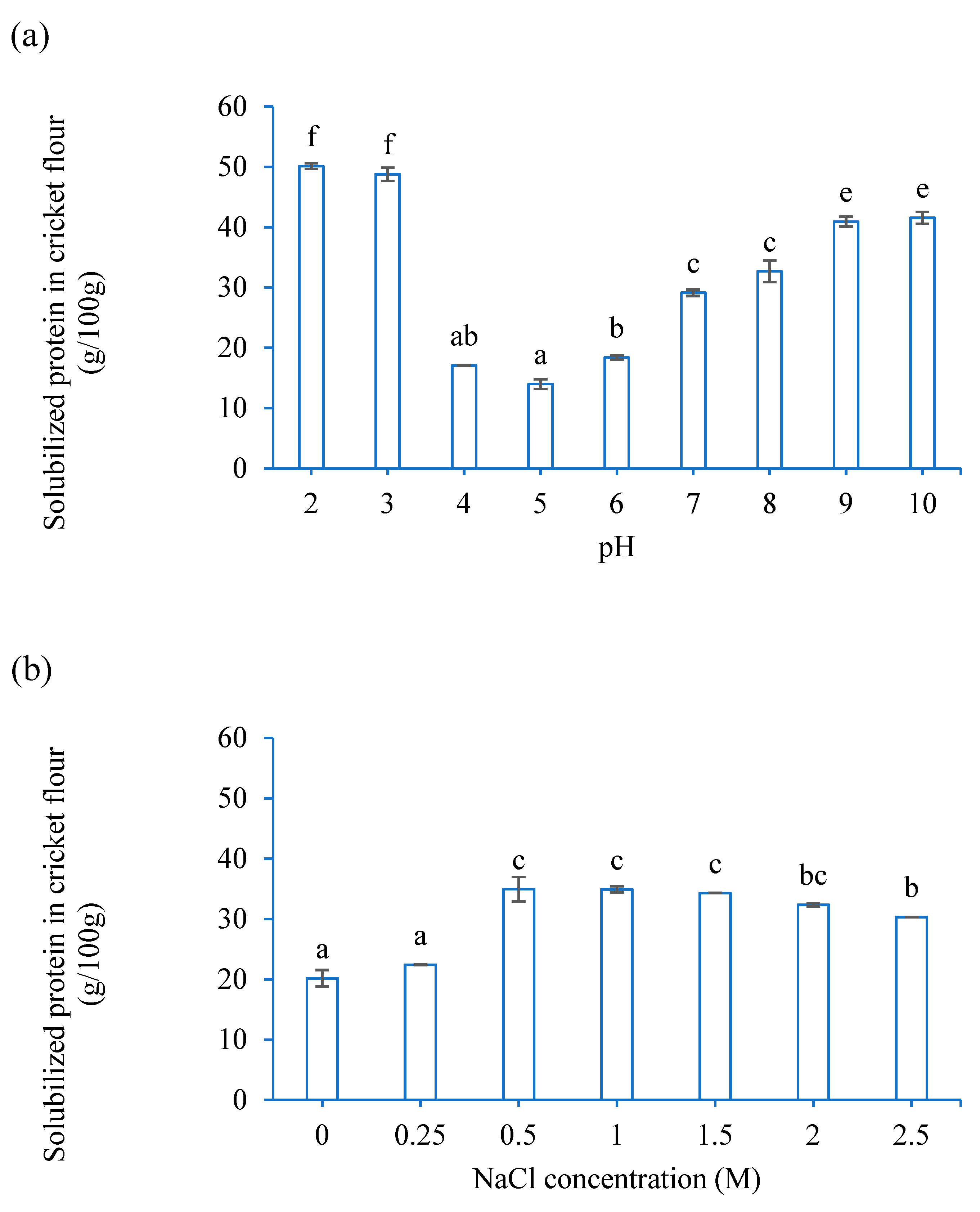
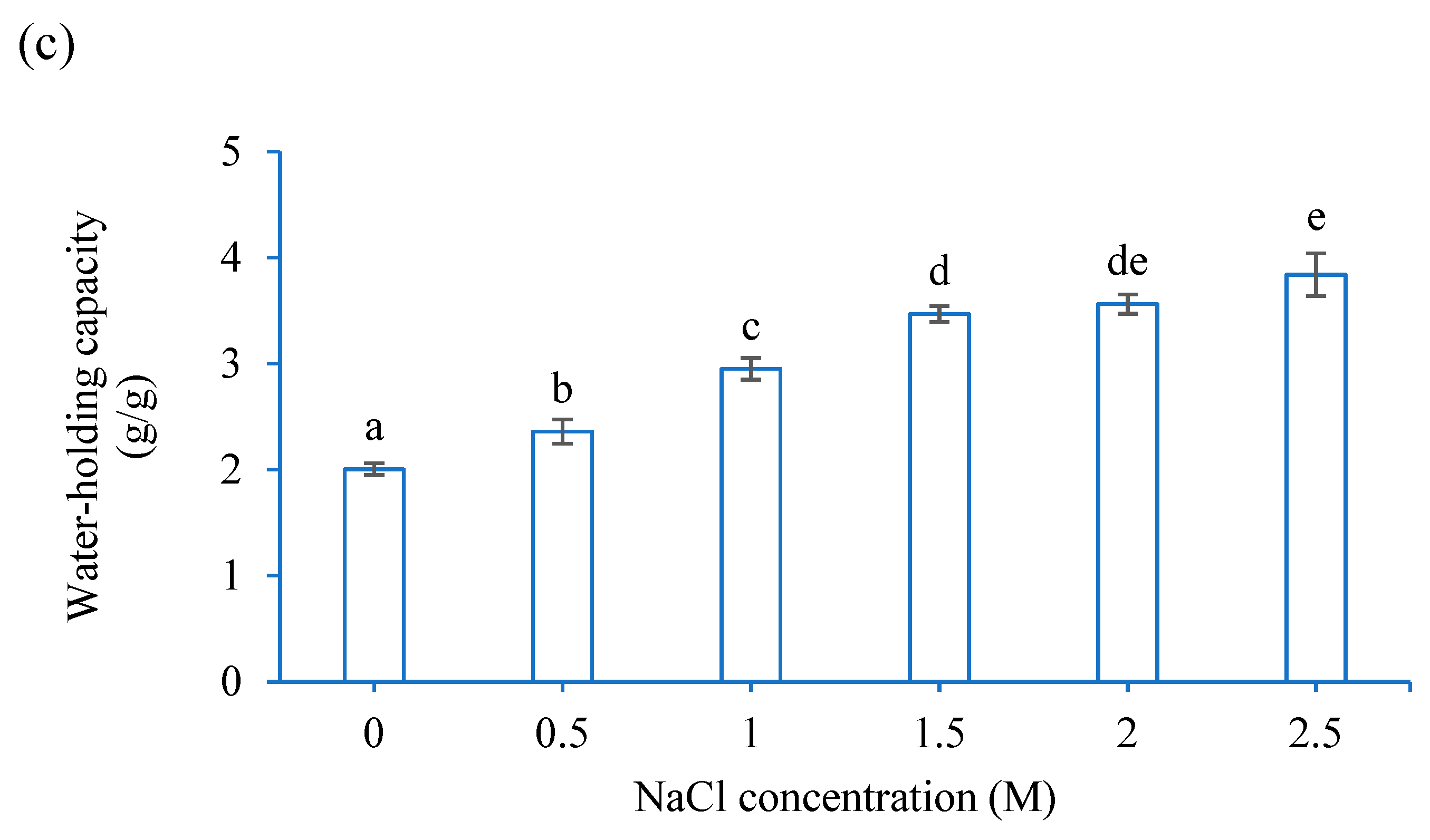
3.1. Functional Properties of CF
3.2. Proximate Compositions
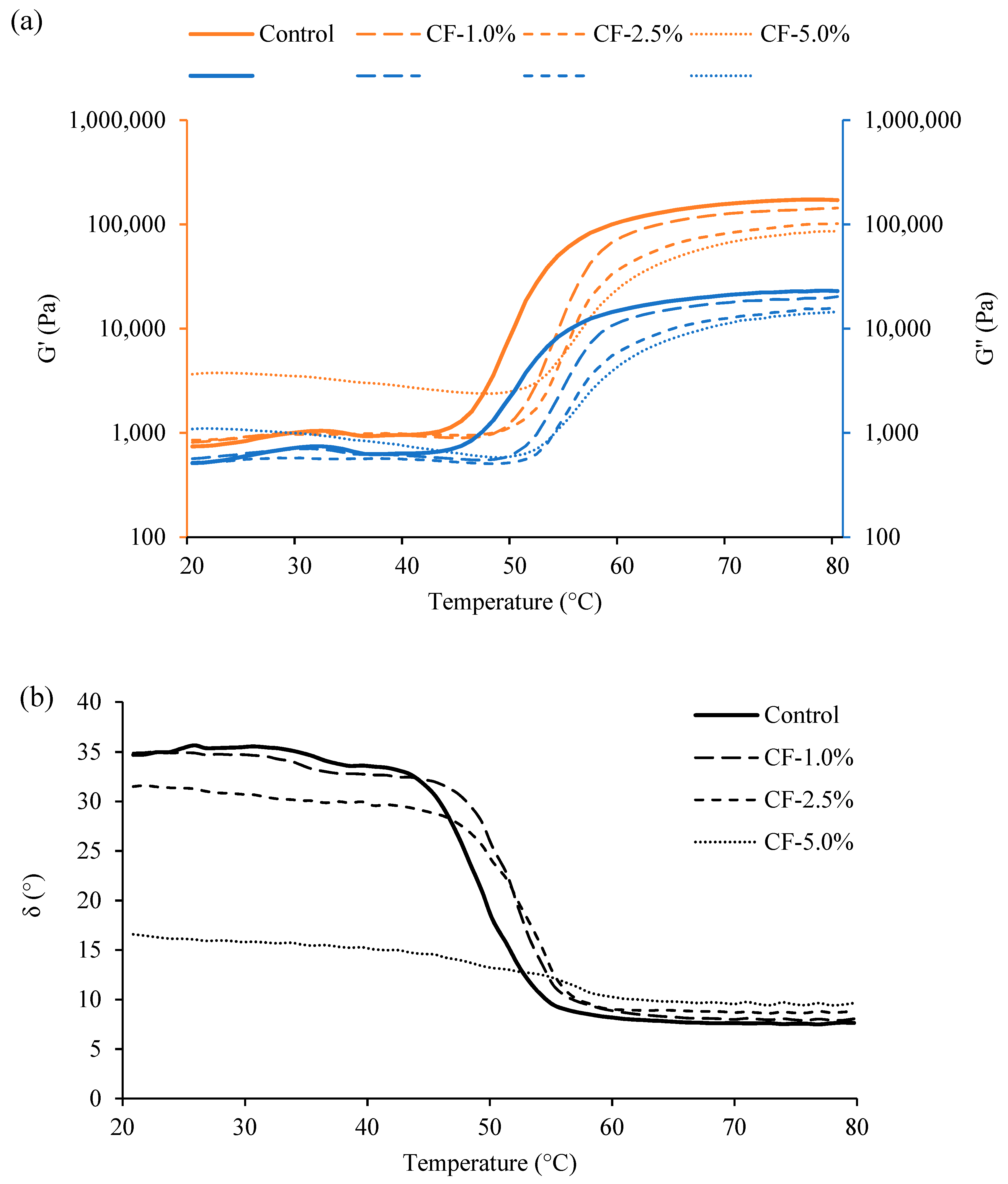
3.3. Rheological Properties of Raw Sausage Batter by Heating
3.4. Color and Texture
3.4.1. Color of Cooked Sausages
3.4.2. Texture of Cooked Sausages
3.5. Oxidative Stability of Cooked Sausages during Storage
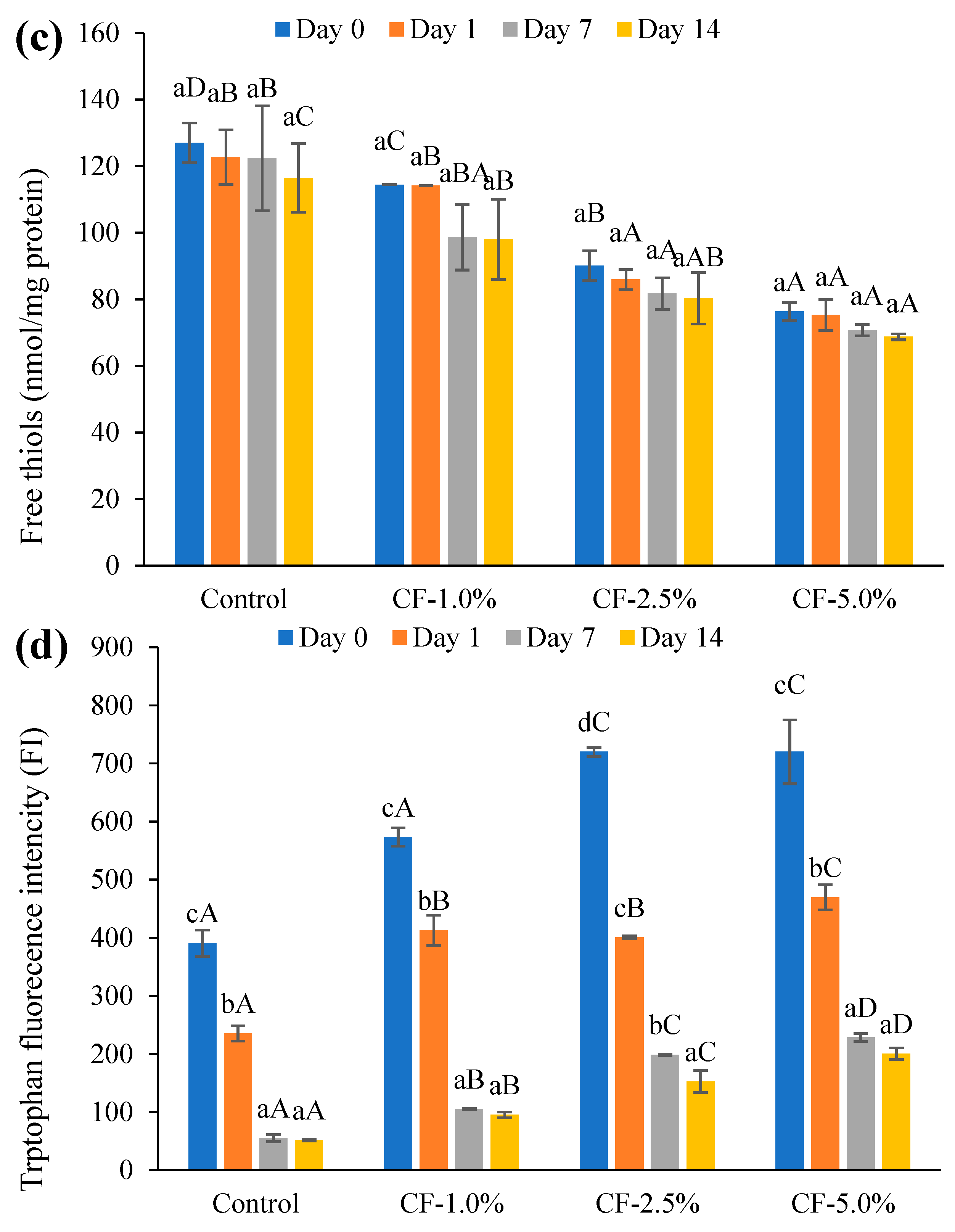
3.5.1. Lipid Oxidation
3.5.2. Protein Oxidation
3.5.3. Correlations of Oxidation Markers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | cricket flour |
| DNPH | 2,4-dinitrophenylhydrazine |
| DTNB | 5,5′-dithio-2-nitrobenzoate |
| FI | fluorescence intensity |
| r.t. | room temperature |
| TBARS | thiobarbituric acid-reactive substances |
References
