The Anti-Fatigue Effect of Glycoprotein from Hairtail Fish (Trichiurus lepturus) on BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HGP
2.3. Antioxidant Activity of HGP
2.4. Animals
2.5. Behavioral Tests of Mice
2.5.1. Weight-Loaded Swimming Test
2.5.2. Pole Climbing Test
2.5.3. Passive Avoidance Test
2.5.4. Elevated Cross Maze Test
2.6. Determination of Biochemical Indexes Related to Anti-Fatigue Activity
2.7. Statistical Analysis
3. Results
3.1. Antioxidant Activity of HGP In Vitro
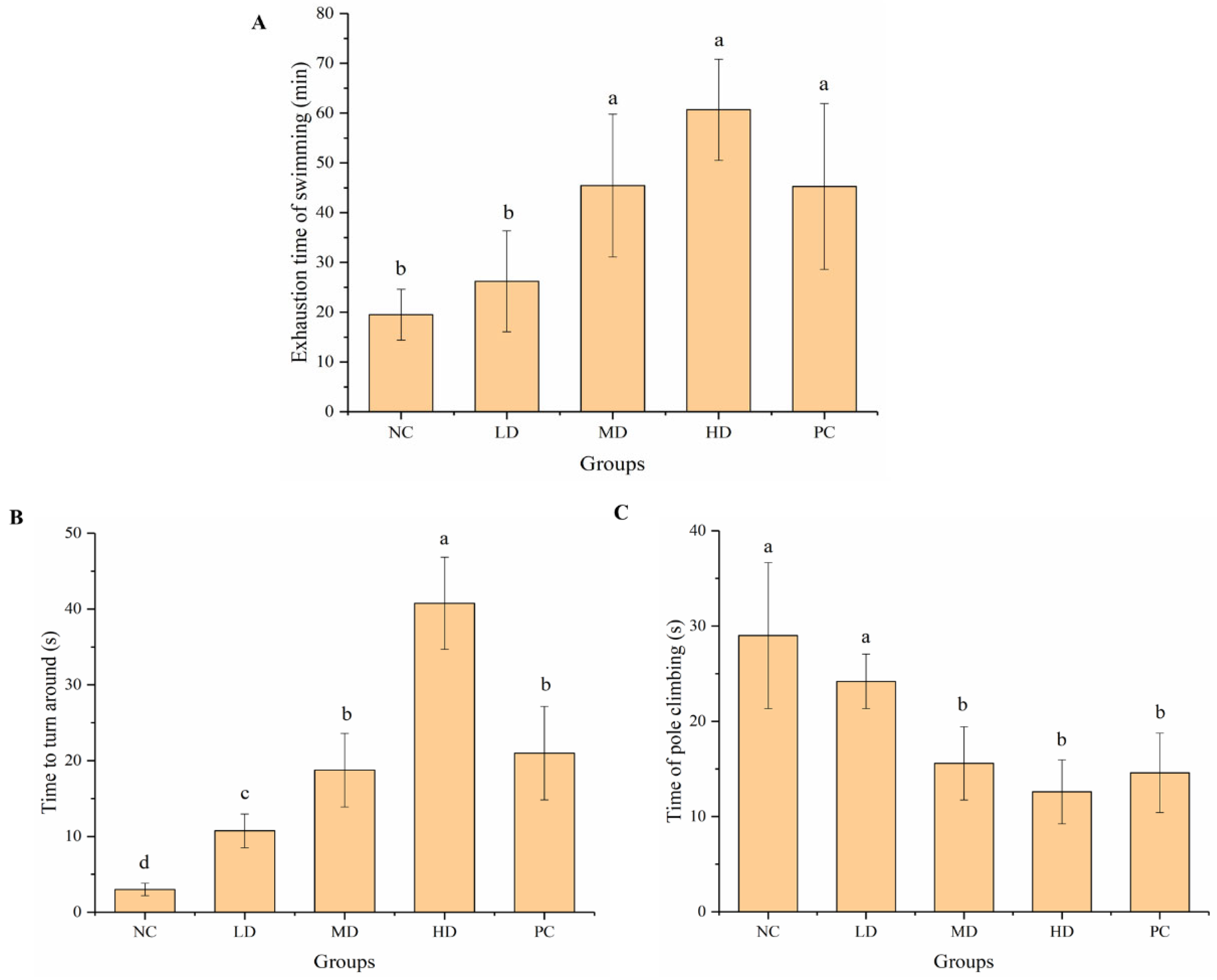
3.2. Effects on Behavior of Experimental Mice
3.2.1. Weight-Loaded Swimming Test and Pole Climbing Test
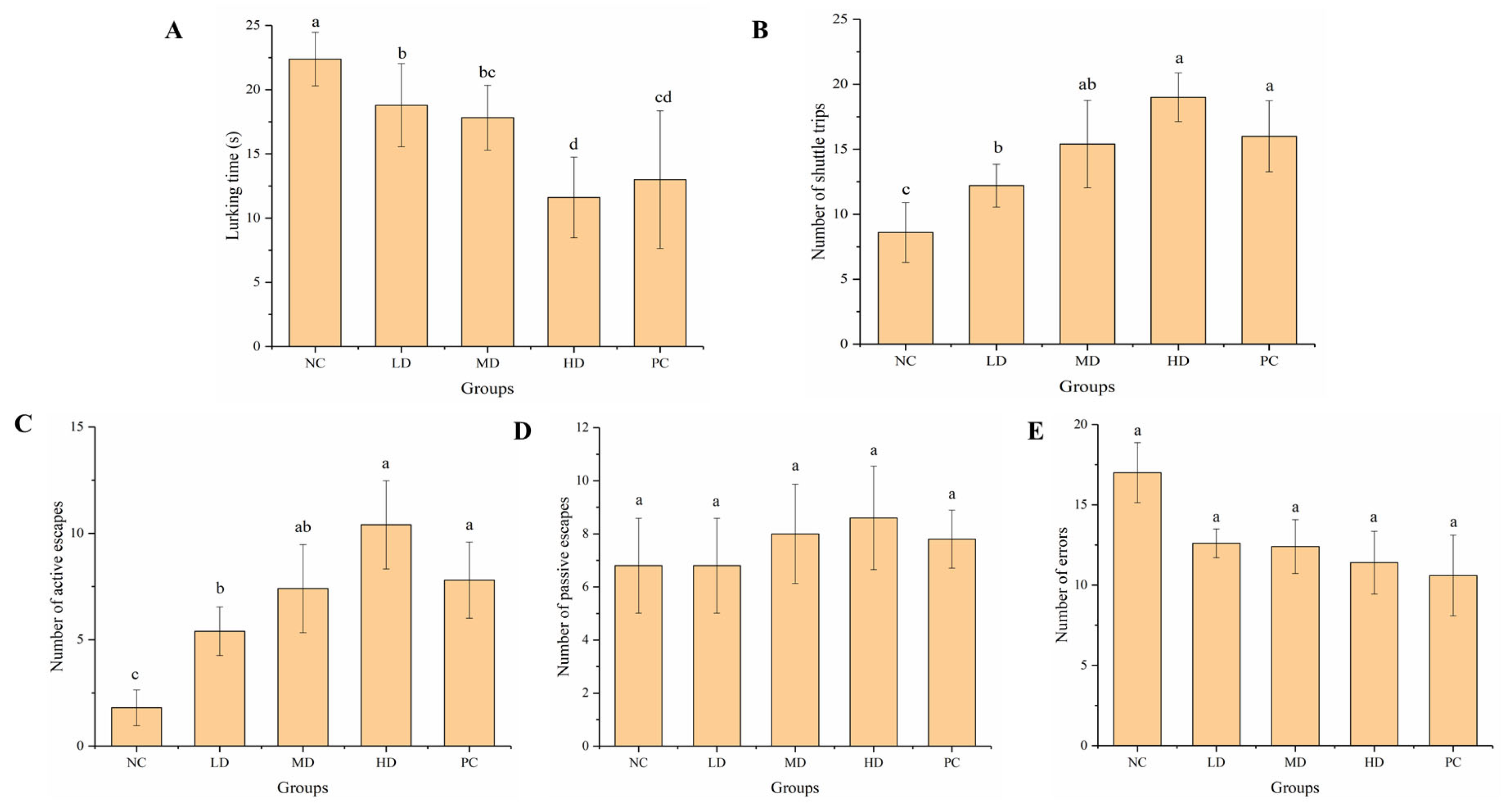
3.2.2. Passive Avoidance Test
3.2.3. Elevated Cross Maze Test
3.3. General Health and Body and Organ Weights
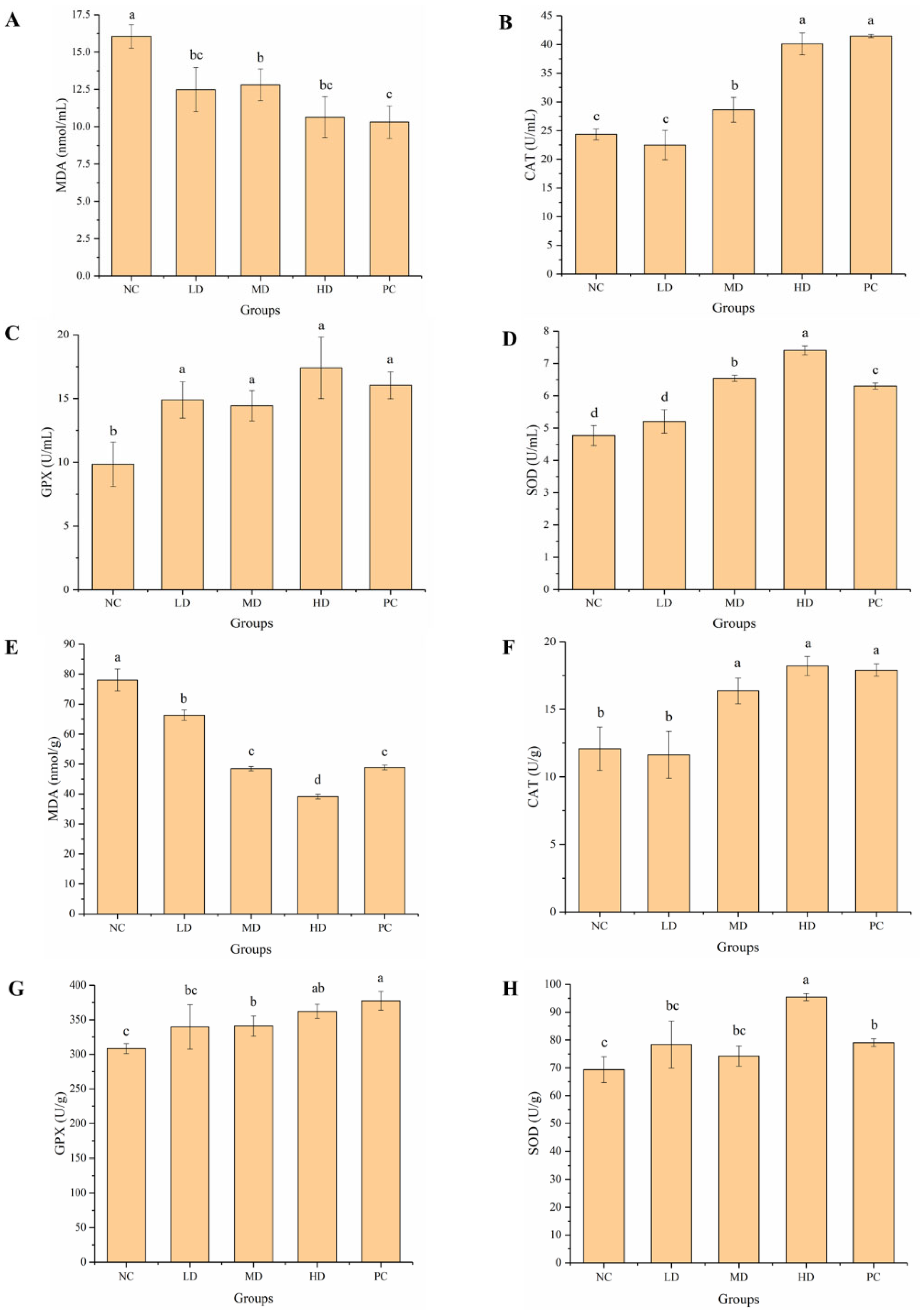
3.4. Effect of HGP on Serum Biochemical Indexes
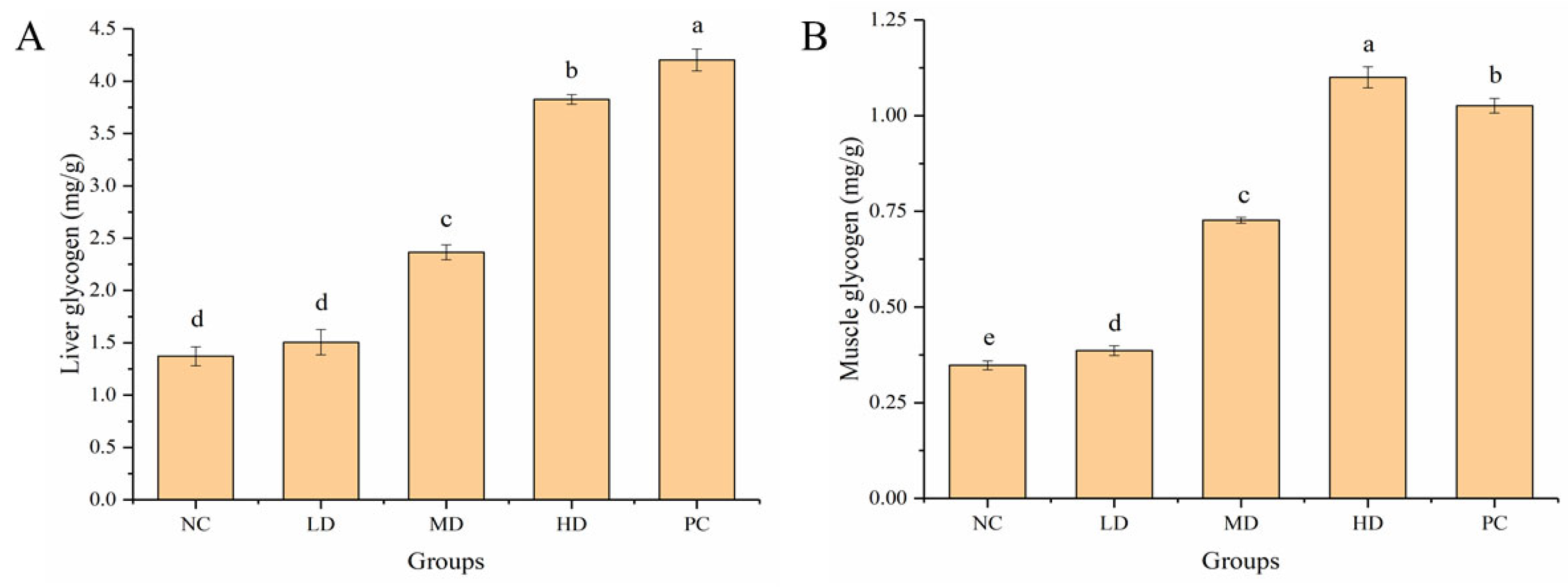
3.5. Effect of HGP on LG and MG
3.6. Effect of HGP on Serum and Brain Antioxidant Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HGP | Hairtail fish (Trichiurus lepturus) glycoprotein |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPX | Glutathione peroxidase |
| MDA | Malonaldehyde |
| LDH | Lactate dehydrogenase |
| BLA | Blood lactic acid |
| BUN | Blood urea nitrogen |
| CK | Creatine kinase |
| LG | Liver glycogen |
| MG | Muscle glycogen |
References
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Xiao, B.; Shui, S.; Yang, J.; Huang, R.; Dong, J. Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. J. Pharm. Biomed. Anal. 2018, 151, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, E. Fatigue in industry. Occup. Environ. Med. 1979, 36, 175–186. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Knutsson, A.; Westerholm, P.; Theorell, T.; Alfredsson, L.; Kecklund, G. Mental fatigue, work and sleep. J. Psychosom. Res. 2004, 57, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liang, R.; Li, Y.; Wang, J. Anti-fatigue effects of dietary nucleotides in mice. Food Nutr. Res. 2017, 61, 1334485. [Google Scholar] [CrossRef]
