Using OPLS-DA to Fingerprint Key Free Amino and Fatty Acids in Understanding the Influence of High Pressure Processing in New Zealand Clams
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. High Pressure Processing
2.3. Free Amino Acid Analysis
2.4. Fatty Acid Analysis
2.5. Statistical Analysis
3. Results & Discussion
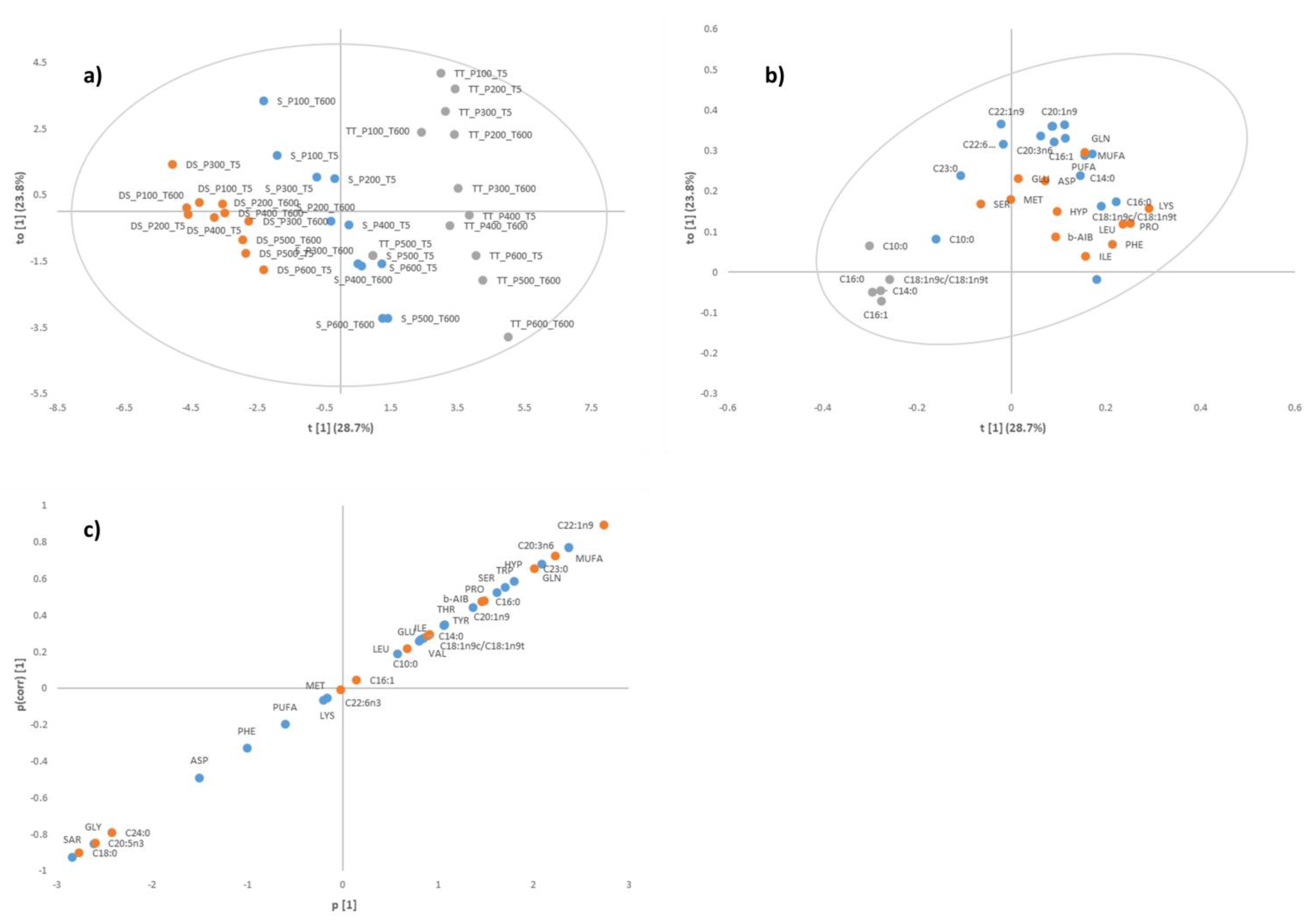
3.1. Identification of Key Differentiating Amino Acids and Fatty Acids
3.2. Mixed Model ANOVA Analysis for Diamond Shell Clam
3.3. Canonical Variate Analysis for Diamond Shell Clam
| Time (s) | Amino Acid | Pressure (MPa) | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | Processing | Time | Processing * Time | ||
| 5 | ASP | 333.48 aw | 417.83 aw | 832.57 by | 533.8 bx | 170.02 bx | 180.79 av | <0.0001 | <0.0001 | <0.0001 |
| 600 | 588.67 bx | 58.41 ax | 205.44 avw | 253.13 aw | 163.27 aw | 171.24 av | ||||
| 5 | ILE | 92.34 awx | 172.3 av | 113.93 ay | 107.64 ay | 95.82 ay | 83.3 bw | <0.0001 | 0.155 | <0.0001 |
| 600 | 78.99 aw | 447.13 ay | 132.98 by | 113.66 axy | 92.96 axy | 54.91 av | ||||
| 5 | LEU | 147.78 bwx | 108.59 av | 169.72 ay | 156.66 axy | 145.03 axy | 135.29 bw | <0.0001 | 0.002 | <0.0001 |
| 600 | 123.48 av | 62.3 aw | 194.97 bw | 186.53 bw | 170.39 bw | 108.25 av | ||||
| 5 | LYS | 85.98 awx | 114.19 bvw | 94.69 axy | 73.98 avwx | 64.1 avwx | 64.56 bv | <0.0001 | 0.947 | 0.068 |
| 600 | 92.89 ax | 163.37 bx | 80.59 ax | 77.51 awx | 68.97 awx | 48.92 av | ||||
| 5 | MET | 45.56 bx | 90.68 av | 47.28 axy | 41.41 awx | 38.31 awx | 34.64 bv | <0.0001 | 0.165 | 0.001 |
| 600 | 34.08 avw | 31.46 av | 52.51 av | 44.25 axy | 42.03 axy | 26 av | ||||
| 5 | SER | 2365.56 aw | 2272.99 aw | 2816.26 bxy | 2477.28 bwx | 1992.72 bwx | 1884.61 av | <0.0001 | <0.0001 | <0.0001 |
| 600 | 2415.51 aw | 2094.06 av | 2042.82 av | 1946.59 av | 1869.59 av | 1884.1 av | ||||
| 5 | THR | 79.72 av | 101.75 av | 170.45 axy | 184.05 ax | 161.67 ax | 136.1 bw | <0.0001 | 0.108 | <0.0001 |
| 600 | 88.47 av | 174.08 bwx | 189.18 bx | 186.85 bx | 151.51 bx | 94.34 bv | ||||
| 5 | VAL | 143.12 bx | 111.43 av | 168.62 ay | 168.28 ay | 147.64 ay | 126.42 bw | <0.0001 | 0.087 | <0.0001 |
| 600 | 122.56 aw | 170.47 bxy | 198.86 by | 173.14 axy | 147.37 axy | 92.73 av | ||||
| Time (s) | Fatty Acid | Pressure (MPa) | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | Processing | Time | Processing * Time | ||
| 5 | C18:0 | 6 avw | 0.66 bw | 5.91 av | 5.84 av | 6.41 aw | 6.13 avw | <0.0001 | 0.012 | 0.001 |