- FAO. The State of Food Security and Nutrition in the World 2022. Repurposing food and agricultural policies to make healthy diets more affordable. In The State of Food Security and Nutrition in the World (SOFI); FAO: Rome, Italy, 2022. [Google Scholar]
- Ahmad, R.S.; Imran, A.; Hussain, M.B. Nutritional composition of meat. In Meat Science and Nutrition; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D. Could consumption of insects, cultured meat or imitation meat reduce global agricultural land use? Glob. Food Secur. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Machovina, B.; Feeley, K.J.; Ripple, W.J. Biodiversity conservation: The key is reducing meat consumption. Sci. Total. Environ. 2015, 536, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; FAO: Rome, Italy, 2006. [Google Scholar]
- Hedenus, F.; Wirsenius, S.; Johansson, D.J.A. The importance of reduced meat and dairy consumption for meeting stringent climate change targets. Clim. Chang. 2014, 124, 79–91. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunde, H.; Merten, E.; Hallora, A.; Muir, G.; Vantomm, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Nakagaki, B.J.; DeFoliart, G.R. Comparison of Diets for Mass-Rearing Acheta domesticus (Orthoptera: Gryllidae) as a Novelty Food, and Comparison of Food Conversion Efficiency with Values Reported for Livestock. J. Econ. Entomol. 1991, 84, 891–896. [Google Scholar] [CrossRef]
- Ververis, E.; Boué, G.; Poulsen, M.; Pires, S.M.; Niforou, A.; Thomsen, S.T.; Tesson, V.; Federighi, M.; Naska, A. A systematic review of the nutrient composition, microbiological and toxicological profile of Acheta domesticus (house cricket). J. Food Compos. Anal. 2022, 114, 104859. [Google Scholar] [CrossRef]
- Oonincx, D.; Finke, M. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed. 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Barrón-Hoyos, J.M.; Archuleta, A.R.; Falcón-Villa, M.D.R.; Canett-Romero, R.; Cinco-Moroyoqui, F.J.; Romero-Barancini, A.L.; Rueda-Puente, E.O. Protein Quality Evaluation of Animal Food Proteins by In-Vitro Methodologies. Food Nutr. Sci. 2013, 4, 376–384. [Google Scholar]
- Malla, N.; Nørgaard, J.V.; Lærke, H.N.; Heckmann, L.-H.L.; Roos, N. Some Insect Species Are Good-Quality Protein Sources for Children and Adults: Digestible Indispensable Amino Acid Score (DIAAS) Determined in Growing Pigs. J. Nutr. 2022, 152, 1042–1051. [Google Scholar] [CrossRef]
- del Mastro, N.L. Evolution of the interest on edible Insects. Am. J. Biol. Environ. Stat. 2021, 7, 52–56. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World (1 April 2017); Laboratory of Entomology, Wageningen University: Wageningen, The Netherlands, 2017; Available online: https://www.wur.nl/en/research-results/chair-groups/plant-sciences/laboratory-of-entomology/edible-insects/worldwide-species-list.htm (accessed on 14 February 2023).