- Zhu, H.; Han, G. Chronic Fatigue Stress and Sudden Death. In Sudden Death: Advances in Diagnosis and Treatment; Springer: Singapore, 2021; pp. 117–135. [Google Scholar]
- Dawson, D.; McCulloch, K. Managing fatigue: It’s about sleep. Sleep Med. Rev. 2005, 9, 365–380. [Google Scholar] [CrossRef]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef]
- Welch, J.F.; Kipp, S.; Sheel, A.W. Respiratory muscles during exercise: Mechanics, energetics, and fatigue. Curr. Opin. Physiol. 2019, 10, 102–109. [Google Scholar] [CrossRef]
- Sundberg, C.W.; Fitts, R.H. Bioenergetic basis of skeletal muscle fatigue. Curr. Opin. Physiol. 2019, 10, 118–127. [Google Scholar] [CrossRef]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Zhou, J.; Xu, J.-D.; Shen, H.; Kong, M.; Yip, K.-M.; Han, Q.-B.; Zhao, Z.-Z.; Xu, J.; Chen, H.-B.; et al. Ginseng ameliorates exercise-induced fatigue potentially by regulating the gut microbiota. Food Funct. 2021, 12, 3954–3964. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, S.; Wu, L.; Wei, L.-L.; Wang, H. Main Title: Fatigue Exercise Alters the Profiles of Phys-iological Biomarkers in As-sociation with Four Metabolic Pathways: A Metabolomic Study. J. Nutr. Obes. 2021, 2, 104. [Google Scholar]
- Wu, L.; Jiayi, W.; Cao, X.; Tian, Y.; Li, J. Effect of Acute High-Intensity Exercise on Rat Myocardium metabolic Profiles. LC-MS Based Metab. Study 2022, 12, 6791. [Google Scholar]
- Peng, S.; Ji, H.; Song, W.; Wei, L.; Zhan, S.; Qu, Y.; Chen, M.; Zhang, D.; Liu, S. Anti-fatigue effect of small molecule oligopeptides from tilapia (Oreochromis Mossambicus) in mice. Food Sci. Technol. 2022, 42, e93021. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, H.; Lin, S.; Zhang, Z.; Zhang, Y.; Hu, J. Anti-fatigue activities of hairtail (Trichiurus lepturus) hydrolysate in an endurance swimming mice model. J. Funct. Foods 2020, 74, 104207. [Google Scholar] [CrossRef]
