Quantification and Diversity Analyses of Major Glucosinolates in Conserved Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Germplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Glucosinolate Standards
2.2. Brassica Germplasms and Growth Condition
2.3. Sample Pretreatment and Extraction
2.4. Identification and Quantification of GSLs Using UPLC-MS/MS
2.5. Statistical Analysis
3. Results and Discussion
3.1. Variation of GSL Contents and Selection of Candidate Germplasms for the Breeding Program
3.2. Correlation Analysis among the GSLs
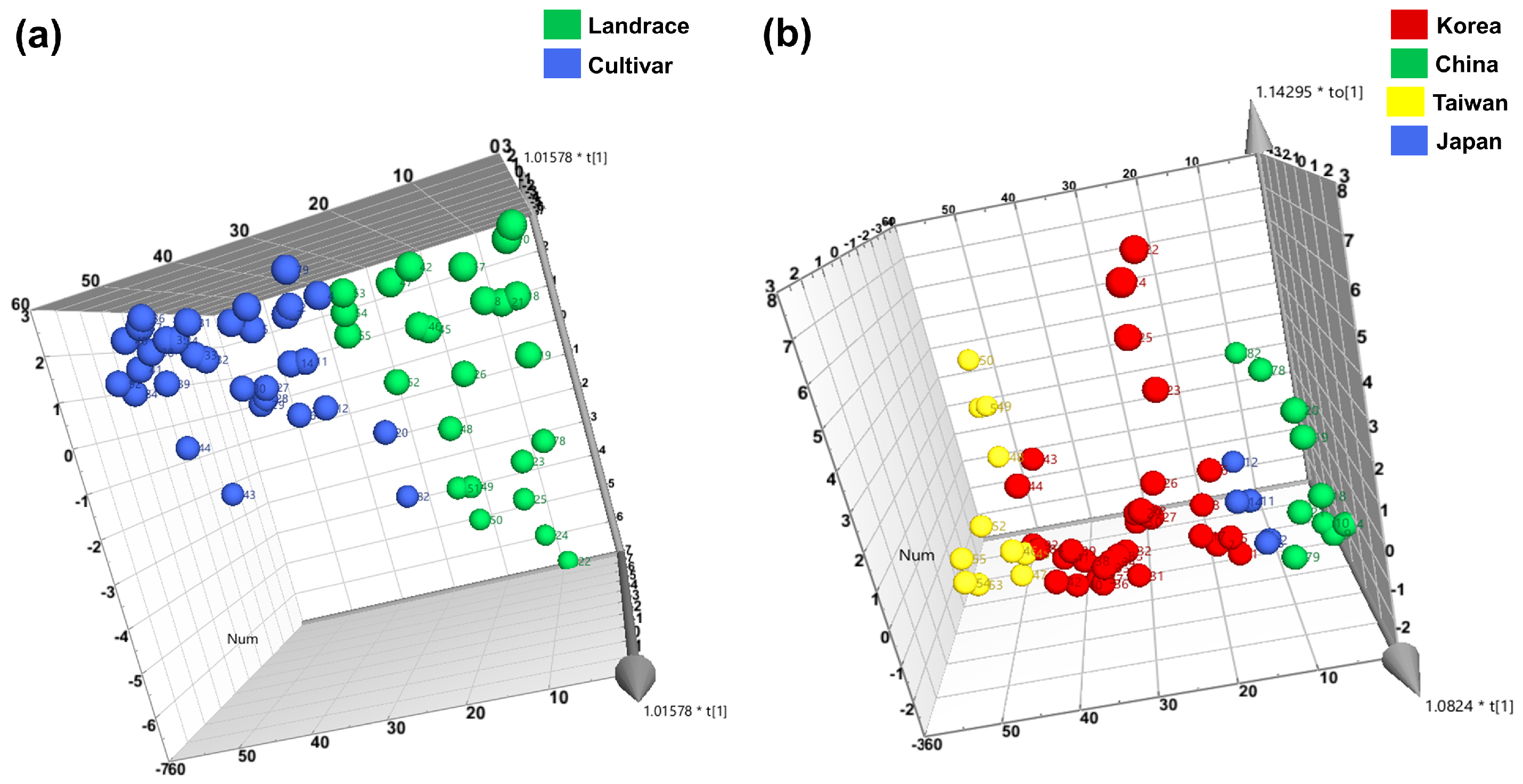
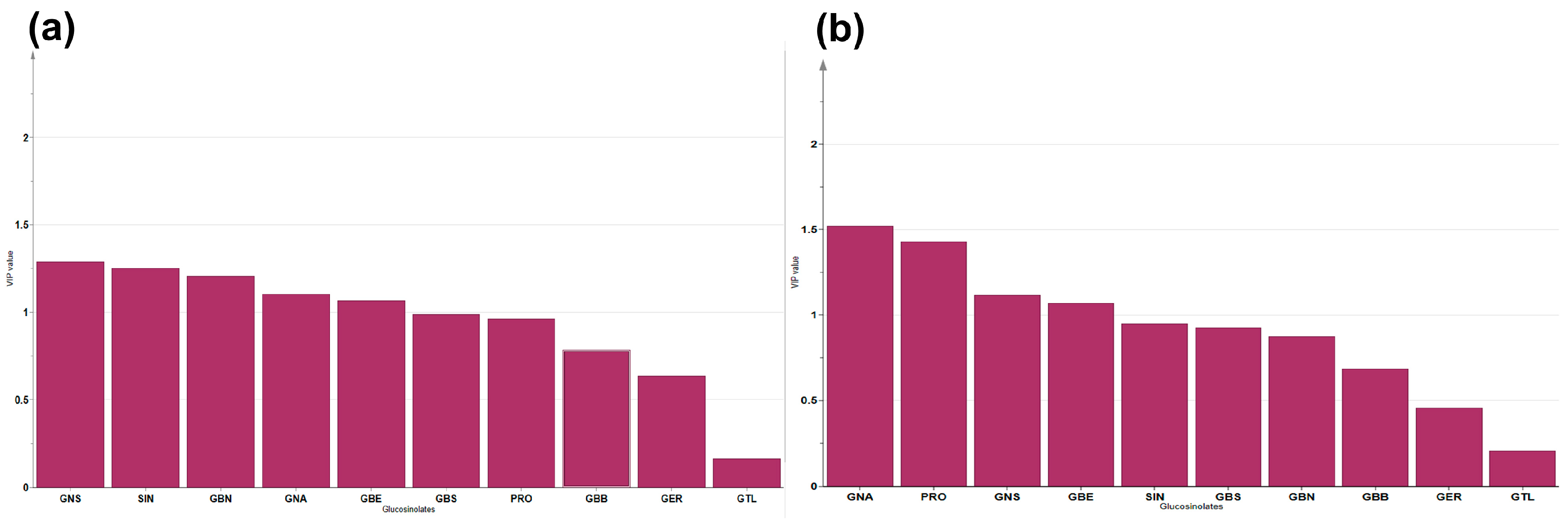
3.3. Clustering and Diversity Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Coz, C.; Ducombs, G. Plants and plant products. In Contact Dermatitis, 4th ed.; Frosch, P.J., Lepoittevin, J.-P., Eds.; Springer: New York, NY, USA, 2006; p. 769. [Google Scholar]
- Gadapati, W.R.; Macfie, S.M. Phytochelatins are only partially correlated with Cd-stress in two species of Brassica. Plant Sci. 2006, 170, 471–480. [Google Scholar] [CrossRef]
- Dixon, G. Origins and diversity of Brassica and its relatives. In Vegetable Brassicas and Related Crucifers; CABI: Wallingford, UK, 2006; pp. 1–33. [Google Scholar]
- Bonnema, G.; Del Carpio, D.P.; Zhao, J. Diversity analysis and molecular taxonomy of Brassica vegetable crops. Genet. Genom. Breed. Veg. Brassicas 2011, 1, 81–124. [Google Scholar]
