Taste Responses and Ingestive Behaviors to Ingredients of Fermented Milk in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Short-Term Lick Test
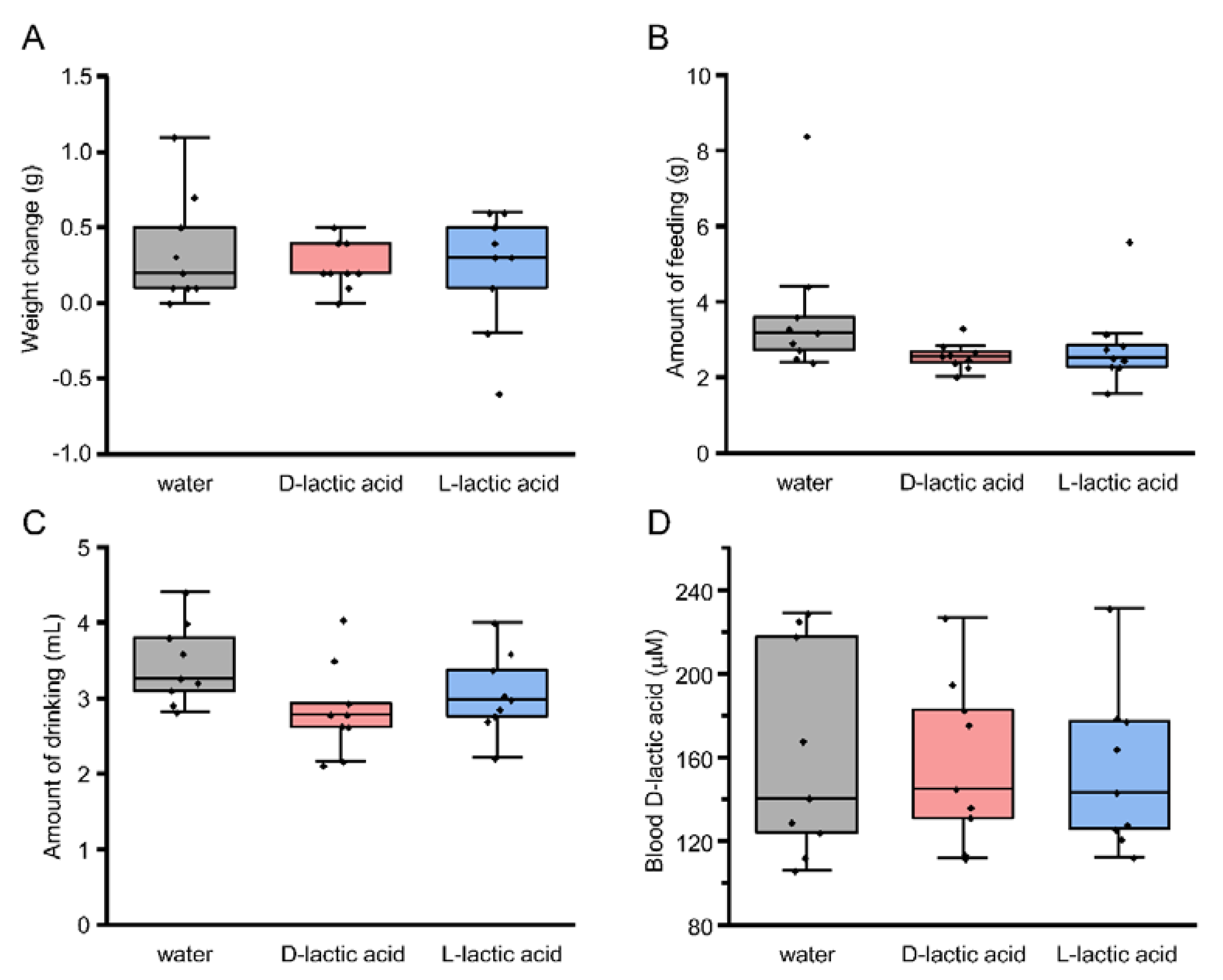
2.4. Long-Term Intake Test: The 48 Hour 2-Bottle Test
2.5. Measurement of Blood d-Lactic Acid
2.6. Statistical Analysis
3. Results
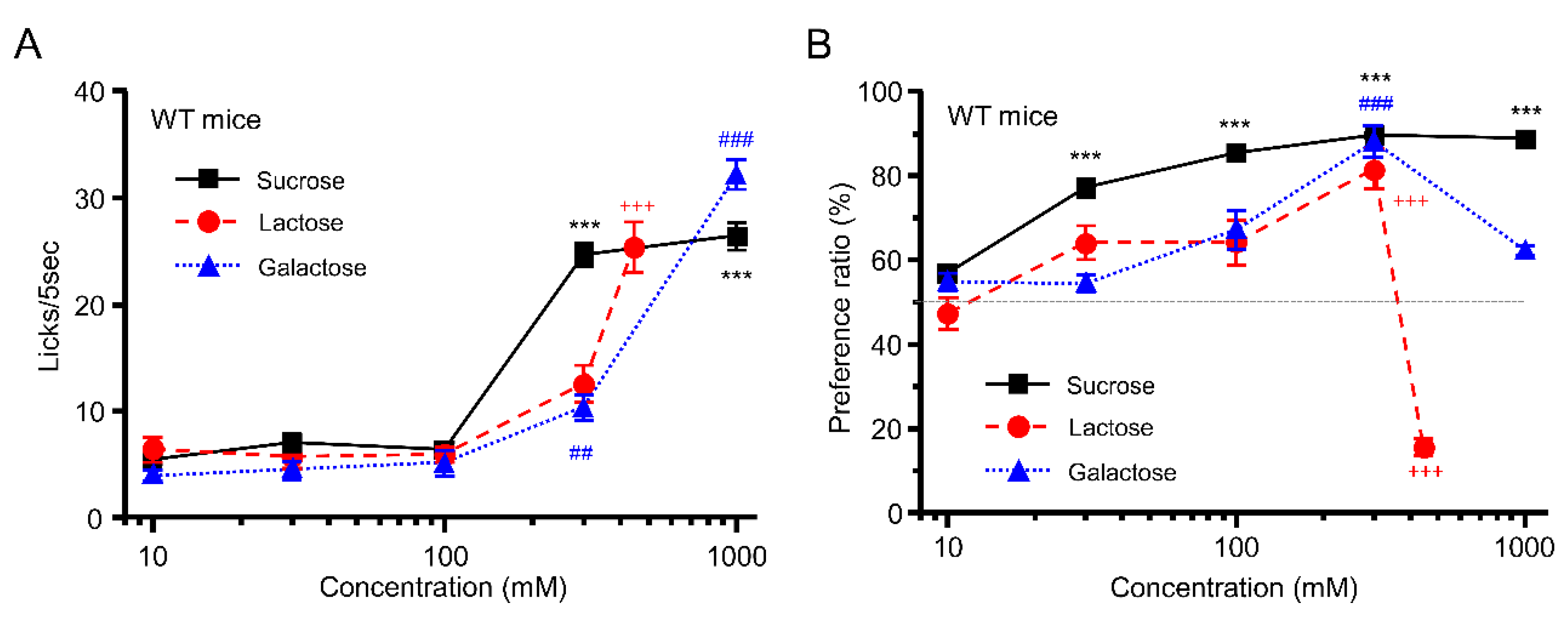
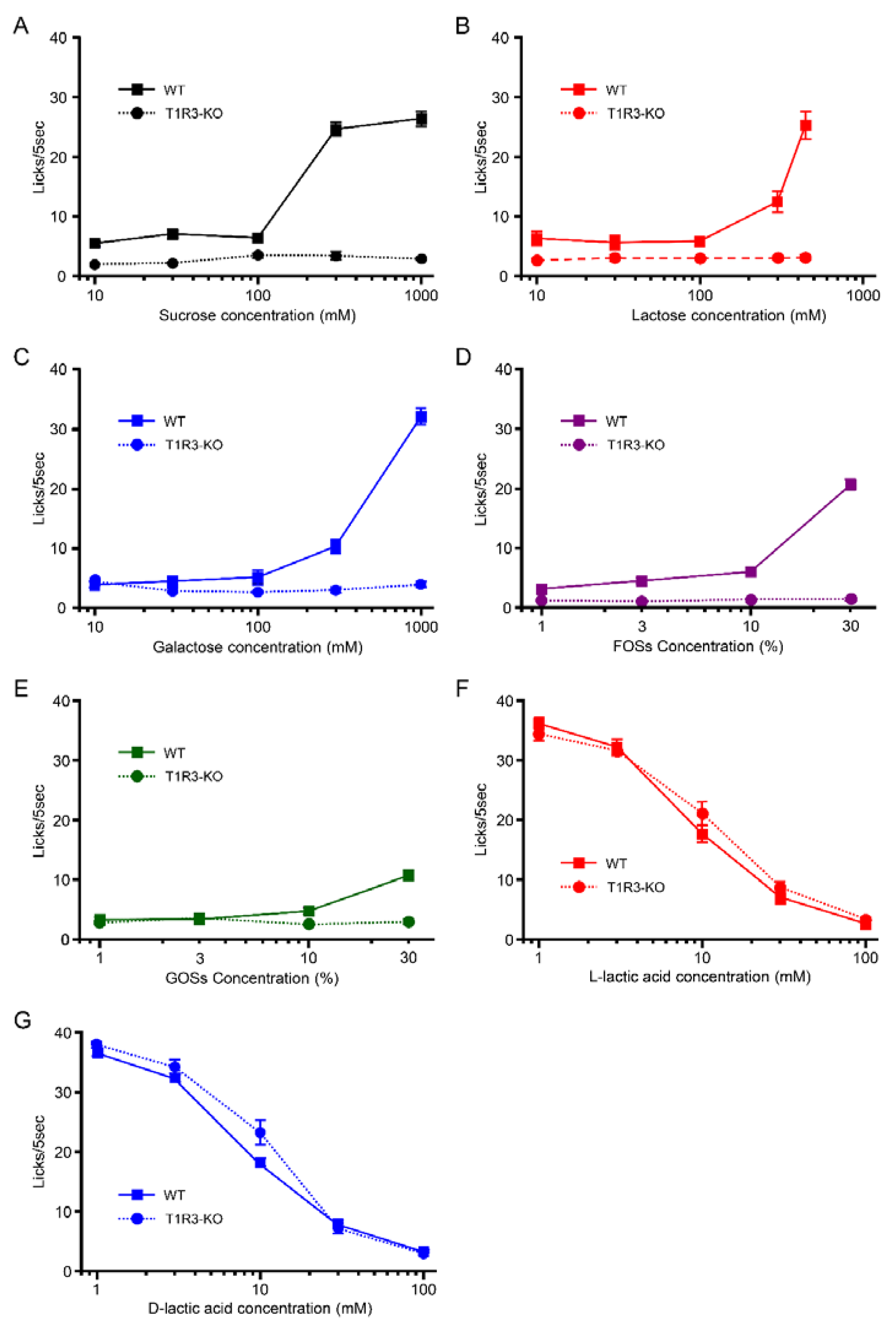
3.1. Taste Response and Ingestive Behaviors to Sugars in WT Mice
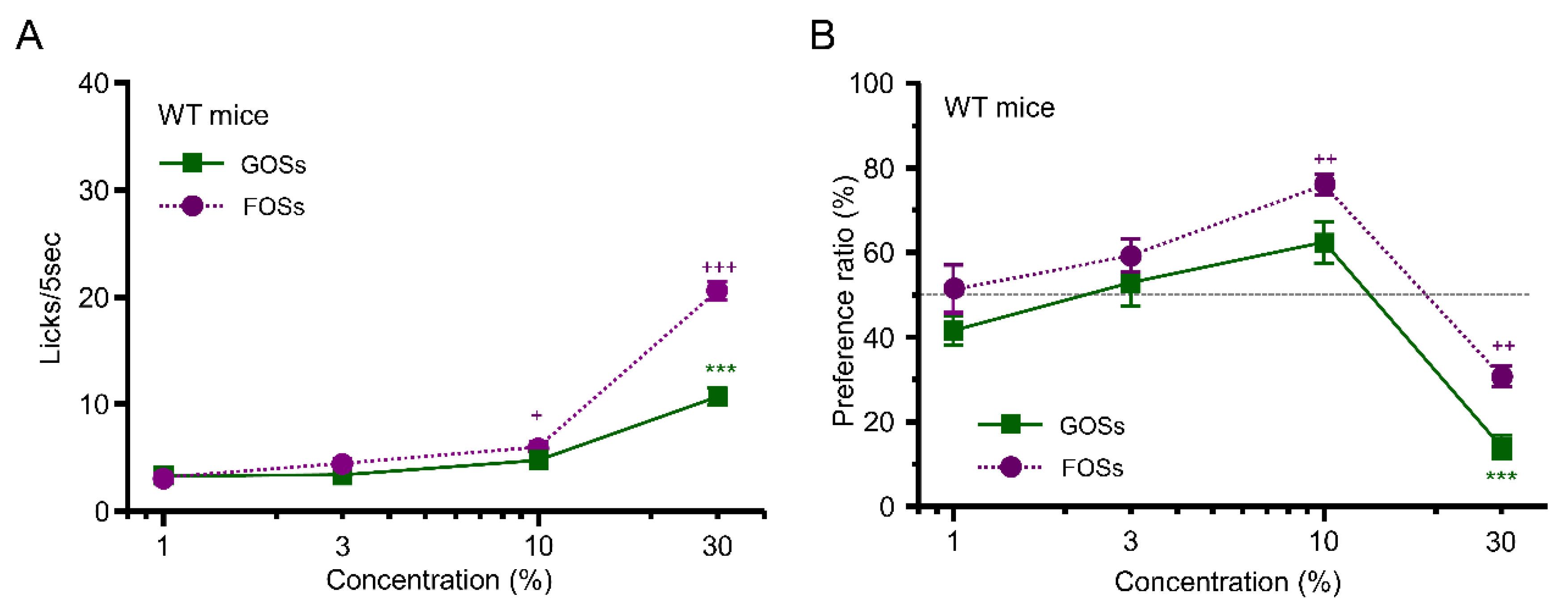
3.2. Taste Response and Ingestive Behaviors to Oligosaccharides in WT Mice
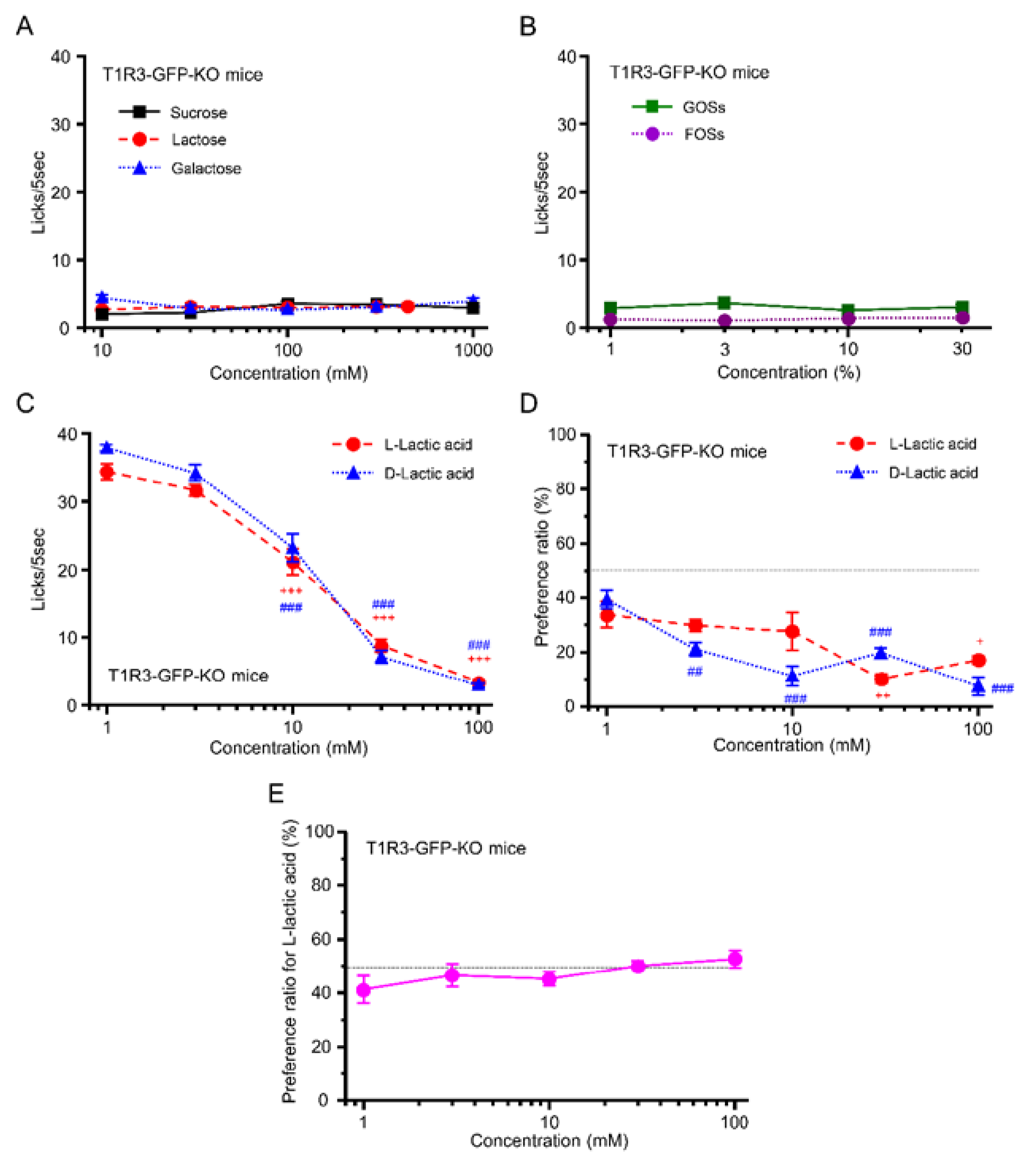
3.3. Taste Responses and Ingestive Behaviors to Acids in WT Mice
3.4. Preference for l- over d-Lactic Acid in T1R3-GFP-KO Mice
3.5. Blood d-Lactic Acid Levels after Ingestion of d- and l-Lactic Acid
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FOSs | fructo-oligosaccharides |
| GOSs | galacto-oligosaccharides |
| GFP | green fluorescent protein |
| HSD | highest-significant-difference |
| KO | knock-out |
| PR | preference ratio |
| T1R3 | Tas1r3 |
| WT | wild-type |
References
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef]