- Qiao, Y.; Ye, Y.; Cai, T.; Li, S.; Liu, X. Anti-fatigue activity of the polysaccharides isolated from Ribes stenocarpum Maxim. J. Funct. Foods 2022, 89, 104947. [Google Scholar] [CrossRef]
- Kang, C.; Liu, Y.; Chi, A.; Zhang, Z. The anti-fatigue potential of water-soluble polysaccharides of Semen cassiae on BALB/c mice. Cell. Mol. Biol. 2021, 67, 148–154. [Google Scholar] [CrossRef]
- Qi, B.; Liu, L.; Zhang, H.; Zhou, G.-X.; Wang, S.; Duan, X.-Z.; Bai, X.-Y.; Wang, S.-M.; Zhao, D. Anti-fatigue effects of proteins isolated from Panax quinquefolium. J. Ethnopharmacol. 2014, 153, 430–434. [Google Scholar] [CrossRef]
- Kornfeld, R.; Kornfeld, S. Comparative aspects of glycoprotein structure. Annu. Rev. Biochem. 1976, 45, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.-Z.; Wang, X.; Yang, R.; Qu, L.-B.; Zeng, H.-J. Studies on the effect of a Fupenzi glycoprotein on the fibrillation of bovine serum albumin and its antioxidant activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118387. [Google Scholar] [CrossRef]
- Shi, J.; Xu, Y.; Guo, J.; Mu, X.; Wang, C.; Chen, X.; Zhang, J. Extraction, purification, characterization, and rheological properties of a glycoprotein from Cynomorium songaricum Rupr. Biotechnol. Appl. Biochem. 2021, 68, 41–51. [Google Scholar] [CrossRef]
- Li, T.; Meng, X.; Wu, C.; Fan, G.; Yang, J.; Pan, W. Anticancer activity of a novel glycoprotein from Camellia oleifera Abel seeds against hepatic carcinoma in vitro and in vivo. Int. J. Biol. Macromol. 2019, 136, 284–295. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Osman, A.; Al-Mohammadi, A.-R.; Enan, G.; Kamal, N.; Sitohy, M. Biochemical, biological characteristics and antibacterial activity of glycoprotein extracted from the epidermal mucus of African catfish (Clarias gariepinus). Int. J. Biol. Macromol. 2019, 138, 773–780. [Google Scholar] [CrossRef]