| 600 | 6.27 ax | 6.08 avw | 5.77 avw | 6.45 bwx | 6.84 bv | 6.55 bvw | ||||
| 5 | C20:5n3 | 5.67 av | 6.6 bw | 5.94 avw | 6.19 avw | 6.28 avw | 6.14 avw | 0 | 0.554 | 0 |
| 600 | 6.08 ax | 5.58 ax | 5.4 ax | 6.62 awx | 6.92 aw | 6.63 av | ||||
| 5 | C22:1n9 | 1.51 aw | 1.86 bx | 1.5 aw | 1.33 av | 1.71 bx | 1.52 bw | <0.0001 | 0.145 | <0.0001 |
| 600 | 1.62 avw | 1.56 av | 1.49 av | 1.62 bxy | 1.52 awx | 1.38 av | ||||
| 5 | C24:0 | 2.05 avwx | 2.26 bwx | 1.8 av | 1.97 avw | 2.37 ax | 1.91 avw | 0.006 | 0.119 | 0.007 |
| 600 | 1.84 ay | 1.79 az | 1.77 az | 2.31 ax | 2.04 aw | 2.02 av | ||||
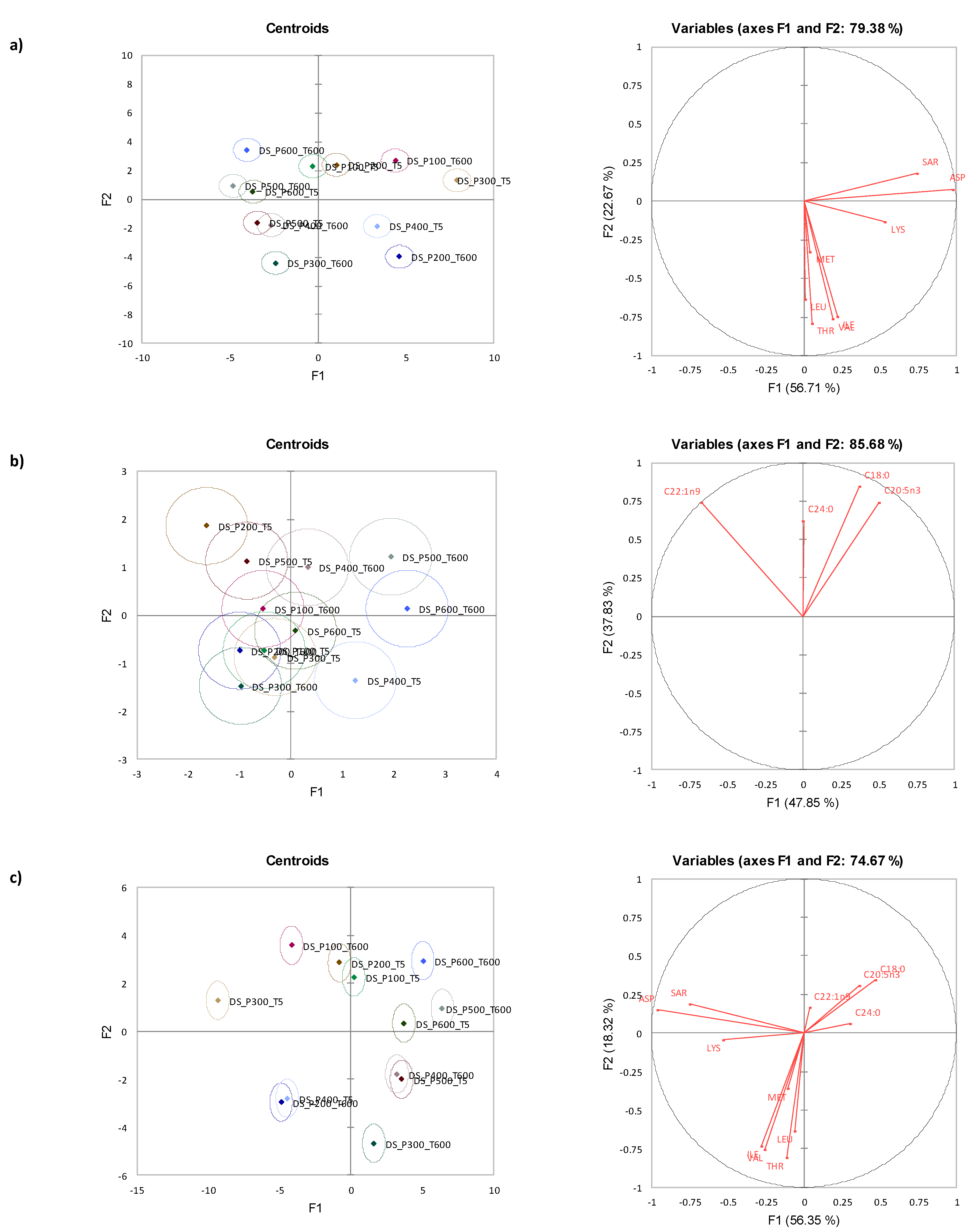
3.4. Mixed Model ANOVA Analysis for Storm Shell Clam
3.5. Canonical Variate Analysis for Storm Shell Clams
3.6. Mixed Model ANOVA Analysis for Tua Tua
3.7. Canonical Variate Analysis for Tua Tua Clams
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wheaton, F.; Schaffer, G.; Ingling, A.; Douglass, L. Physical properties of soft shell clams, Mya arenaria. Aquac. Eng. 2008, 38, 181–188. [Google Scholar] [CrossRef]
- Tamburini, E.; Turolla, E.; Lanzoni, M.; Moore, D.; Castaldelli, G. Manila clam and Mediterranean mussel aquaculture is sustainable and a net carbon sink. Sci. Total. Environ. 2022, 848, 157508. [Google Scholar] [CrossRef]
- Bejaoui, S.; Ghribi, F.; Chetoui, I.; Aouini, F.; Bouaziz, M.; Houas-Gharsallah, I.; Soudani, N.; El Cafsi, M. Effect of storage temperature and time on the fatty acids and nutritional quality of the commercial mussel (Mytilus galloprovincialis). J. Food Sci. Technol. 2021, 58, 3493–3503. [Google Scholar] [CrossRef]
- Sabagh, E.; Taha, N.; Saleh, E.; ElFar, A.; Sadek, K. Effect of Freezing and Frozen Storage on Amino Acid Profile and Fatty Acid Pattern in Imported and Local Meat. Alex. J. Vet. Sci. 2016, 49, 113–121. [Google Scholar] [CrossRef]
- Farkas, D.F.; Hoover, D.G. High pressure processing. J. Food Sci. 2000, 65, 47–64. [Google Scholar] [CrossRef]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Samson, C.T.; Welti-Chanes, J.; Torres, J.A. High-pressure processing tech-nologies for the pasteurization and sterilization of foods. Food Bioprocess Technol. 2011, 4, 969. [Google Scholar] [CrossRef]
- Luo, Q.; Hamid, N.; Oey, I.; Leong, S.Y.; Kantono, K.; Alfaro, A.; Lu, J. Physicochemical changes in New Zealand abalone (Haliotis iris) with pulsed electric field (PEF) processing and heat treatments. LWT 2019, 115, 108438. [Google Scholar] [CrossRef]
