An Innovative Mei-Gin Formula Exerts Anti-Adipogenic and Anti-Obesity Effects in 3T3-L1 Adipocyte and High-Fat Diet-Induced Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition Analysis of the Mei-Gin Formula (MGF)
2.2. Cell Culture and Treatment
2.3. Animals and Diet
2.4. Oil Red O Lipid Staining
2.5. Determination of Glycerol-3-Phosphate Dehydrogenase (GPDH) Activity
2.6. Statistical Analysis
3. Results
3.1. Determination of Phenolic Contents of the Mei-Gin Formulas
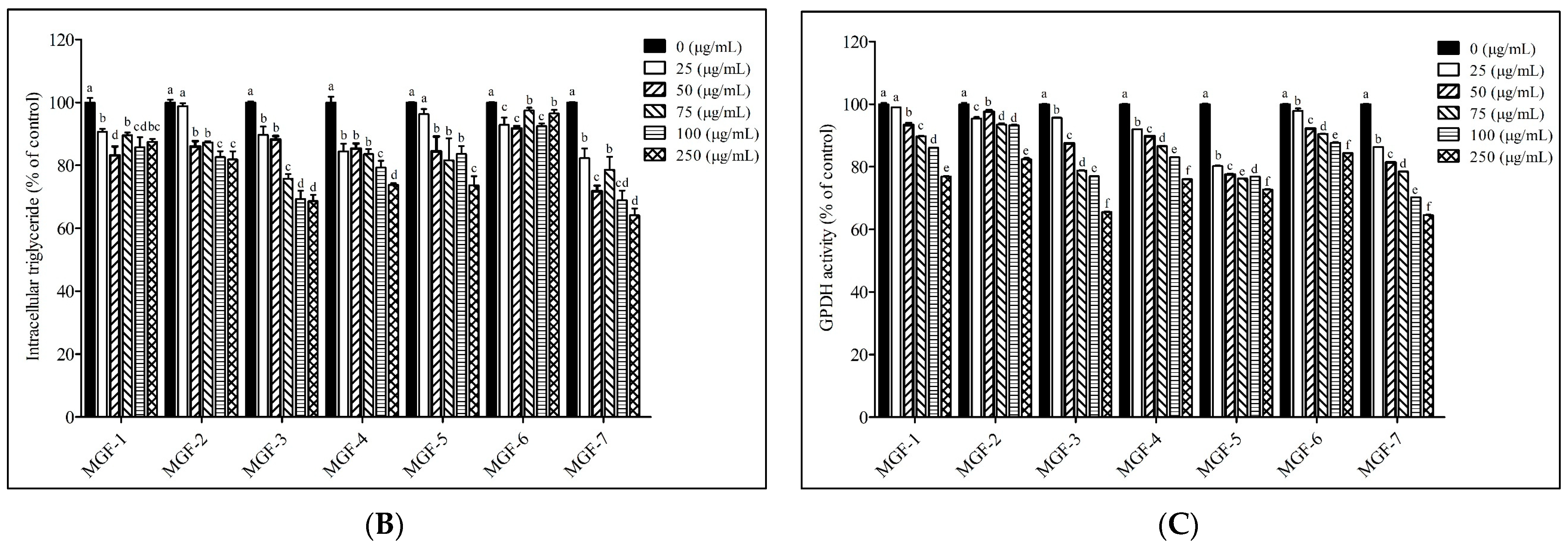
3.2. Effects of Mei-Gin Formulas on Lipid Accumulation in 3T3-L1 Adipocytes
3.3. Effect of the Mei-Gin Formula on Body Weight, Feed Intake, Energy Intake, Feed Efficiency, and Mass of Selected Organs in HFD-Induced Obesity
3.4. Effect of the Mei-Gin Formula on Body Fat Mass and Adipose Tissue in Rates with HFD-Induced Obesity Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Alter, D.A. The economic burden of obesity worldwide: A systematic review of the direct costs of obesity. Obes. Rev. 2011, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Harbron, C.G.; Murgatroyd, P.R.; Prentice, A.M. Covert manipulation of dietary fat and energy density: Effect on substrate flux and food intake in men eating ad libitum. Am. J. Clin. Nutr. 1995, 62, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Engin, A. Fat Cell and Fatty Acid Turnover in Obesity. Adv. Exp. Med. Biol. 2017, 960, 135–160. [Google Scholar] [CrossRef]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef]
- Schoettl, T.; Fischer, I.P.; Ussar, S. Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 2018, 221, jeb162958. [Google Scholar] [CrossRef]
- Atzmon, G.; Yang, X.M.; Muzumdar, R.; Ma, X.H.; Gabriely, I.; Barzilai, N. Differential gene expression between visceral and subcutaneous fat depots. Horm. Metab. Res. 2002, 34, 622–628. [Google Scholar] [CrossRef]
- Gealekman, O.; Guseva, N.; Hartigan, C.; Apotheker, S.; Gorgoglione, M.; Gurav, K.; Tran, K.V.; Straubhaar, J.; Nicoloro, S.; Czech, M.P.; et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 2011, 123, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Herz, C.T.; Kiefer, F.W. Adipose tissue browning in mice and humans. J. Endocrinol. 2019, 241, R97–R109. [Google Scholar] [CrossRef]
- Sun, N.N.; Wu, T.Y.; Chau, C.F. Natural Dietary and Herbal Products in Anti-Obesity Treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Gonzalez-Castejon, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharm. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Ishihara, Y.; Tsuji, M.; Vogel, C.F.A. Suppressive effects of aryl-hydrocarbon receptor repressor on adipocyte differentiation in 3T3-L1 cells. Arch. Biochem. Biophys. 2018, 642, 75–80. [Google Scholar] [CrossRef]
- Chyau, C.C.; Chu, C.C.; Chen, S.Y.; Duh, P.D. The Inhibitory Effects of Djulis (Chenopodium formosanum) and Its Bioactive Compounds on Adipogenesis in 3T3-L1 Adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef]
- Lee, M.S.; Shin, Y.; Jung, S.; Kim, S.Y.; Jo, Y.H.; Kim, C.T.; Yun, M.K.; Lee, S.J.; Sohn, J.; Yu, H.J.; et al. The Inhibitory Effect of Tartary Buckwheat Extracts on Adipogenesis and Inflammatory Response. Molecules 2017, 22, 1160. [Google Scholar] [CrossRef]