- Oh, P.-S.; Lim, K.-T. Antioxidant activity of Dioscorea batatas Decne glycoprotein. Eur. Food Res. Technol. 2007, 226, 507–515. [Google Scholar] [CrossRef]
- Shao, S.; Wang, M.-X.; Zhang, H.-Y.; Fan, L.; Han, R.-X.; Shen, Y.-X.; Yan, M.-M.; Zhao, D.-Q. Antifatigue activity of glycoprotein from Schisandra chinensis functions by reducing oxidative stress. Evidence-Based Complement. Altern. Med. 2020, 2020, 8. [Google Scholar] [CrossRef]
- Virto, L.R. A preliminary assessment of the indicators for Sustainable Development Goal (SDG) 14 “Conserve and sustainably use the oceans, seas and marine resources for sustainable development”. Mar. Policy 2018, 98, 47–57. [Google Scholar] [CrossRef]
- Hu, F.; Zhong, H.; Wu, C.; Wang, S.; Guo, Z.; Tao, M.; Zhang, C.; Gong, D.; Gao, X.; Tang, C. Development of fisheries in China. Reprod. Breed. 2021, 1, 64–79. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Yuan, C.; Morioka, K.; Chen, S.; Liu, D.; Ye, X. Enhancement of the gelation properties of hairtail (Trichiurus haumela) muscle protein with curdlan and transglutaminase. Food Chem. 2015, 176, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lin, Y.; Wu, H.; Lin, J.; Chen, Y.; Hamzah, S.S.; Zeng, H.; Zhang, Y.; Hu, J. Preparation of antioxidant peptides from hairtail surimi using hydrolysis and evaluation of its antioxidant stability. Food Sci. Technol. 2020, 40, 945–955. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zeng, L.; Yang, Z.-S.; Huang, F.-F.; Ding, G.-F.; Wang, B. Anti-fatigue effect by peptide fraction from protein hydrolysate of croceine croaker (Pseudosciaena crocea) swim bladder through inhibiting the oxidative reactions including DNA damage. Mar. Drugs 2016, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liao, W.; Kang, M.; Jia, Y.; Wang, Q.; Duan, S.; Xiao, S.; Cao, Y.; Ji, H. Anti-fatigue and anti-oxidant activities of oyster (Ostrea rivularis) hydrolysate prepared by compound protease. Food Funct. 