- Kantono, K.; How, M.S.; Wang, Q.J. Design of experiments meets immersive environment: Optimising eating atmosphere using artificial neural network. Appetite 2022, 176, 122. [Google Scholar] [CrossRef]
- How, M.S.; Jones, J.R.; Morgenstern, M.P.; Gray-Stuart, E.; Bronlund, J.E.; Saint-Eve, A.; Trelea, I.C.; Souchon, I. Modelling the role of oral processing on in vivo aroma release of white rice: Conceptual model and experimental validation. LWT 2021, 141, 110918. [Google Scholar] [CrossRef]
- How, M.S.; Jones, J.R.; Morgenstern, M.P.; Gray-Stuart, E.; Bronlund, J.E. A mechanistic approach to model the breakdown of solid food during chewing. J. Food Eng. 2021, 317, 110871. [Google Scholar] [CrossRef]
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.; Sulaiman, A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Appl. Sci. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Cheftel, J.C. High-pressure, microbial inactivation and food preservation. Rev. Agaroquimica Technol. Ali-Mentos 1995, 1, 75–90. [Google Scholar] [CrossRef]
- Borggaard, C.; Andersen, J.R. Measurement of Meat Quality, Instrumental. In Encyclopedia of Meat Sciences; Jensen, W.K., Devine, C., Dikeman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 675–680. [Google Scholar]
- Simonin, H.; Duranton, F.; De Lamballerie, M. New Insights into the High-Pressure Processing of Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Impact of high-pressure processing on vitamin E (α-, γ-, and δ-tocopherol), vitamin D (cholecalciferol and ergocalciferol), and fatty acid profiles in liquid foods. J. Agric. Food Chem. 2012, 60, 3763–3768. [Google Scholar] [CrossRef] [PubMed]
- Figueirêdo, B.C.; Bragagnolo, N.; Skibsted, L.H.; Orlien, V. Inhibition of Cholesterol and Polyunsaturated Fatty Acids Oxidation through the Use of Annatto and Bixin in High-Pressure Processed Fish. J. Food Sci. 2015, 80, C1646–C1653. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Munoz, A.; Chevalier-Lucia, D.; LeBail, A.; Ramaswamy, H.S.; Simpson, B.K. Physicochemical changes induced in carp (Cyprinus carpio) fillets by high pressure processing at low temperature. Innov. Food Sci. Emerg. Technol. 2006, 7, 13–18. [Google Scholar] [CrossRef]