- Looy, H.; Dunkel, F.V.; Wood, J.R. How then shall we eat? Insect-eating attitudes and sustainable foodways. Agric. Hum. Values 2013, 31, 131–141. [Google Scholar] [CrossRef]
- Meshulam-Pascoviche, D.; David-Birman, T.; Refael, G.; Lesmes, U. Big opportunities for tiny bugs: Processing effects on the techno-functionality and digestibility of edible insects. Trends Food Sci. Technol. 2022, 122, 265–274. [Google Scholar] [CrossRef]
- Megido, R.C.; Gierts, C.; Blecker, C.; Brostaux, Y.; Haubruge, É.; Alabi, T.; Francis, F. Consumer acceptance of insect-based alternative meat products in Western countries. Food Qual. Prefer. 2016, 52, 237–243. [Google Scholar] [CrossRef]
- Choi, J.-H.; Yong, H.I.; Ku, S.-K.; Kim, T.-K.; Choi, Y.-S. The Quality Characteristics of Pork Patties According to the Replacement of Mealworm (Tenebrio molitor L.). Korean J. Food Cook. Sci. 2019, 35, 441–449. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, T.-K.; Choi, H.-D.; Park, J.-D.; Sung, J.-M.; Jeon, K.-H.; Paik, H.-D.; Kim, Y.-B. Optimization of peplacing pork meat with yellow eorm (Tenebrio molitor L.) for frankfurters. Korean J. Food Sci. Anim. Resour. 2017, 37, 617–625. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.; Jones, O.G.; Kim, Y.H.B. Effect of House Cricket (Acheta domesticus) Flour Addition on Physicochemical and Textural Properties of Meat Emulsion Under Various Formulations. J. Food Sci. 2017, 82, 2787–2793. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lee, M.H.; Yong, H.I.; Jung, S.; Paik, H.-D.; Jang, H.W.; Choi, Y.-S. Effect of Interaction between Mealworm Protein and Myofibrillar Protein on the Rheological Properties and Thermal Stability of the Prepared Emulsion Systems. Foods 2020, 9, 1443. [Google Scholar] [CrossRef]
- Scholliers, J.; Steen, L.; Fraeye, I. Structure and physical stability of hybrid model systems containing pork meat and superworm (Zophobas morio larvae): The influence of heating regime and insect: Meat ratio. Innov. Food Sci. Emerg. Technol. 2020, 65, 102452. [Google Scholar] [CrossRef]
- Scholliers, J.; Steen, L.; Fraeye, I. Partial replacement of meat by superworm (Zophobas morio larvae) in cooked sausages: Effect of heating temperature and insect: Meat ratio on structure and physical stability. Innov. Food Sci. Emerg. Technol. 2020, 66, 102535. [Google Scholar] [CrossRef]
- Scholliers, J.; Steen, L.; Fraeye, I. Gelation of a combination of insect and pork proteins as affected by heating temperature and insect:meat ratio. Food Res. Int. 2020, 137, 109703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, C.; Kong, B.; Sun, F.; Shen, X.; Yao, X.; Liu, Q. Pre-dried mealworm larvae flour could partially replace lean meat in frankfurters: Effect of pre-drying methods and replacement ratios. Meat Sci. 2022, 188, 108802. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.R.; Scampicchio, M.; Angeli, S.; Ferrentino, G. Effect of hot melt extrusion on physical and functional properties of insect based extruded products. J. Food Eng. 2019, 259, 44–51. [Google Scholar] [CrossRef]
- Aleman, R.S.; Marcia, J.; Pournaki, S.K.; Borrás-Linares, I.; Lozano-Sanchez, J.; Fernandez, I.M. Formulation of protein-rich chocolate chip cookies rsing cricket (Acheta domesticus) powder. Foods 2022, 11, 3275. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar]