2018, 9, 6577–6585. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Regenstein, J.; Su, X. In vitro and in vivo anti-oxidation and anti-fatigue effect of monkfish liver hydrolysate. Food Biosci. 2017, 18, 9–14. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Z.; Wu, J.; Jiang, Y.; Zhang, H.; Zhan, X. Structural elucidation of N-glycans and bioactivity of sialoglycoprotein from crucian carp eggs structure and bioactivity of crucian egg SGP. Food Biosci. 2020, 38, 100776. [Google Scholar] [CrossRef]
- Lu, X.; Zhong, R.; Hu, L.; Huang, L.; Chen, L.; Cheng, W.; Zheng, B.; Liang, P. DHA-enriched phospholipids from large yellow croaker roe regulate lipid metabolic disorders and gut microbiota imbalance in SD rats with a high-fat diet. Food Funct. 2021, 12, 4825–4841. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Q.; Qu, Y.; Ye, F. Antioxidant activity and anti-exercise-fatigue effect of highly denatured soybean meal hydrolysate prepared using neutrase. J. Food Sci. Technol. 2015, 52, 1982–1992. [Google Scholar] [CrossRef]
- Yang, Q.; Jin, W.; Lv, X.; Dai, P.; Ao, Y.; Wu, M.; Deng, W.; Yu, L. Effects of macamides on endurance capacity and anti-fatigue property in prolonged swimming mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-F.; Li, Y.-Y.; Xu, J.-J.; Su, X.-R.; Gao, X.; Yue, F.-P. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocoll. 2011, 25, 1350–1353. [Google Scholar] [CrossRef]
- Farokhi, M.R.; Taherifard, E.; SoukhakLari, R.; Moezi, L.; Pirsalami, F.; Savardashtaki, A.; Moosavi, M. The memory modulatory effect of agmatine in passive avoidance task coincides with alterations of hippocampal CaMKII-α and ERK signaling in mice. Eur. J. Pharmacol. 2022, 923, 174928. [Google Scholar] [CrossRef]