- Kole, C. Wild Crop Relatives: Genomic and Breeding Resources: Plantation and Ornamental Crops; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Cho, H.S. Food and nationalism: Kimchi and Korean national identity. Korean J. Int. Stud. 2006, 4, 207–229. [Google Scholar]
- Schonhof, I.; Krumbein, A.; Brückner, B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Food/Nahrung 2004, 48, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordás, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Chumala, P.; Suchy, M. Phytoalexins from Thlaspi arvense, a wild crucifer resistant to virulent Leptosphaeria maculans: Structures, syntheses and antifungal activity. Phytochemistry 2003, 64, 949–956. [Google Scholar]
- Wittstock, U.; Halkier, B.A. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 2002, 7, 263–270. [Google Scholar] [CrossRef]
- Giamoustaris, A.; Mithen, R. Genetics of aliphatic glucosinolates. IV. Side-chain modification in Brassica oleracea. Theor. Appl. Genet. 1996, 93, 1006–1010. [Google Scholar] [CrossRef]
- Mithen, R.; Faulkner, K.; Magrath, R.; Rose, P.; Williamson, G.; Marquez, J. Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor. Appl. Genet. 2003, 106, 727–734. [Google Scholar] [CrossRef] [PubMed]
- RDA-Genebank. RDA-Genebank passport data. Available online: http://genebank.rda.go.kr/ (accessed on 23 February 2023).