- Cho, S.Y.; Ryu, G.H. Effects of mealworm larva composition and selected process parameters on the physicochemical properties of extruded meat analog. Food Sci. Nutr. 2021, 9, 4408–4419. [Google Scholar] [CrossRef]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Kiiru, S.M.; Kinyuru, J.N.; Kiage, B.N.; Martin, A.; Marel, A.; Osen, R. Extrusion texturization of cricket flour and soy protein isolate: Influence of insect content, extrusion temperature, and moisture-level variation on textural properties. Food Sci. Nutr. 2020, 8, 4112–4120. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Cha, J.Y.; Park, S.-Y.; Jung, S.; Choi, Y.-S. Drying-induced restructured jerky analog developed using a combination of edible insect protein and textured vegetable protein. Food Chem. 2021, 373, 131519. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Pasini, G.; Cullere, M.; Vegro, M.; Simonato, B.; Zotte, A.D. Potentiality of protein fractions from the house cricket (Acheta domesticus) and yellow mealworm (Tenebrio molitor) for pasta formulation. LWT 2022, 16, 113638. [Google Scholar] [CrossRef]
- Severini, C.; Azzollini, D.; Albenzio, M.; Derossi, A. On printability, quality and nutritional properties of 3D printed cereal based snacks enriched with edible insects. Food Res. Int. 2018, 106, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Larki, N.A.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure design of insect-based meat analogs with high-moisture extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- ISO 1442: 1997; Meat and Meat Products—Determination of Moisture Content (Reference Method). International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species—Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- Han, X.; Heinonen, M. Development of ultra-high performance liquid chromatographic and fluorescent method for the analysis of insect chitin. Food Chem. 2020, 334, 127577. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, X.; Sun, F.; Jiang, S.; Liu, H.; Liu, Q.; Kong, B. Using a stable pre-emulsified canola oil system that includes porcine plasma protein hydrolysates and oxidized tannic acid to partially replace pork fat in frankfurters. Meat Sci. 2019, 160, 107968. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Sci. 2015, 110, 174–179. [Google Scholar] [CrossRef]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified Extraction 2-Thiobarbituric Acid Method for Measuring Lipid Oxidation in Poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; pp. 464–478. [Google Scholar]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016, 197, 670–675. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 2008, 80, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Ansah, P.; Besiwah, E.K.; Bonah, E.; Amagloh, F.K. Non-meat ingredients in meat products: A scoping review. Appl. Food Res. 2022, 2, 100044. [Google Scholar] [CrossRef]
- Brogan, E.N.; Park, Y.-L.; Matak, K.E.; Jaczynski, J. Characterization of protein in cricket (Acheta domesticus), locust (Locusta migratoria), and silk worm pupae (Bombyx mori) insect powders. LWT 2021, 152, 112314. [Google Scholar] [CrossRef]
- Adeyeye, E.; Emmanuel, A. Chemical composition of female and male giant African crickets, Brachytrypes Membranaceus L. Int. J. Pharm. Biol. Sci. 2010, 1, 125–136. [Google Scholar]
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.; Lewis, R.; Kaplan, D. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A 2005, 82, 223–233. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.; Chen, J. pH Shifting Alters Solubility Characteristics and Thermal Stability of Soy Protein Isolate and Its Globulin Fractions in Different pH, Salt Concentration, and Temperature Conditions. J. Agric. Food Chem. 2010, 58, 8035–8042. [Google Scholar] [CrossRef]