- Fathalizade, F.; Baghani, M.; Khakpai, F.; Fazli-Tabaei, S.; Zarrindast, M.R. GABA-ergic agents modulated the effects of histamine on the behaviour of male mice in the elevated plus maze test. Exp. Physiol. 2022, 107, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.S.; Roberts, T.K.; Hugh Dunstan, R.; McGregor, N.R.; Butt, H.L. Free radicals in chronic fatigue syndrome: Cause or effect? Redox Rep. 2000, 5, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, R.; Chen, Z.; Yu, H.; Li, R.; Li, P. Effect and mechanism of mackerel (Pneumatophorus japonicus) peptides for anti-fatigue. Food Funct. 2014, 5, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, R.; Fernández-Teruel, A.; Gil, L.; Aguilar, R.; Tobeña, A. Driscoll, Inbred Roman high-and low-avoidance rats: Differences in anxiety, novelty-seeking, and shuttlebox behaviors. Physiol. Behav. 1999, 67, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S. Renewal of signaled shuttle box avoidance in rats. Learn. Motiv. 2014, 46, 27–43. [Google Scholar] [CrossRef]
- Duan, F.-F.; Guo, Y.; Li, J.-W.; Yuan, K. Antifatigue effect of luteolin-6-C-neohesperidoside on oxidative stress injury induced by forced swimming of rats through modulation of Nrf2/ARE signaling pathways. Oxidative Med. Cell. Longev. 2017, 2017, 13. [Google Scholar] [CrossRef]
- Chi, A.; Li, H.; Kang, C.; Guo, H.; Wang, Y.; Guo, F.; Tang, L. Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang green tea. Int. J. Biol. Macromol. 2015, 80, 566–572. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Lyu, C.; Li, Q.; Kou, F.; Jiang, M.; Wei, H. Metabolomics study on the intervention effect of Radix Salviae Miltiorrhizae extract in exercise-induced exhaustion rat using gas chromatography coupled to mass spectrometry. J. Chromatogr. B 2021, 1178, 122805. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhao, M.; Wang, H.; Cui, C.; You, L. Effects of supplementation with grass carp protein versus peptide on swimming endurance in mice. Nutrition 2011, 27, 789–795. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.-W.; Chen, Q.-H.; Li, D.; Mao, R.-X.; Liu, X.-R.; Li, Y. Small molecule oligopeptides isolated from walnut (Juglans regia L.) and their anti-fatigue effects in mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef]