- Cormier, H.; Thifault, É.; Garneau, V.; Tremblay, A.; Drapeau, V.; Pérusse, L.; Vohl, M.-C. Association between yogurt consumption, dietary patterns, and cardio-metabolic risk factors. Eur. J. Nutr. 2016, 55, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Givens, D.I.; Astrup, A.; Bakker, S.J.L.; Goossens, G.H.; Kratz, M.; Marette, A.; Pijl, H.; Soedamah-Muthu, S.S. The Impact of Dairy Products in the Development of Type 2 Diabetes: Where Does the Evidence Stand in 2019? Adv. Nutr. 2019, 10, 1066–1075. [Google Scholar] [CrossRef]
- Gil, Á.; Ortega, R.M. Introduction and Executive Summary of the Supplement, Role of Milk and Dairy Products in Health and Prevention of Noncommunicable Chronic Diseases: A Series of Systematic Reviews. Adv. Nutr. 2019, 10, S67–S73. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. D-Lactic Acid as a Metabolite: Toxicology, Diagnosis, and Detection. BioMed Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef] [PubMed]
- Alm, I. Effect of Fermentation on l(+) and d(−) Lactic Acid in Milk. J. Dairy Sci. 1982, 65, 515–520. [Google Scholar] [CrossRef]
- Vicente, J.; Baran, Y.; Navascués, E.; Santos, A.; Calderón, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological management of acidity in wine industry: A review. Int. J. Food Microbiol. 2022, 375, 109726. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Koseki, T.; Hirayama, A.; Abe, S.; Sano, T.; Tomita, M.; Soga, T. Correlation between Sensory Evaluation Scores of Japanese Sake and Metabolome Profiles. J. Agric. Food Chem. 2010, 58, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A. Interaction of Saliva and Taste. J. Dent. Res. 1990, 69, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.; Ghosh, B.; Weiss, Z.F.; Christiaanse, J.; Gordon, M.D. Mechanisms of lactic acid gustatory attraction in Drosophila. Curr. Biol. 2021, 31, 3525–3537.e6. [Google Scholar] [CrossRef]
- Behrens, M.; Blank, K.; Meyerhof, W. Blends of Non-caloric Sweeteners Saccharin and Cyclamate Show Reduced Off-Taste due to TAS2R Bitter Receptor Inhibition. Cell Chem. Biol. 2017, 24, 1199–1204. [Google Scholar] [CrossRef]
- Teng, B.; Wilson, C.E.; Tu, Y.-H.; Joshi, N.R.; Kinnamon, S.C.; Liman, E.R. Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1. Curr. Biol. 2019, 29, 3647–3656.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, H.; Zhang, W.; Ding, C.; O’Keeffe, S.; Ye, M.; Zuker, C.S. Sour Sensing from the Tongue to the Brain. Cell 2019, 179, 392–402.e15. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Slupsky, C.M.; He, X.; Hernell, O.; Andersson, Y.; Rudolph, C.; Lönnerdal, B.; West, C.E. Postprandial metabolic response of breast-fed infants and infants fed lactose-free vs. regular infant formula: A randomized controlled trial. Sci. Rep. 2017, 7, 3640. [Google Scholar] [CrossRef] [PubMed]
- Bayless, T.M.; Brown, E.; Paige, D.M. Lactase Non-persistence and Lactose Intolerance. Curr. Gastroenterol. Rep. 2017, 19, 23. [Google Scholar] [CrossRef]
- Knol, J.; Scholtens, P.; Kafka, C.; Steenbakkers, J.; Gro, S.; Helm, K.; Klarczyk, M.; Schöpfer, H.; Böckler, H.-M.; Wells, J. Colon Microflora in Infants Fed Formula with Galacto- and Fructo-Oligosaccharides: More Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 36–42. [Google Scholar] [CrossRef]
- Yoshida, R.; Ninomiya, Y. Taste information derived from T1R-expressing taste cells in mice. Biochem. J. 2016, 473, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.P.; Deng, D.; Breslin, P.A. Bitter taste receptors (T2Rs) are sentinels that coordinate metabolic and immunological defense responses. Curr. Opin. Physiol. 