- Pazos, M.; Méndez, L.; Fidalgo, L.; Vázquez, M.; Torres, J.A.; Aubourg, S.P.; Saraiva, J.A. Effect of High-Pressure Processing of Atlantic Mackerel (Scomber scombrus) on Biochemical Changes during Commercial Frozen Storage. Food Bioprocess Technol. 2015, 8, 2159–2170. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Montero, P.; Giménez, B.; Gómez-Guillén, M. Effect of functional edible films and high pressure processing on microbial and oxidative spoilage in cold-smoked sardine (Sardina pilchardus). Food Chem. 2007, 105, 511–520. [Google Scholar] [CrossRef]
- Kaur, B.P.; Kaushik, N.; Rao, P.S.; Chauhan, O.P. Effect of High-Pressure Processing on Physical, Biochemical, and Microbiological Characteristics of Black Tiger Shrimp (Penaeus monodon). Food Bioprocess Technol. 2012, 6, 1390–1400. [Google Scholar] [CrossRef]
- Chevalier, D.; Le Bail, A.; Ghoul, M. Effects of high pressure treatment (100–200 MPa) at low temperature on turbot (Scophthalmus maximus) muscle. Food Res. Int. 2001, 34, 425–429. [Google Scholar] [CrossRef]
- Cruz-Romero, M.C.; Kerry, J.P.; Kelly, A. Fatty acids, volatile compounds and colour changes in high-pressure-treated oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2008, 9, 54–61. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Ralat, M.; Marshall, M.R. Effect of high pressure processing and cooking treatment on the quality of Atlantic salmon. Food Chem. 2009, 116, 828–835. [Google Scholar] [CrossRef]
- Xiong, Y.L. Protein Oxidation and Implications for Muscle Food Quality. Antioxidants in Muscle Foods: Nutritional Strategies to Improve Quality; John Wiley and Sons: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Campus, M.; Flores, M.; Martinez, A.; Toldra, F. Effect of high pressure treatment on colour, microbial and chemical char-acteristics of dry cured loin. Meat Sci. 2008, 80, 1174–1181. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, Y.; Jin, Y.; Deng, Y.; Zhao, Y. Impact of high hydrostatic pressure on non-volatile and volatile com-pounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Narwankar, S.P.; Flimlin, G.E.; Schaffner, D.W.; Tepper, B.J.; Karwe, M.V. Microbial Safety and Consumer Ac-ceptability of High-Pressure Processed Hard Clams (Mercenaria mercenaria). J. Food Sci. 2011, 76, M375–M380. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Kung, H.-F.; Cheng, Q.-L.; Lin, C.-S.; Tseng, C.-H.; Chiu, K.; Tsai, Y.-H. Effects of high-hydrostatic-pressure processing on the chemical and microbiological quality of raw ready-to-eat hard clam marinated in soy sauce during cold storage. LWT 2022, 159, 113229. [Google Scholar] [CrossRef]