- Pather, S.; Gerlai, R. Shuttle box learning in zebrafish (Danio rerio). Behav. Brain Res. 2009, 196, 323–327. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, J.; Kang, Q.; Huang, J.; Cui, Y.; Zhang, G.; Wang, Z.; Zhang, L.; Zheng, Z.; Lu, J.; et al. Anti-fatigue activity of hemp leaves water extract and the related biochemical changes in mice. Food Chem. Toxicol. 2021, 150, 112054. [Google Scholar] [CrossRef]
- Tan, W.; Yu, K.-Q.; Liu, Y.-Y.; Ouyang, M.-Z.; Yan, M.-H.; Luo, R.; Zhao, X.-S. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int. J. Biol. Macromol. 2012, 50, 59–62. [Google Scholar] [CrossRef]
- Narkhede, A.N.; Jagtap, S.; Nirmal, P.S.; Giramkar, S.A.; Nagarkar, B.E.; Kulkarni, O.; Harsulkar, A.M. Anti-fatigue effect of Amarkand on endurance exercise capacity in rats. BMC Complement. Altern. Med. 2015, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K. Mechanisms and effects of lipid peroxidation. Mol. Asp. Med. 1993, 14, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Cand, F.; Verdetti, J. Superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation in the major organs of the aging rats. Free. Radic. Biol. Med. 1989, 7, 59–63. [Google Scholar] [CrossRef] [PubMed]
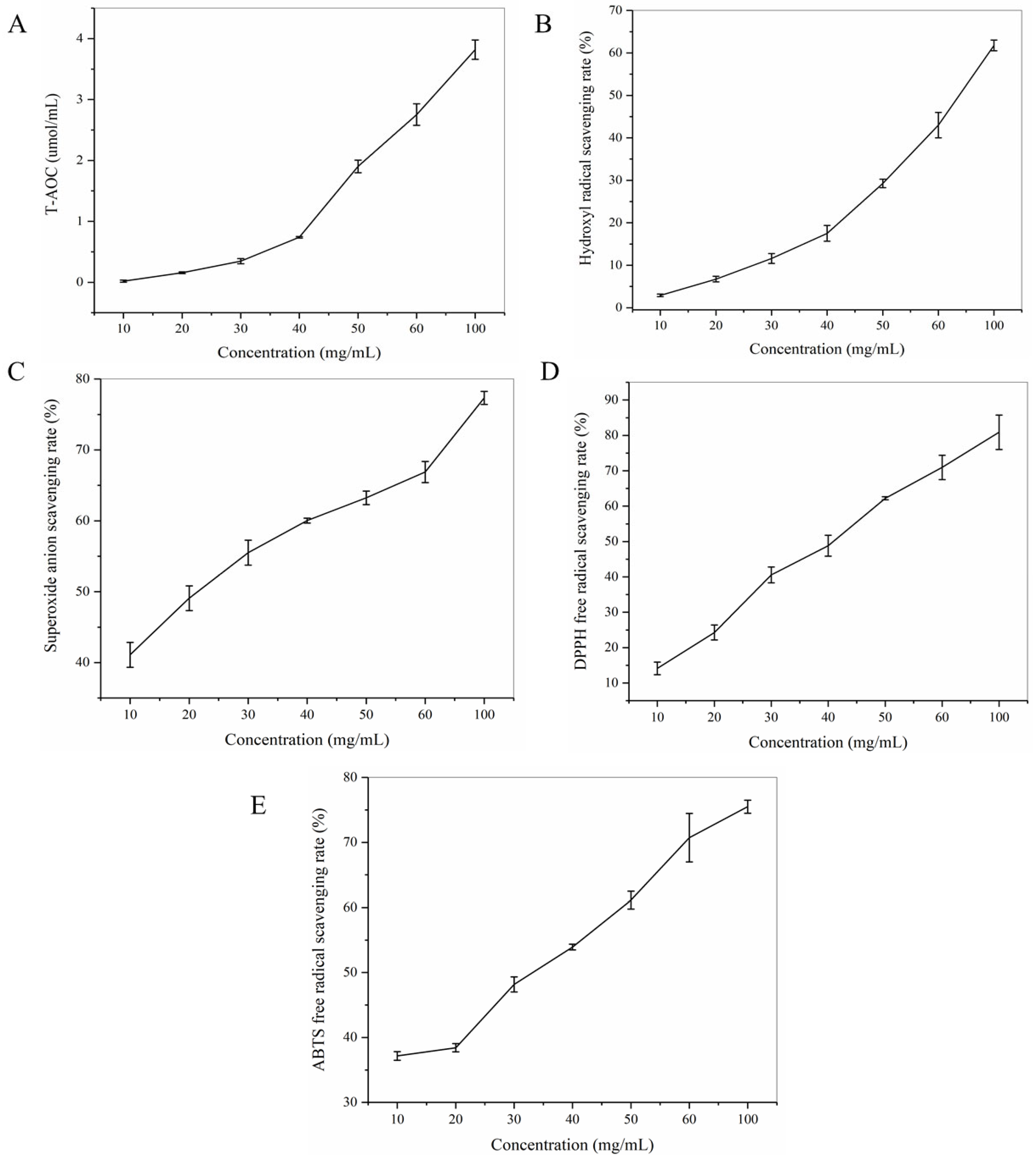


| T-AOC (µmol/mL) | Hydroxyl Radical Scavenging Rate (%) | Superoxide Anion Scavenging Rate (%) | DPPH Free Radical Scavenging Rate (%) | ABTS Free Radical Scavenging Rate (%) |
|---|---|---|---|---|
| 7.41 ± 0.03 | 64.29 ± 3.72 | 98.79 ± 0.26 | 92.31 ± 0.63 | 84.46 ± 1.07 |
| Group | 0 Weeks | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | 5 Weeks | 6 Weeks | 7 Weeks |
|---|---|---|---|---|---|---|---|---|
| Weight (g) | ||||||||
| NC | 20.78 ± 0.80 a | 23.44 ± 1.03 a | 24.93 ± 1.20 a | 26.40 ± 1.55 a | 27.16 ± 1.49 a | 28.53 ± 1.50 a | 29.34 ± 1.44 a | 29.42 ± 1.71 a |