- Ishida, M.; Kakizaki, T.; Ohara, T.; Morimitsu, Y. Development of a simple and rapid extraction method of glucosinolates from radish roots. Breed. Sci. 2011, 61, 208–211. [Google Scholar] [CrossRef]
- Rhee, J.-H.; Choi, S.; Lee, J.-E.; Hur, O.-S.; Ro, N.-Y.; Hwang, A.-J.; Ko, H.-C.; Chung, Y.-J.; Noh, J.-J.; Assefa, A.D. Glucosinolate content in Brassica genetic resources and their distribution pattern within and between inner, middle, and outer leaves. Plants 2020, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Tobias, S.; Carlson, J.E. Brief report: Bartlett’s test of sphericity and chance findings in factor analysis. Multivar. Behav. Res. 1969, 4, 375–377. [Google Scholar] [CrossRef]
- Shrestha, N. Factor analysis as a tool for survey analysis. Am. J. Appl. Math. Stat. 2021, 9, 4–11. [Google Scholar] [CrossRef]
- Kim, S.-H.; Subramanian, P.; Hahn, B.-S.; Ha, B.-K. High-Throughput Phenotypic Characterization and Diversity Analysis of Soybean Roots (Glycine max L.). Plants 2022, 11, 2017. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jo, J.W.; Wang, X.; Shin, M.-J.; Hur, O.S.; Ha, B.-K.; Hahn, B.-S. Diversity Characterization of Soybean Germplasm Seeds Using Image Analysis. Agronomy 2022, 12, 1004. [Google Scholar] [CrossRef]