- Lin, C.-S.; Tsai, Y.-H.; Chen, P.-W.; Chen, Y.-C.; Wei, P.-C.; Tsai, M.-L.; Kuo, C.-H.; Lee, Y.-C. Impacts of high-hydrostatic pressure on the organoleptic, microbial, and chemical qualities and bacterial community of freshwater clam during storage studied using high-throughput sequencing. LWT 2022, 171. [Google Scholar] [CrossRef]
- Juárez, M.; Polvillo, O.; Contò, M.; Ficco, A.; Ballico, S.; Failla, S. Comparison of four extraction/methylation ana-lytical methods to measure fatty acid composition by gas chromatography in meat. J. Chromatogr. A 2008, 1190, 327–332. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Liu, R.-J.; Zheng, J.-J.; Qing, X.-D.; Yang, K.-L.; Zhang, Y.-Q.; Pan, L.-Y.; Nie, J.-F. Authentication of the production season of Xinyang Maojian green tea using two-dimensional fingerprints coupled with chemometric multivariate calibration and pattern recognition analysis. LWT 2023, 176. [Google Scholar] [CrossRef]
- Richards, G.P.; McLeod, C.; Le Guyader, F.S. Processing Strategies to Inactivate Enteric Viruses in Shellfish. Food Environ. Virol. 2010, 2, 183–193. [Google Scholar] [CrossRef]
- Ohmori, T.; Shigehisa, T.; Taji, S.; Hayashi, R. Effect of high pressure on the protease activities in meat. Agric. Biol. Chem. 1991, 55, 357–361. [Google Scholar]
- Ma, Q.; Hamid, N.; Oey, I.; Kantono, K.; Farouk, M. The Impact of High-Pressure Processing on Physicochemical Properties and Sensory Characteristics of Three Different Lamb Meat Cuts. Molecules 2020, 25, 2665. [Google Scholar] [CrossRef] [PubMed]
- Kantono, K.; Hamid, N.; Oey, I.; Wu, Y.C.; Ma, Q.; Farouk, M.; Chadha, D. Effect of high hydrostatic pressure pro-cessing on the chemical characteristics of different lamb cuts. Foods 2020, 9, 1444. [Google Scholar] [CrossRef]
- Ahmed, J.; Habeebullah, S.; Alagarsamy, S.; Mulla, M.; Thomas, L. Impact of high-pressure treatment on amino acid profile, fatty acid compositions, and texture of yellowfin seabream (Acanthopagrus latus) fillets. Front. Sustain. Food Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Ren, Y.; Fan, E.; Chang, H.; Wu, H. Effects of high pressure treatment and temperature on lipid oxidation and fatty acid composition of yak (Poephagus grunniens) body fat. Meat Sci. 2013, 94, 489–494. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.; Kelly, A. Changes in the microbiological and physicochemical quality of high-pressure-treated oysters (Crassostrea gigas) during chilled storage. Food Control 2008, 19, 1139–1147. [Google Scholar] [CrossRef]