- Ye, X.-Q.; Liu, D.-H.; Hu, C. Some factors’ effects on the solubility of protein from yellow mealworm (Tenebrio molitor L.) larvae. J. Zhejiang Univ.-Sci. A 2001, 2, 436–438. [Google Scholar] [CrossRef]
- Hamm, R. Kolloidchemie des Fleisches. Das Wasserbindungsvermögen des Muskeleiweißes in Theorie und Praxis. 275 Seiten. Paul-Parey-Verlag, Berlin und Hamburg. Food/Nahr 1972, 16, 689–690. [Google Scholar]
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Adebowalea, Y.A.; Adebowal, K.O.; Oguntokunc, M.O. Evaluation of nutritive properties of the large African cricket (Gryllidae sp.). Pak. J. Sci. Ind. Res. 2005, 48, 274–278. [Google Scholar]
- Ishioroshi, M.; Jima, K.S.; Yasui, T. Heat-induced gelation of myosin: Factors of pH and salt concentrations. J. Food Sci. 1979, 44, 1280–1284. [Google Scholar] [CrossRef]
- Kim, J.H.; Seong, P.N.; Cho, S.H.; Park, B.Y.; Hah, K.H.; Yu, L.H.; Lim, D.G.; Hwang, I.H.; Kim, D.H.; Lee, J.M.; et al. Characterization of Nutritional Value for Twenty-one Pork Muscles. Asian-Australas. J. Anim. Sci. 2008, 21, 138–143. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Jin, G.; Wang, Y.; Wang, J.; Puolanne, E.; Cao, J. Role of low molecular additives in the myofibrillar protein gelation: Underlying mechanisms and recent applications. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Visessanguan, W.; Ogawa, M.; Nakai, S.; An, H. Physicochemical Changes and Mechanism of Heat-Induced Gelation of Arrowtooth Flounder Myosin. J. Agric. Food Chem. 2000, 48, 1016–1023. [Google Scholar] [CrossRef]
- Jongberg, S.; Terkelsen, L.D.S.; Miklos, R.; Lund, M.N. Green tea extract impairs meat emulsion properties by disturbing protein disulfide cross-linking. Meat Sci. 2015, 100, 2–9. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Beldade, P. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 2009, 20, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. 11—Quinone-Tanned Scleroproteins. In Metabolic Biochemistry and Molecular Biomechanics; Hochachka, P.W., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 467–504. [Google Scholar]
- Park, Y.-S.; Choi, Y.-S.; Hwang, K.-E.; Kim, T.-K.; Lee, C.-W.; Shin, D.-M.; Han, S.G. Physicochemical Properties of Meat Batter Added with Edible Silkworm Pupae (Bombyx mori) and Transglutaminase. Korean J. Food Sci. Anim. Resour. 2017, 37, 351–359. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef]
- Vehovský, K.; Zadinová, K.; Stupka, R.; Čítek, J.; Lebedová, N.; Okrouhlá, M.; Šprysl, M. Fatty acid composition in pork fat: De-novo synthesis, fatty acid sources and influencing factors—A review. Agron. Res. 2018, 16, 2211–2228. [Google Scholar]
- David-Birman, T.; Raften, G.; Lesmes, U. Effects of thermal treatments on the colloidal properties, antioxidant capacity and in-vitro proteolytic degradation of cricket flour. Food Hydrocoll. 2018, 79, 48–54. [Google Scholar] [CrossRef]
- Kurioka, A.; Yamazaki, M. Antioxidant in the Cocoon of the Silkworm, Bombyx mori. J. Insect Biotechnol. Sericol. 2002, 71, 177–180. [Google Scholar]
- Vanqa, N.; Mshayisa, V.V.; Basitere, M. Proximate, physicochemical, techno-functional and antioxidant properties of three edible insect (Gonimbrasia belina, Hermetia illucens and Macrotermes subhylanus) flours. Foods 2022, 11, 976. [Google Scholar] [CrossRef]
- Heinonen, M.; Gürbüz, G.; Ertbjerg, P. Chapter 3—Oxidation of proteins. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 85–123. [Google Scholar]