- Arumugam, A.; Ibrahim, M.D.; Kntayya, S.B.; Mohd Ain, N.; Iori, R.; Galletti, S.; Ioannides, C.; Abdull Razis, A.F. Induction of Apoptosis by Gluconasturtiin-Isothiocyanate (GNST-ITC) in human hepatocarcinoma HepG2 cells and human breast adenocarcinoma MCF-7 cells. Molecules 2020, 25, 1240. [Google Scholar] [CrossRef]
- Pedras, M.; Jha, M.; Ahiahonu, P. The synthesis and biosynthesis of phytoalexins produced by cruciferous plants. Curr. Org. Chem. 2003, 7, 1635–1647. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. JNCI J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef]
- Engelen-Eigles, G.; Holden, G.; Cohen, J.D.; Gardner, G. The effect of temperature, photoperiod, and light quality on gluconasturtiin concentration in watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2006, 54, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, S.-Y.; Kim, J.K.; Park, S.U. Comparative phytochemical analyses and metabolic profiling of different phenotypes of Chinese cabbage (Brassica rapa ssp. pekinensis). Foods 2019, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Mishra, A. Glucosinolates in animal nutrition: A review. Anim. Feed Sci. Technol. 2007, 132, 1–27. [Google Scholar] [CrossRef]
- Kim, B.-R.; Hu, R.; Keum, Y.-S.; Hebbar, V.; Shen, G.; Nair, S.S.; Kong, A.T. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003, 63, 7520–7525. [Google Scholar]
- Prindiville, S.A.; Byers, T. 38. CANCER PREVENTION. Hematol./Oncol. Secrets 2003, 171. [Google Scholar]