- Sato, N.; Quitain, A.T.; Kang, K.; Daimon, H.; Fujie, K. Reaction Kinetics of Amino Acid Decomposition in High-Temperature and High-Pressure Water. Ind. Eng. Chem. Res. 2004, 43, 3217–3222. [Google Scholar] [CrossRef]
- Kang, G.; Cho, S.; Seong, P.; Park, B.; Kim, S.; Kim, D.; Kim, Y.; Kang, S.; Park, K. Effects of high pressure processing on fatty acid composition and volatile compounds in Korean native black goat meat. Meat Sci. 2013, 94, 495–499. [Google Scholar] [CrossRef] [PubMed]
- McArdle, R.M.; Marcos, B.; Kerry, J.P.; Mullen, A. Monitoring the effects of high pressure processing and temperature on selected beef quality attributes. Meat Sci. 2010, 86, 629–634. [Google Scholar] [CrossRef]
- Limbad, M.; Maddox, N.G.; Hamid, N.; Kantono, K. Sensory and Physicochemical Characterization of Sourdough Bread Prepared with a Coconut Water Kefir Starter. Foods 2020, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Niaura, T.; Kantono, K. How does wine ageing influence perceived complexity? Tem-poral-choose-all-that-apply (TCATA) reveals temporal drivers of complexity in experts and novices. Food Qual. Prefer. 2021, 92, 104230. [Google Scholar] [CrossRef]
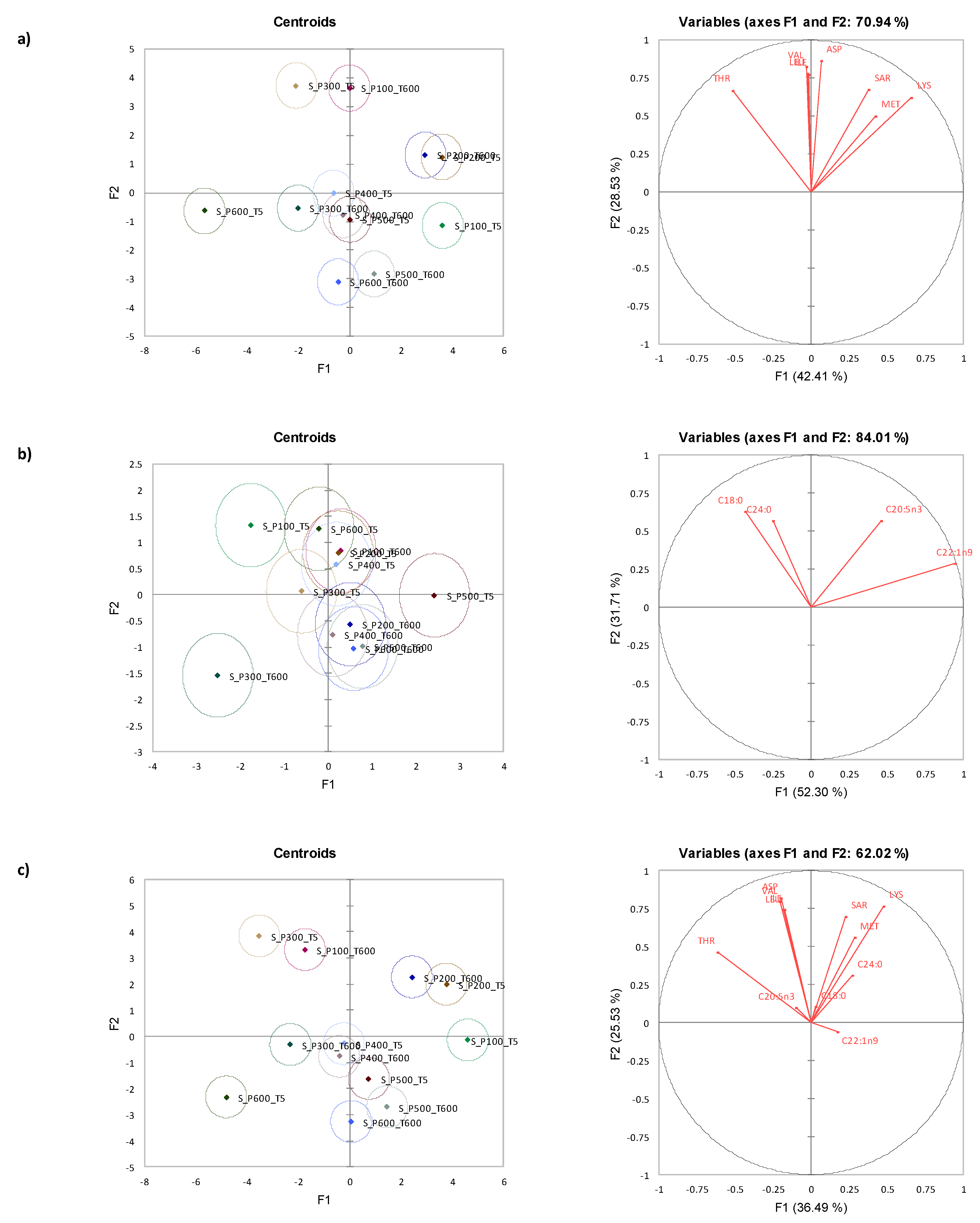
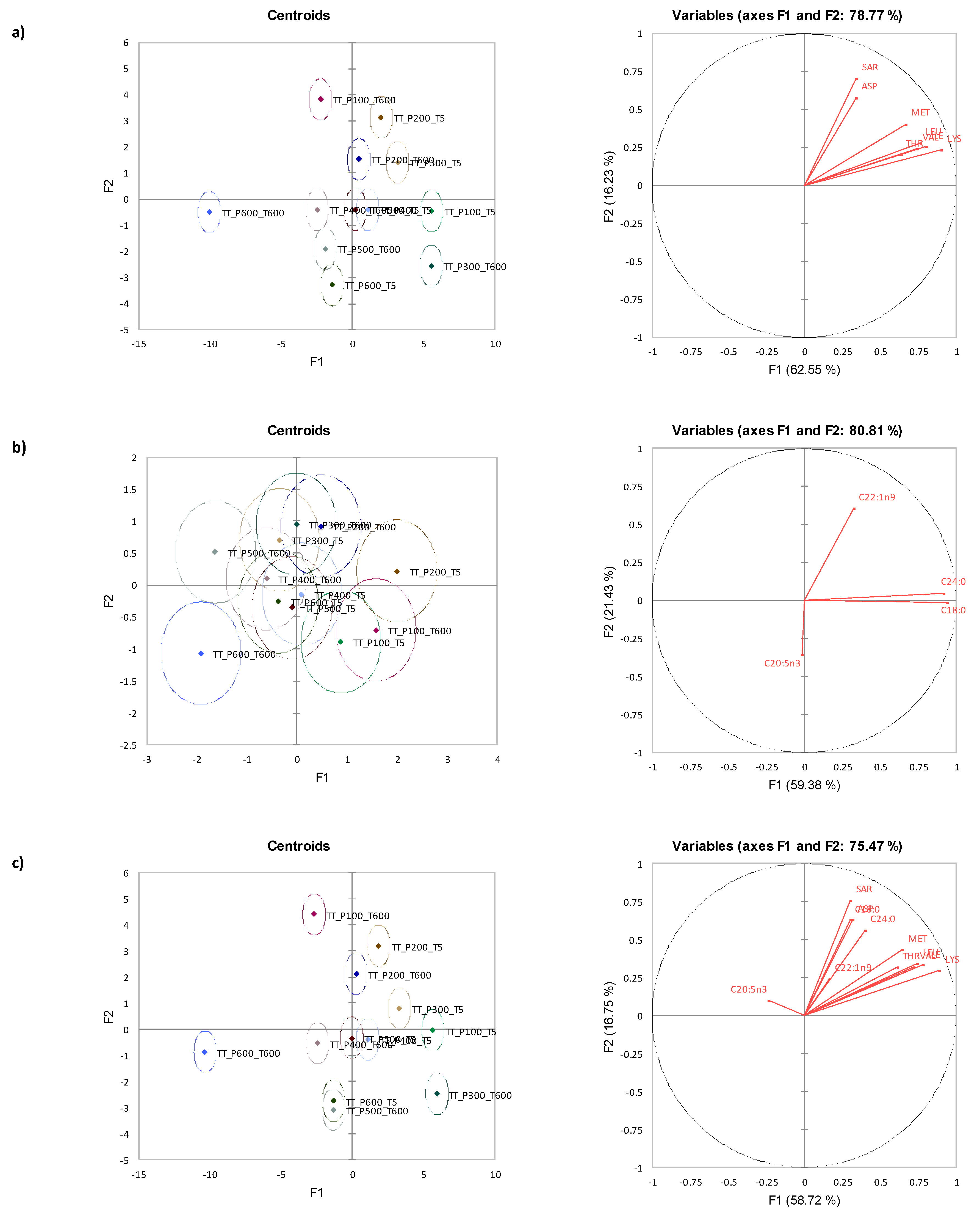
| Time (s) | Amino Acid | Pressure (MPa) | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | Processing | Time | Processing * Time | ||
| 5 | ASP | 355.35 av | 546.06 ax | 787.6 by | 469.1 awx | 396.88 avw | 406.27 avw | <0.0001 | 0.024 | <0.0001 |
| 600 | 839.98 by | 679.18 ax | 368.76 av | 509.19 bw | 418.86 avw | 385.85 av | ||||
| 5 | ILE | 65.57 av | 96.52 awx | 112.5 ax | 79.26 bvw | 70.01 bv | 71.67 bv | <0.0001 | 0 | 0.001 |
| 600 | 88.15 bx | 86.69 ax | 81.63 ax | 64.24 aw | 44.18 av | 40.5 av | ||||
| 5 | LEU | 92.63 av | 126.47 awx | 130.51 ax | 98.69 bvw | 88.15 bv | 88.81 bv | <0.0001 | <0.0001 | <0.0001 |
| 600 | 125.49 bx | 91.54 aw | 120.23 ax | 77.66 aw | 41.47 av | 42.7 av | ||||
| 5 | LYS | 65.29 ax | 79.19 ay | 52.18 awx | 45.32 aw | 39.92 aw | 18.91 av | <0.0001 | 0.022 | <0.0001 |
| 600 | 72.31 az | 61.35 ay | 46.04 ax | 42.11 awx | 33.45 avw | 26.3 bv | ||||
| 5 | MET | 23.11 awx | 30.25 bx | 15.19 avw | 15.78 bvw | 10.15 av | 10.51 av | <0.0001 | 0.24 | <0.0001 |
| 600 | 32.22 ax | 15.43 aw | 13.92 avw | 14.77 aw | 11.89 avw | 10.67 av | ||||
| 5 | SAR | 1729.13 aw | 1601.77 aw | 1684.01 bw | 1511.8 bw | 1586.72 bw | 1262.01 av | <0.0001 | <0.0001 | 0 |
| 600 | 1847.52 ay | 1626.16 ax | 1238.17 avw | 1356.17 aw | 1213.05 avw | 1160.61 av | ||||