- Baron, C.P.; Andersen, H.J. Myoglobin-induced lipid oxidation. A Review. J. Agric. Food Chem. 2002, 50, 3887–3897. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Keto, L.; Mäkinen, S.; Seppä, L.; Puolanne, E.; Mäki, M. Lipid oxidation in cooked pork meat sausages as affected by natural antioxidant combinations. In Proceedings of the 65th International Congress of Meat Sciences and Technology, Potsdam, Germany, 4–8 August 2019. [Google Scholar]

| Ingredients | Control | CF-1.0% | CF-2.5% | CF-5.0% |
|---|---|---|---|---|
| Lean pork | 2100 (35.0%) | 2100 (34.7%) | 2100 (34.1%) | 2100 (33.3%) |
| Pork fat | 1200 (20.0%) | 1200 (19.8%) | 1200 (19.5%) | 1200 (19.0%) |
| NaCl | 120 (2.0%) | 120 (2.0%) | 120 (2.0%) | 120 (1.9%) |
| Phosphate * | 30 (0.5%) | 30 (0.5%) | 30 (0.5%) | 30 (0.5%) |
| Ice-water | 2550 (42.5%) | 2550 (42.1%) | 2550 (41.5%) | 2550 (40.5%) |
| CF | - | 60 (1.0%) | 150 (2.4%) | 300 (4.8%) |
| Total weight | 6000 | 6060 | 6150 | 6300 |
| Moisture | Crude Fat | Protein | Chitin | Others # | ||
|---|---|---|---|---|---|---|
| % | % | % | % | % | ||
| Ingredients | Lean pork | 74.9 ± 0.2 | 3.3 ± 0.8 | 20.3 ± 0.4 | - | 1.5 ± 0.4 |
| CF * | 8.2 ± 1.3 | 23.5 ± 0.5 | 56.0 ± 0.5 | 8.1 ± 0.8 | 4.2 ± 1.0 | |
| Sausages | Control | 68.7 ± 0.1 | 21.2 ± 0.3 | 7.1 ± 0.1 | 0.0 | 3.0 ± 0.2 |
| CF-1.0% | 68.1 ± 0.1 | 21.2 ± 0.3 | 7.6 ± 0.1 | 0.1 ± 0.0 | 3.0 ± 0.2 | |
| CF-2.5% | 67.2 ± 0.1 | 21.2 ± 0.3 | 8.3 ± 0.1 | 0.2 ± 0.0 | 3.0 ± 0.2 | |
| CF-5.0% | 65.8 ± 0.1 | 21.3 ± 0.3 | 9.4 ± 0.1 | 0.4 ± 0.0 | 3.1 ± 0.2 |
| Control | CF-1.0% | CF-2.5% | CF-5.0% | ||
|---|---|---|---|---|---|
| Color | L* | 74.8 ± 0.3 d | 71.2 ± 0.1 c | 69.1 ± 0.5 b | 66.0 ± 0.5 a |
| a* | 5.4 ± 0.0 a | 5.5 ± 0.2 a | 6.5 ± 0.2 b | 6.7 ± 0.4 b | |
| b* | 11.4 ± 0.1 a | 11.7 ± 0.2 a | 12.3 ± 0.2 b | 12.9 ± 0.1 c | |
| ΔE* | 4.1 ± 0.1 a | 6.5 ± 0.5 b | 9.7 ± 0.9 c | ||
| Texture | Hardness (g) | 743.9 ± 21.1 c | 573.2 ± 19.4 b | 245.7 ± 8.5 a | 271.0 ± 18.0 a |
| Springiness (ratio) | 0.9 ± 0.0 d | 0.8 ± 0.0 c | 0.7 ± 0.0 b | 0.5 ± 0.0 a | |
| Cohesiveness | 0.7 ± 0.0 d | 0.6 ± 0.0 c | 0.5 ± 0.0 b | 0.4 ± 0.0 a | |
| Chewiness (N) | 475.7 ± 23.9 c | 254.5 ± 5.5 b | 80.3 ± 4.5 a | 56.8 ± 2.6 a | |
| Resilience | 0.4 ± 0.0 d | 0.2 ± 0.0 c | 0.2 ± 0.0 b | 0.1 ± 0.0 a | |
| Fracturability | 14.9 ± 0.5 d | 13.5 ± 0.8 c | 10.3 ± 0.2 b | 7.4 ± 0.1 a | |
| TBARS | Carbonyls | Free Thiols | Tryptophan | |
|---|---|---|---|---|
| Storage days | ** | ** | ** | ** |
| CF% | ** | ** | ** | ** |
| Storage day × CF% | ** | NS | NS | ** |
| Correlation | TBARS | Carbonyls | Free Thiols | Tryptophan |
|---|---|---|---|---|
| TBARS | 1 | |||
| Carbonyls | 0.566 ** | 1 | ||
| Free thiols | −0.640 ** | −0.854 ** | 1 | |
| Tryptophan | −0.243 | 0.152 | −0.184 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Li, B.; Puolanne, E.; Heinonen, M. Hybrid Sausages Using Pork and Cricket Flour: Texture and Oxidative Storage Stability. Foods 2023, 12, 1262. https://doi.org/10.3390/foods12061262
Han X, Li B, Puolanne E, Heinonen M. Hybrid Sausages Using Pork and Cricket Flour: Texture and Oxidative Storage Stability. Foods. 2023; 12(6):1262. https://doi.org/10.3390/foods12061262
Chicago/Turabian StyleHan, Xiaocui, Binbin Li, Eero Puolanne, and Marina Heinonen. 2023. "Hybrid Sausages Using Pork and Cricket Flour: Texture and Oxidative Storage Stability" Foods 12, no. 6: 1262. https://doi.org/10.3390/foods12061262
APA StyleHan, X., Li, B., Puolanne, E., & Heinonen, M. (2023). Hybrid Sausages Using Pork and Cricket Flour: Texture and Oxidative Storage Stability. Foods, 12(6), 1262. https://doi.org/10.3390/foods12061262