- Lee, J.; Kwak, J.; Eom, Y.; Lee, S.; Jang, Y.; Choi, J. Korean brocooli (Brassica oleracea L. var. italica) variation in glucosinolate content in genetic resources. Korea Hortic. Sci. Technol. J. 2012, 30, 743–750. [Google Scholar] [CrossRef]
- Yan, X.; Chen, S. Regulation of plant glucosinolate metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef]
- Goodacre, R.; Shann, B.; Gilbert, R.J.; Timmins, E.M.; McGovern, A.C.; Alsberg, B.K.; Kell, D.B.; Logan, N.A. Detection of the dipicolinic acid biomarker in Bacillus spores using Curie-point pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. Anal. Chem. 2000, 72, 119–127. [Google Scholar] [CrossRef]
- Kim, J.K.; Bamba, T.; Harada, K.; Fukusaki, E.; Kobayashi, A. Time-course metabolic profiling in Arabidopsis thaliana cell cultures after salt stress treatment. J. Exp. Bot. 2007, 58, 415–424. [Google Scholar] [CrossRef]
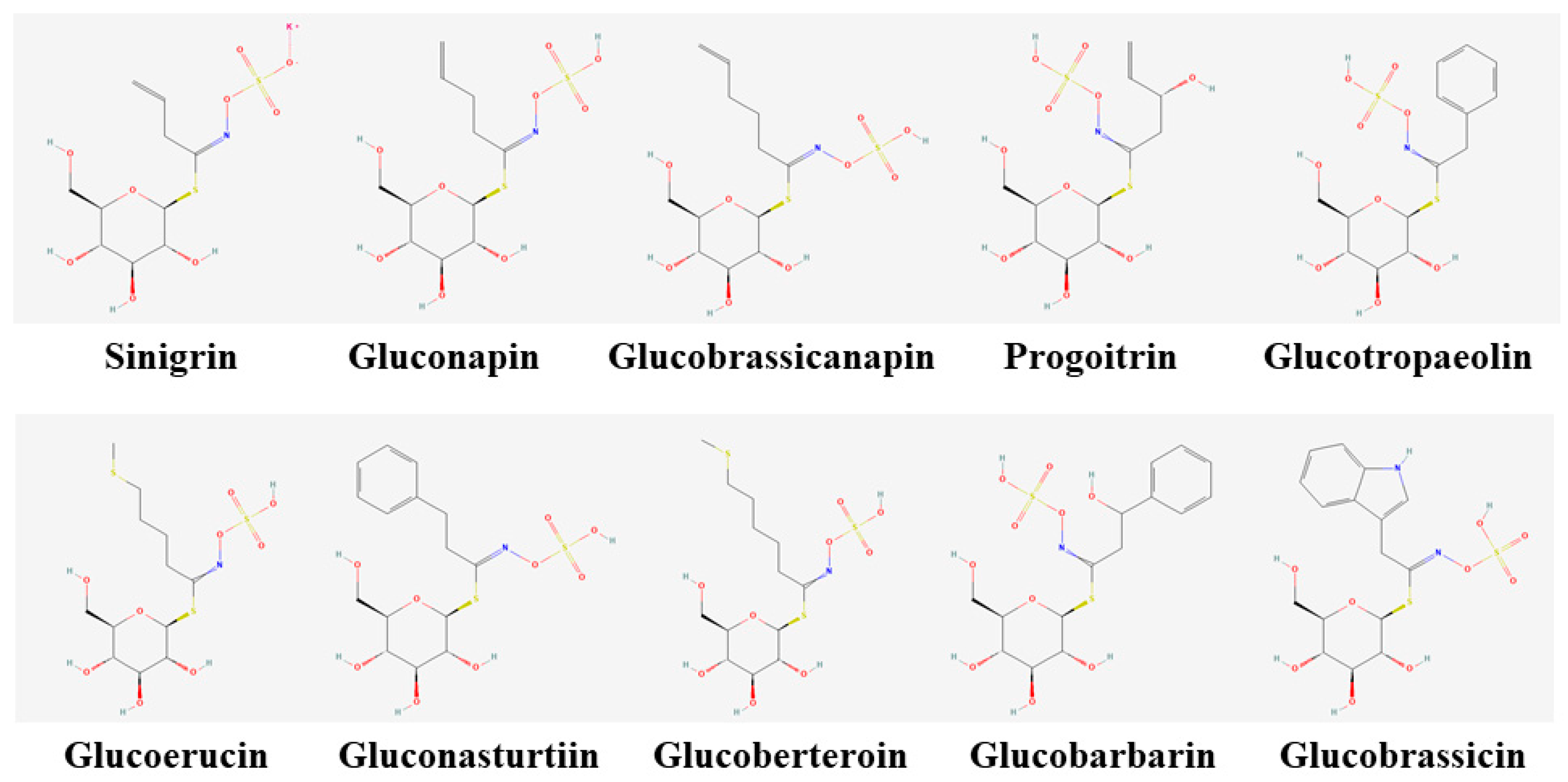
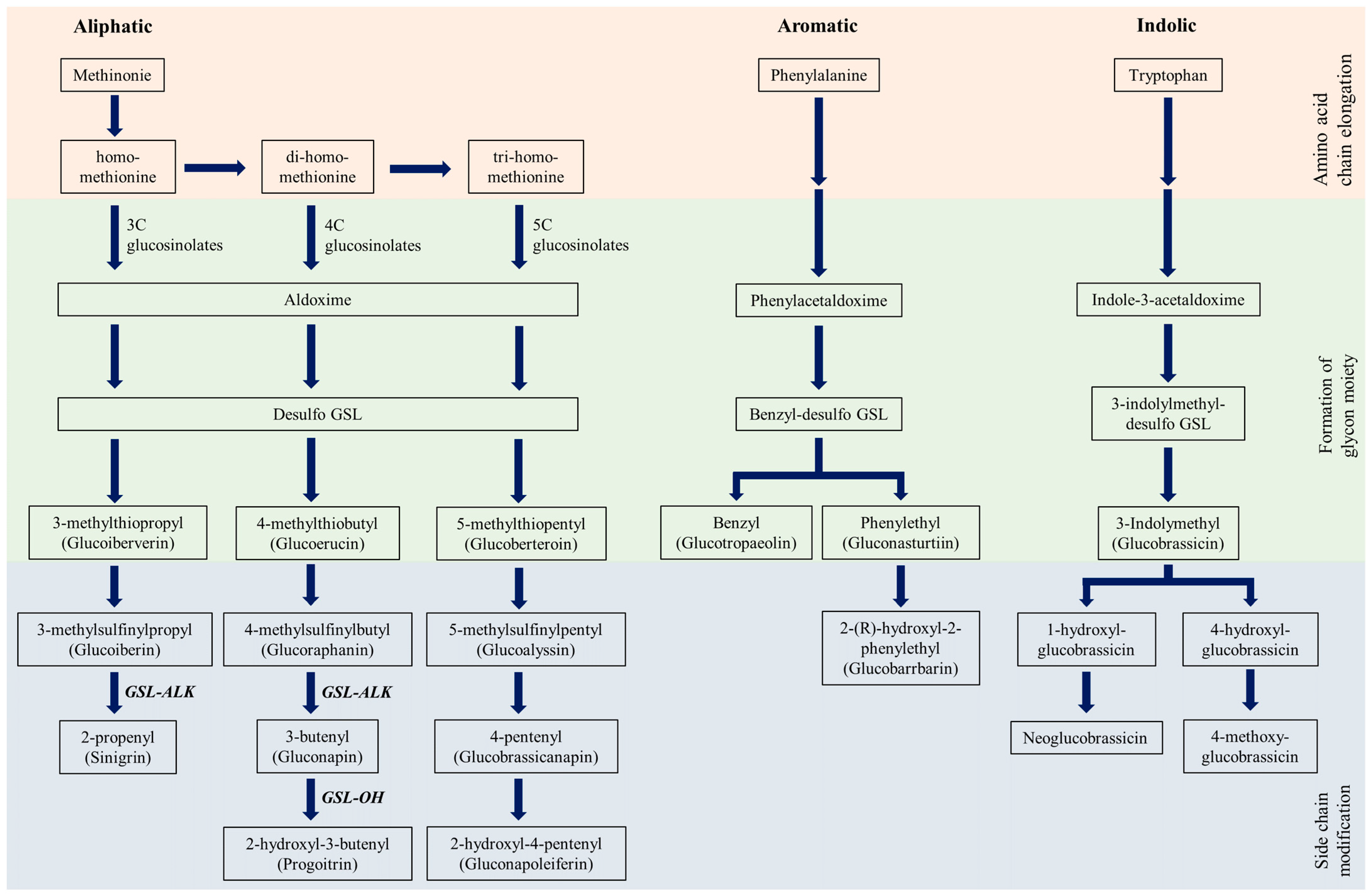
| Name | Abbreviation | Group | RT (min) | MRM Transition | CID (ev) | Dwell Time (sec) | Calibration Curve Parameters |
|---|---|---|---|---|---|---|---|
| Progoitrin | PRO | Aliphatic | 5.96 | 387.77 > 194.85 | 25 | 0.029 | Y = 8.2526X + 28.1501 (r2 = 0.962) |
| Sinigrin | SIN | Aliphatic | 6.55 | 357.75 > 161.84 | 25 | 0.029 | Y = 12.7878X − 11.1181 (r2 = 0.999) |
| Gluconapin | GNA | Aliphatic | 7.77 | 371.74 > 258.74 | 25 | 0.029 | Y = 8.36216X + 29.5397 (r2 = 0.994) |