| 5 | THR | 154.44 avw | 135.23 av | 217.12 bx | 173.74 bw | 159.31 bvw | 218.57 bx | <0.0001 | <0.0001 | <0.0001 |
| 600 | 226.48 bx | 137.22 aw | 162.62 awx | 143.83 aw | 101.62 av | 107.17 av | ||||
| 5 | VAL | 104.18 av | 154.55 awx | 171.83 ax | 123.07 bvw | 105.87 bv | 113.94 bv | <0.0001 | <0.0001 | <0.0001 |
| 600 | 138.73 bx | 134.88 ax | 143.47 ax | 100.22 aw | 62.32 av | 56.7 av | ||||
| Time (s) | Fatty Acid | Pressure (MPa) | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | Processing | Time | Processing * Time | ||
| 5 | C18:0 | 4.66 bx | 3.75 avwx | 3.09 avwx | 3.59 bvwx | 2.59 av | 4.3 awx | 0.014 | <0.0001 | 0.017 |
| 600 | 2.99 avw | 2.84 avw | 3.36 aw | 2.65 avw | 2.32 av | 2.23 av | ||||
| 5 | C20:5n3 | 1.26 av | 1.45 av | 1.41 bv | 1.43 bv | 1.47 av | 1.55 av | 0.118 | 0.019 | 0.012 |
| 600 | 1.58 bx | 1.28 aw | 0.94 av | 1.3 aw | 1.32 aw | 1.33 aw | ||||
| 5 | C22:1n9 | 3.15 av | 3.69 av | 3.33 bv | 3.65 bv | 4.41 aw | 3.5 av | <0.0001 | 0.028 | <0.0001 |
| 600 | 3.79 bx | 3.66 awx | 2.44 av | 3.47 awx | 3.68 awx | 3.61 awx | ||||
| 5 | C24:0 | 0.83 aw | 0.58 av | 0.57 bv | 0.57 bv | 0.53 av | 0.47 av | 0 | 0.028 | 0.923 |
| 600 | 0.68 aw | 0.53 av | 0.48 av | 0.49 av | 0.45 av | 0.45 av | ||||
| Time (s) | Amino Acid | Pressure (MPa) | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | Processing | Time | Processing * Time | ||
| 5 | ASP | 193.12 avw | 212.39 w | 175.24 avw | 172.33 av | 168.57 av | 157.34 av | <0.0001 | 0.104 | 0.009 |
| 600 | 245.88 bz | 215.51 ay | 196.72 axy | 181.82 bwx | 163.11 aw | 132.89 av | ||||
| 5 | ILE | 173.82 bx | 157.6 wx | 168.53 ax | 136.42 bw | 131.02 bvw | 103.84 bv | <0.0001 | <0.0001 | <0.0001 |
| 600 | 128.89 ax | 183.55 ay | 192.82 ay | 116.83 ax | 77.21 aw | 54.36 av | ||||
| 5 | LEU | 255.47 by | 224.56 wxy | 240.17 axy | 198.03 bvwx | 180.92 bvw | 157.04 bv | <0.0001 | <0.0001 | <0.0001 |
| 600 | 207.39 awx | 230.35 axy | 264.07 ay | 172.97 aw | 128.77 av | 102.27 av | ||||
| 5 | LYS | 127.95 by | 98.53 wx | 108.31 ax | 89.68 bw | 84.81 bvw | 68.61 bv | <0.0001 | <0.0001 | <0.0001 |
| 600 | 92.11 ax | 101.78 axy | 115.14 ay | 68.59 aw | 64.01 aw | 9.71 av | ||||
| 5 | MET | 65.12 ax | 58.69 x | 55.23 ax | 40.23 bw | 33.17 aw | 16.7 av | <0.0001 | 0.032 | <0.0001 |
| 600 | 59.62 ayz | 43.46 axy | 69.77 az | 36.85 awx | 24.35 avw | 14.82 av | ||||
| 5 | SAR | 1109.24 aw | 1148.45 w | 1161.47 bw | 1012.66 avw | 1038.09 bvw | 862.02 av | <0.0001 | 0.226 | <0.0001 |