2021, 20, 70–76. [Google Scholar] [CrossRef]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of Sweet and Umami Taste in the Absence of Taste Receptor T1r3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Damak, S.; Mosinger, B.; Margolskee, R.F. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Ohkuri, T.; Jyotaki, M.; Yasuo, T.; Horio, N.; Yasumatsu, K.; Sanematsu, K.; Shigemura, N.; Yamamoto, T.; Margolskee, R.F.; et al. Endocannabinoids selectively enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Kawai, M.; Sekine-Hayakawa, Y.; Okiyama, A.; Ninomiya, Y. Gustatory sensation of l- and d-amino acids in humans. Amino Acids 2012, 43, 2349–2358. [Google Scholar] [CrossRef]
- Zukerman, S.; Ackroff, K.; Sclafani, A. Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R840–R853. [Google Scholar] [CrossRef] [PubMed]
- Forsgård, R.A. Lactose digestion in humans: Intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable. Am. J. Clin. Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef]
- Blake, H.H.; Henning, S.J. Basis for lactose aversion in the weanling rat. Physiol. Behav. 1985, 35, 313–316. [Google Scholar] [CrossRef]
- Welzl, H.; D’Adamo, P.; Lipp, H.-P. Conditioned taste aversion as a learning and memory paradigm. Behav. Brain Res. 2001, 125, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sebastio, G.; Villa, M.; Sartorio, R.; Guzzetta, V.; Poggi, V.; Auricchio, S.; Boll, W.; Mantei, N.; Semenza, G. Control of lactase in human adult-type hypolactasia and in weaning rabbits and rats. Am. J. Hum. Genet. 1989, 45, 489–497. [Google Scholar] [PubMed]
- Fernández-Bañares, F. Carbohydrate Maldigestion and Intolerance. Nutrients 2022, 14, 1923. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Gearry, R.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | Effect | Degree of Freedom | F Value | p Value |
|---|---|---|---|---|
| Sucrose | genotype | 1.65 | 712 | <0.001 |
| concentration | 4.65 | 136 | <0.001 | |
| interaction | 4.65 | 120 | <0.001 | |
| Lactose | genotype | 1.65 | 154 | <0.001 |
| concentration | 4.65 | 33.4 | <0.001 | |
| interaction | 4.65 | 31.6 | <0.001 | |
| Galactose | genotype | 1.65 | 262 | <0.001 |
| concentration | 4.65 | 128 | <0.001 | |
| interaction | 4.65 | 118 | <0.001 | |
| GOSs | genotype | 1.52 | 206 | <0.001 |
| concentration | 3.52 | 128 | <0.001 | |
| interaction | 3.52 | 108 | <0.001 | |
| FOSs | genotype | 1.52 | 49.1 | <0.001 |
| concentration | 3.52 | 22.8 | <0.001 | |
| interaction | 3.52 | 24.5 | <0.001 | |