| Glucobrassicanapin | GBN | Aliphatic | 8.6 | 385.71 > 258.87 | 25 | 0.029 | Y = 7.2514 X + 47.2841 (r2 = 0.992) |
| Glucobarbarin | GBB | Aromatic | 8.65 | 437.71 > 274.75 | 25 | 0.029 | Y = 9.29915 X − 0.454779 (r2 = 0.999) |
| Glucoerucin | GER | Aliphatic | 8.76 | 419.69 > 258.74 | 25 | 0.029 | Y = 6.77393 X + 73.6679 (r2 = 0.985) |
| Glucotropaeolin | GTL | Aromatic | 8.88 | 407.72 > 258.87 | 25 | 0.029 | Y = 18.2122X − 3.93949 (r2 = 0.999) |
| Glucoberteroin | GBE | Aliphatic | 9.18 | 433.72 > 275.06 | 25 | 0.029 | Y = 6.09397 X + 63.1212 (r2 = 0.971) |
| Glucobrassicin | GBC | Indolyl | 9.32 | 446.69 > 204.94 | 25 | 0.029 | Y = 6.39827 X + 2.6232 (r2 = 0.998) |
| Gluconasturtiin | GNS | Aromatic | 9.36 | 421.69 > 274.87 | 25 | 0.029 | Y = 4.36109 X + 90.233 (r2 = 0.961) |
| Variable | SIN | GNA | GBN | PRO | GTL | GER | GNS | GBE | GBB | GBS |
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | 0.30 | 333.26 | 545.60 | 155.28 | 0.55 | 0.26 | 109.48 | 2.15 | ND | 72.89 |
| Maximum | 20.00 | 23,501.58 | 10,344.70 | 8536.51 | 40.77 | 207.29 | 1494.47 | 2109.97 | 150.69 | 2213.95 |
| Mean | 3.42 | 3432.88 | 4065.17 | 1740.44 | 12.49 | 28.18 | 514.58 | 248.18 | 49.82 | 633.95 |
| Std. deviation | 4.36 | 4088.64 | 2616.67 | 1499.76 | 7.45 | 39.00 | 336.82 | 387.90 | 33.79 | 490.51 |
| SIN | GNA | GBN | PRO | GTL | GER | GNS | GBE | GBB | |