| LD | 20.61 ± 1.03 a | 21.77 ± 1.18 c | 22.97 ± 1.29 b | 24.58 ± 1.24 b | 25.91 ± 1.27 b | 26.92 ± 1.28 b | 27.90 ± 1.23 b | 28.09 ± 1.28 b |
| MD | 20.29 ± 0.78 a | 22.20 ± 1.07 bc | 23.15 ± 0.97 b | 24.44 ± 1.07 b | 25.58 ± 1.30 b | 27.07 ± 1.24 b | 27.80 ± 1.14 b | 28.16 ± 1.25 b |
| HD | 20.53 ± 1.00 a | 22.40 ± 0.92 bc | 23.13 ± 1.20 b | 24.66 ± 1.54 b | 25.73 ± 1.93 b | 27.25 ± 1.71 b | 28.19 ± 1.70 ab | 28.36 ± 1.75 ab |
| PC | 20.57 ± 0.92 a | 22.91 ± 1.21 ab | 23.26 ± 1.30 b | 24.54 ± 1.28 b | 25.88 ± 1.37 b | 27.42 ± 1.06 b | 27.91 ± 1.17 b | 28.26 ± 1.14 b |
| Group | Brain Index (%) | Liver Index (%) | Gastrocnemius Index (%) |
|---|---|---|---|
| NC | 1.32 ± 0.09 a | 4.72 ± 0.21 a | 1.12 ± 0.03 b |
| LD | 1.33 ± 0.11 a | 4.75 ± 0.24 a | 1.12 ± 0.08 ab |
| MD | 1.36 ± 0.10 a | 4.76 ± 0.18 a | 1.14 ± 0.06 ab |
| HD | 1.36 ± 0.12 a | 4.80 ± 0.18 a | 1.17 ± 0.08 a |
| PC | 1.34 ± 0.09 a | 4.71 ± 0.25 a | 1.14 ± 0.11 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Chen, J.; Huang, L.; Ou, Y.; Wu, J.; Guo, Z.; Zheng, B. The Anti-Fatigue Effect of Glycoprotein from Hairtail Fish (Trichiurus lepturus) on BALB/c Mice. Foods 2023, 12, 1245. https://doi.org/10.3390/foods12061245
Lu X, Chen J, Huang L, Ou Y, Wu J, Guo Z, Zheng B. The Anti-Fatigue Effect of Glycoprotein from Hairtail Fish (Trichiurus lepturus) on BALB/c Mice. Foods. 2023; 12(6):1245. https://doi.org/10.3390/foods12061245
Chicago/Turabian StyleLu, Xiaodan, Jiaqi Chen, Luyao Huang, Yujia Ou, Jingru Wu, Zebin Guo, and Baodong Zheng. 2023. "The Anti-Fatigue Effect of Glycoprotein from Hairtail Fish (Trichiurus lepturus) on BALB/c Mice" Foods 12, no. 6: 1245. https://doi.org/10.3390/foods12061245
APA StyleLu, X., Chen, J., Huang, L., Ou, Y., Wu, J., Guo, Z., & Zheng, B. (2023). The Anti-Fatigue Effect of Glycoprotein from Hairtail Fish (Trichiurus lepturus) on BALB/c Mice. Foods, 12(6), 1245. https://doi.org/10.3390/foods12061245