| 600 | 1287.37 bx | 1378.6 ay | 964.09 aw | 988.13 aw | 770.51 av | 796.04 av | ||||
| 5 | THR | 270.91 bw | 206.95 v | 240.52 avw | 207.88 av | 197.4 bv | 197.85 bv | <0.0001 | 0.072 | <0.0001 |
| 600 | 236.16 aw | 303.21 bx | 295.73 ax | 196.32 aw | 109.25 av | 111.43 av | ||||
| 5 | VAL | 254.51 bx | 230.57 wx | 244.92 awx | 204.62 bvwx | 203.2 bvw | 162.52 bv | <0.0001 | 0.001 | <0.0001 |
| 600 | 199.35 aw | 288.37 ax | 286.16 ax | 184.49 aw | 127.27 av | 93.92 av | ||||
| Time (s) | Fatty Acid | Pressure (MPa) | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | 600 | Processing | Time | Processing * Time | ||
| 5 | C18:0 | 1.82 awx | 2.13 ax | 1.45 av | 1.6 bvw | 1.46 av | 1.53 bvw | <0.0001 | 0.024 | 0.103 |
| 600 | 1.95 ax | 1.82 ax | 1.51 aw | 1.47 avw | 1.26 avw | 1.21 av | ||||
| 5 | C20:5 n3 | 0.96 aw | 0.91 avw | 0.82 av | 0.89 avw | 0.87 avw | 0.91 avw | 0.163 | 0.223 | 0.871 |
| 600 | 0.93 av | 0.98 av | 0.85 av | 0.93 bv | 0.88 av | 1.01 av | ||||
| 5 | C22:1n9 | 7.11 avw | 8.06 aw | 7.14 avw | 7.07 avw | 6.5 av | 7.05 avw | 0.005 | 0.239 | 0.748 |
| 600 | 7.19 av | 8.67 aw | 7.48 av | 7.34 bv | 7.26 av | 6.65 av | ||||
| 5 | C24:0 | 0.7 avw | 0.79 aw | 0.6 av | 0.63 bvw | 0.64 bvw | 0.58 bv | <0.0001 | 0.006 | 0.004 |
| 600 | 0.77 ay | 0.67 ax | 0.66 awx | 0.57 aw | 0.46 av | 0.44 av | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
How, M.S.; Hamid, N.; Liu, Y.; Kantono, K.; Oey, I.; Wang, M. Using OPLS-DA to Fingerprint Key Free Amino and Fatty Acids in Understanding the Influence of High Pressure Processing in New Zealand Clams. Foods 2023, 12, 1162. https://doi.org/10.3390/foods12061162
How MS, Hamid N, Liu Y, Kantono K, Oey I, Wang M. Using OPLS-DA to Fingerprint Key Free Amino and Fatty Acids in Understanding the Influence of High Pressure Processing in New Zealand Clams. Foods. 2023; 12(6):1162. https://doi.org/10.3390/foods12061162
Chicago/Turabian StyleHow, Muhammad Syahmeer, Nazimah Hamid, Ye Liu, Kevin Kantono, Indrawati Oey, and Mingfei Wang. 2023. "Using OPLS-DA to Fingerprint Key Free Amino and Fatty Acids in Understanding the Influence of High Pressure Processing in New Zealand Clams" Foods 12, no. 6: 1162. https://doi.org/10.3390/foods12061162
APA StyleHow, M. S., Hamid, N., Liu, Y., Kantono, K., Oey, I., & Wang, M. (2023). Using OPLS-DA to Fingerprint Key Free Amino and Fatty Acids in Understanding the Influence of High Pressure Processing in New Zealand Clams. Foods, 12(6), 1162. https://doi.org/10.3390/foods12061162