|---|---|---|---|---|---|---|---|---|---|
| GNA | 0.625 *** | ||||||||
| GBN | 0.759 *** | 0.652 *** | |||||||
| PRO | 0.502 *** | 0.531 *** | 0.358 *** | ||||||
| GTL | −0.217 | −0.227 | −0.010 | −0.203 | |||||
| GER | 0.210 | 0.183 | 0.038 | 0.382 ** | −0.122 | ||||
| GNS | 0.734 *** | 0.683 *** | 0.627 *** | 0.756 *** | −0.238 * | 0.424 ** | |||
| GBE | 0.544 *** | 0.312 * | 0.481 | 0.272 * | 0.015 | 0.573 *** | 0.529 *** | ||
| GBB | 0.224 | 0.345 * | 0.443 | 0.247 | 0.195 | 0.343 | 0.430 * | 0.540 *** | |
| GBS | 0.531 *** | 0.274 * | 0.516 *** | 0.243 | 0.144 | 0.224 | 0.466 * | 0.601 *** | 0.347 ** |
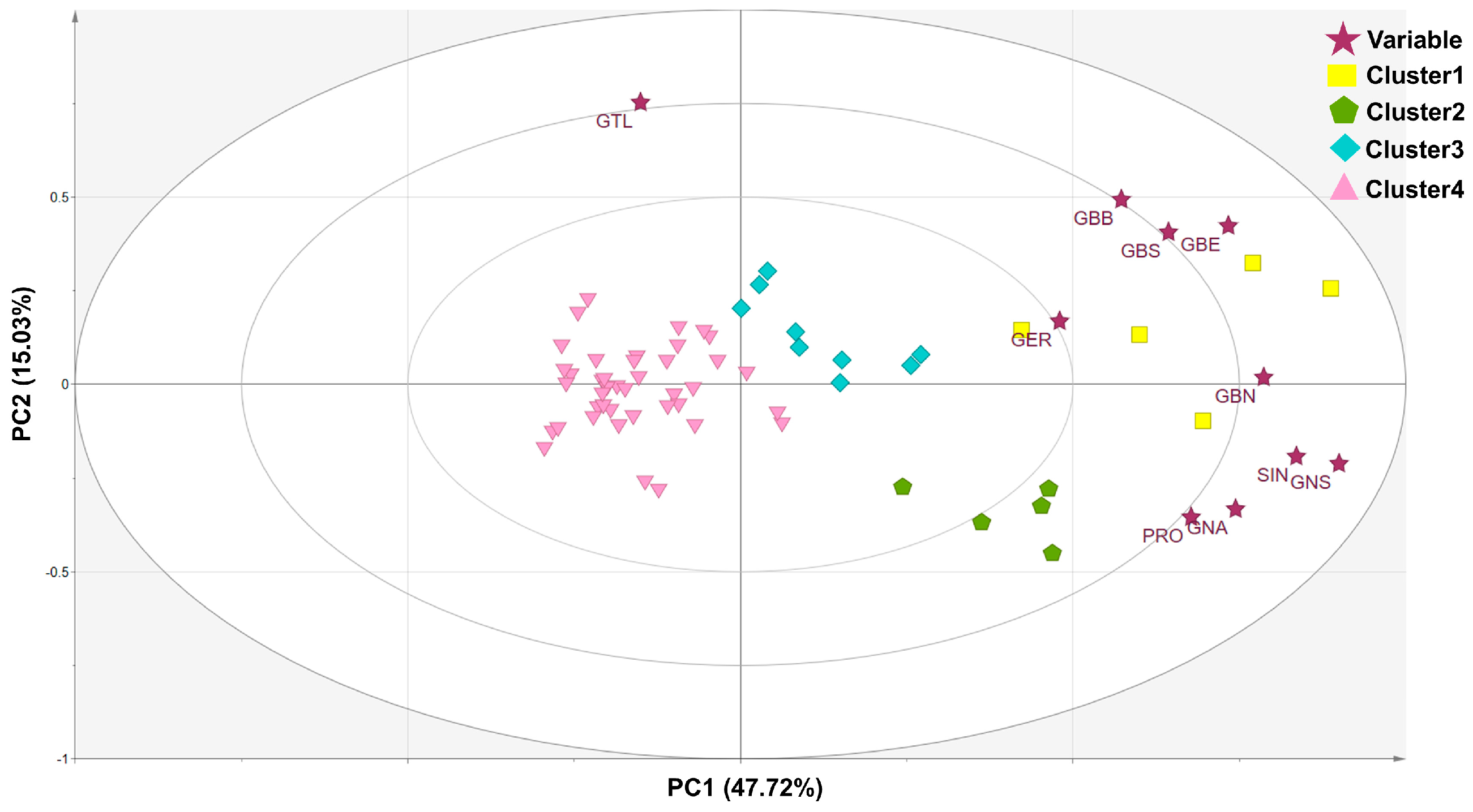
| Glucosinolates | Eigenvector of Principal Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Sinigrin | 0.386 | −0.157 | −0.230 |
| Gluconapin | 0.344 | −0.271 | −0.184 |
| Glucobrassicanapin | 0.364 | 0.015 | −0.447 |
| Progoitrin | 0.314 | −0.289 | 0.205 |
| Glucotropaeolin | −0.070 | 0.615 | −0.257 |
| Glucoerucin | 0.222 | 0.139 | 0.718 |
| Gluconasturtiin | 0.416 | −0.172 | 0.071 |
| Glucoberteroin | 0.340 | 0.345 | 0.214 |
| Glucobarbarin | 0.265 | 0.403 | 0.088 |
| Glucobrassicin | 0.298 | 0.332 | −0.176 |
| Eigenvalue | 4.67 | 1.50 | 1.22 |
| Variability (%) | 46.72 | 15.03 | 12.15 |
| Cumulative (%) | 46.72 | 61.74 | 73.90 |
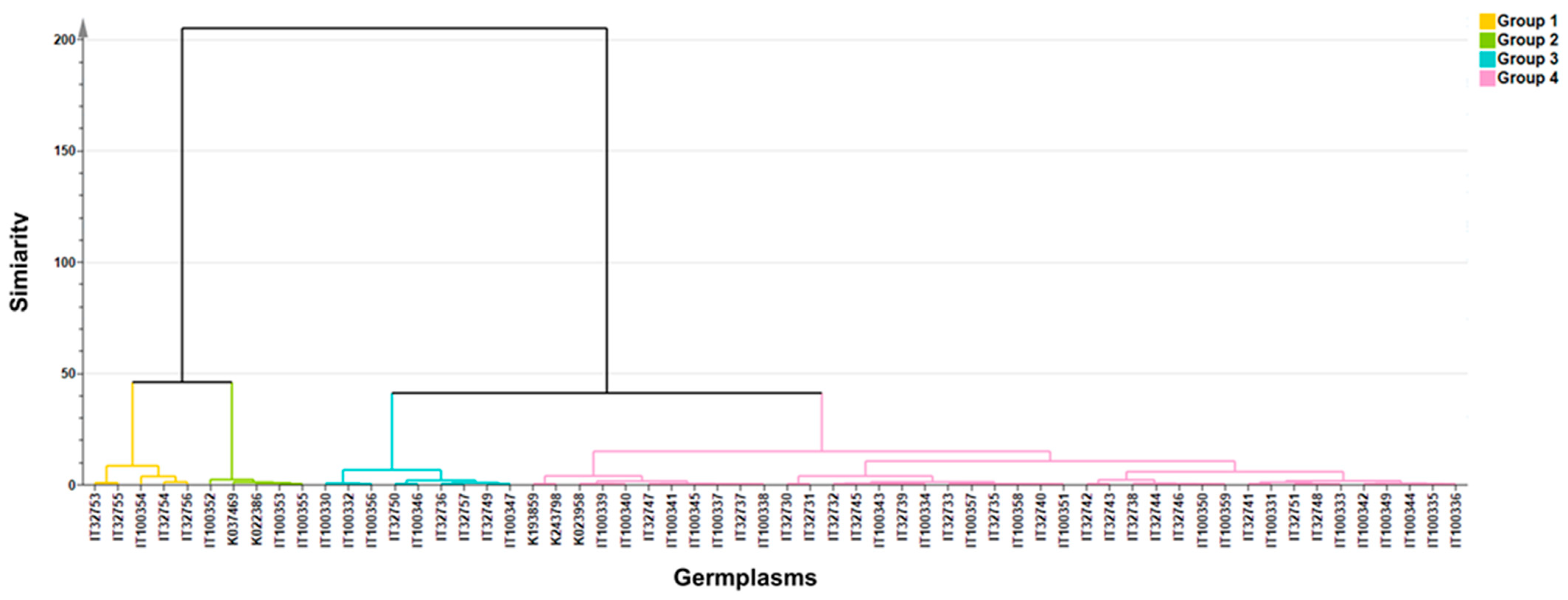
| Clustering | Status | Origin |
|---|---|---|