| l-lactic acid | genotype | 1.65 | 1.04 | 0.31 |
| concentration | 4.65 | 327 | <0.001 | |
| interaction | 4.65 | 1.62 | 0.179 | |
| d-lactic acid | genotype | 1.65 | 2.52 | 0.117 |
| concentration | 4.65 | 452 | <0.001 | |
| interaction | 4.65 | 2.68 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamase, Y.; Huang, H.; Mitoh, Y.; Egusa, M.; Miyawaki, T.; Yoshida, R. Taste Responses and Ingestive Behaviors to Ingredients of Fermented Milk in Mice. Foods 2023, 12, 1150. https://doi.org/10.3390/foods12061150
Yamase Y, Huang H, Mitoh Y, Egusa M, Miyawaki T, Yoshida R. Taste Responses and Ingestive Behaviors to Ingredients of Fermented Milk in Mice. Foods. 2023; 12(6):1150. https://doi.org/10.3390/foods12061150
Chicago/Turabian StyleYamase, Yuko, Hai Huang, Yoshihiro Mitoh, Masahiko Egusa, Takuya Miyawaki, and Ryusuke Yoshida. 2023. "Taste Responses and Ingestive Behaviors to Ingredients of Fermented Milk in Mice" Foods 12, no. 6: 1150. https://doi.org/10.3390/foods12061150
APA StyleYamase, Y., Huang, H., Mitoh, Y., Egusa, M., Miyawaki, T., & Yoshida, R. (2023). Taste Responses and Ingestive Behaviors to Ingredients of Fermented Milk in Mice. Foods, 12(6), 1150. https://doi.org/10.3390/foods12061150