| Cluster 1 (n = 5) | Landrace 5 | KOR 4, TWN 1 |
| Cluster 2 (n = 5) | Landrace 4, Cultivar1 | TWN 3, CHN 2 |
| Cluster 3 (n = 9) | Cultivar 6, Landrace 3 | KOR 6, CHN 2, TWN 1 |
| Cluster 4 (n = 41) | Cultivar 25, Landrace 13, Unknown 3 | KOR 20, CHN 6, TWN 6, JPN 5, THA 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Lee, G.-A.; Subramanian, P.; Hahn, B.-S. Quantification and Diversity Analyses of Major Glucosinolates in Conserved Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Germplasms. Foods 2023, 12, 1243. https://doi.org/10.3390/foods12061243
Kim S-H, Lee G-A, Subramanian P, Hahn B-S. Quantification and Diversity Analyses of Major Glucosinolates in Conserved Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Germplasms. Foods. 2023; 12(6):1243. https://doi.org/10.3390/foods12061243
Chicago/Turabian StyleKim, Seong-Hoon, Gi-An Lee, Parthiban Subramanian, and Bum-Soo Hahn. 2023. "Quantification and Diversity Analyses of Major Glucosinolates in Conserved Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Germplasms" Foods 12, no. 6: 1243. https://doi.org/10.3390/foods12061243
APA StyleKim, S.-H., Lee, G.-A., Subramanian, P., & Hahn, B.-S. (2023). Quantification and Diversity Analyses of Major Glucosinolates in Conserved Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Germplasms. Foods, 12(6), 1243. https://doi.org/10.3390/foods12061243