- Lee, E.J.; Moon, J.Y.; Yoo, B.S. Cadmium inhibits the differentiation of 3T3-L1 preadipocyte through the C/EBPalpha and PPARgamma pathways. Drug Chem. Toxicol. 2012, 35, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hasegawa, M.; Fujita, M.; Kobayashi, I.; Ozasa, K.; Watanabe, Y. Anti-Helicobacter pylori effects of Bainiku-ekisu (concentrate of Japanese apricot juice). Nihon. Shokakibyo. Gakkai. Zasshi. 2002, 99, 379–385. [Google Scholar] [PubMed]
- Nakajima, S.; Fujita, K.; Inoue, Y.; Nishio, M.; Seto, Y. Effect of the folk remedy, Bainiku-ekisu, a concentrate of Prunus mume juice, on Helicobacter pylori infection in humans. Helicobacter 2006, 11, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Chen, H.Y.; Liu, S.C. Study of the Antibacterial Efficacy of Bainiku-Ekisu against Pathogens. Int. J. Bacteriol. 2014, 2014, 460395. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of Anti-Oxidant and Anti-Inflammatory Effects between Fresh and Aged Black Garlic Extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef]
- Kang, O.J. Evaluation of Melanoidins Formed from Black Garlic after Different Thermal Processing Steps. Prev. Nutr. Food. Sci. 2016, 21, 398–405. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.; Wang, L.; Li, X.; Zhang, S.; Wang, X.; Jin, M.; Hsiao, C.D.; Lin, H.; Han, L.; et al. Metabolomics for Biomarker Discovery in Fermented Black Garlic and Potential Bioprotective Responses against Cardiovascular Diseases. J. Agric. Food. Chem. 2019, 67, 12191–12198. [Google Scholar] [CrossRef]
- Amor, S.; Gonzalez-Hedstrom, D.; Martin-Carro, B.; Inarejos-Garcia, A.M.; Almodovar, P.; Prodanov, M.; Garcia-Villalon, A.L.; Granado, M. Beneficial Effects of an Aged Black Garlic Extract in the Metabolic and Vascular Alterations Induced by a High Fat/Sucrose Diet in Male Rats. Nutrients 2019, 11, 153. [Google Scholar] [CrossRef]
- Park, E.; Baek, S.H.; Bang, K.S.; Kim, N.H.; Takimoto, K. Fermented Garlic Extract Increases Oxygen Consumption and UCP-1 mRNA Expression in Human Adipose-Derived Stem Cells. Cell J. 2019, 21, 357–362. [Google Scholar] [CrossRef]
- Huang, H.T.; Liaw, C.C.; Chiou, C.T.; Lee, K.T.; Kuo, Y.H. Mesonosides A-H, primeverose derivatives from Mesona procumbens suppress adipogenesis by downregulating PPARgamma and C/EBPalpha in 3T3-L1 cells. J. Food Drug Anal. 2021, 29, 448–467. [Google Scholar] [CrossRef]
- Jhang, J.J.; Ong, J.W.; Lu, C.C.; Hsu, C.L.; Lin, J.H.; Liao, J.W.; Yen, G.C. Hypouricemic effects of Mesona procumbens Hemsl. through modulating xanthine oxidase activity in vitro and in vivo. Food Funct. 2016, 7, 4239–4246. [Google Scholar] [CrossRef]
- Grant, L.; McBean, D.E.; Fyfe, L.; Warnock, A.M. The inhibition of free radical generation by preparations of Harpagophytum procumbens in vitro. Phytother. Res. 2009, 23, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Cheng, M.C.; Chen, B.Y.; Wang, C.Y. Effects of high pressure extraction on the extraction yield, phenolic compounds, antioxidant and anti-tyrosinase activity of Djulis hull. J. Food Sci. Technol. 2019, 56, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, C.; Segura-Carretero, A.; Del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.C.; Bassett, M.N.; Samman, N.C. Dietary nutritional profile and phenolic compounds consumption in school children of highlands of Argentine Northwest. Food Chem. 2018, 238, 111–116. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential alpha-amylase/alpha-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Nabi, S.A.; Swapna, S.; Apparao, C. Cinnamic acid as one of the antidiabetic active principle(s) from the seeds of Syzygium alternifolium. Food Chem. Toxicol. 2012, 50, 1425–1431. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Johansson, K.; Neovius, K.; DeSantis, S.M.; Rossner, S.; Neovius, M. Discontinuation due to adverse events in randomized trials of orlistat, sibutramine and rimonabant: A meta-analysis. Obes. Rev. 2009, 10, 564–575. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ghosh, I.; Jana, A.; Ghosh, M.; Mukherjee, A. Genotoxicity of antiobesity drug orlistat and effect of caffeine intervention: An in vitro study. Drug Chem. Toxicol. 2017, 40, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Cave, E.; Crowther, N.J. The Use of 3T3-L1 Murine Preadipocytes as a Model of Adipogenesis. Methods Mol. Biol. 2019, 1916, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Chang, W.T.; Hsu, C.L. Inhibitory effect of deep sea water on adipogenesis in 3T3-L1 adipocytes. Chung Shan Med. J. 2015, 26, 35–41. [Google Scholar]
- Papetti, A.; Daglia, M.; Aceti, C.; Sordelli, B.; Spini, V.; Carazzone, C.; Gazzani, G. Hydroxycinnamic acid derivatives occurring in Cichorium endivia vegetables. J. Pharm. Biomed. Anal. 2008, 48, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Groenbaek, M.; Tybirk, E.; Neugart, S.; Sundekilde, U.K.; Schreiner, M.; Kristensen, H.L. Flavonoid Glycosides and Hydroxycinnamic Acid Derivatives in Baby Leaf Rapeseed From White and Yellow Flowering Cultivars With Repeated Harvest in a 2-Years Field Study. Front. Plant. Sci. 2019, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019, 295, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, R.; Chotimarkorn, C.; Shafiqul, I.M.; Hori, M.; Ozaki, H.; Ushio, H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 2007, 358, 615–619. [Google Scholar] [CrossRef]
- Ling, Y.; Li, Y.; Zhu, R.; Qian, J.; Liu, J.; Gao, W.; Meng, C.; Miao, J.; Xiong, B.; Qiu, X.; et al. Hydroxamic Acid Derivatives of beta-Carboline/Hydroxycinnamic Acid Hybrids Inducing Apoptosis and Autophagy through the PI3K/Akt/mTOR Pathways. J. Nat. Prod. 2019, 82, 1442–1450. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Murata, T.; El-Rayes, B.F.; Shoji, M. The flavonoid p-hydroxycinnamic acid exhibits anticancer effects in human pancreatic cancer MIA PaCa-2 cells in vitro: Comparison with gemcitabine. Oncol. Rep. 2015, 34, 3304–3310. [Google Scholar] [CrossRef]
- Salomone, F.; Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Webb, M.; Grosso, G.; Godos, J.; Galvano, F.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Higher phenolic acid intake independently associates with lower prevalence of insulin resistance and non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Shin, S.H.; Seo, S.G.; Min, S.; Yang, H.; Lee, E.; Son, J.E.; Kwon, J.Y.; Yue, S.; Chung, M.Y.; Kim, K.H.; et al. Caffeic acid phenethyl ester, a major component of propolis, suppresses high fat diet-induced obesity through inhibiting adipogenesis at the mitotic clonal expansion stage. J. Agric. Food Chem. 2014, 62, 4306–4312. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Wong, C.N.; Pin, W.K.; Wong, M.H.; Kwok, C.Y.; Chan, R.Y.; Yu, P.H.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-alpha in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kang, S.I.; Shin, H.S.; Yoon, S.A.; Kim, J.H.; Ko, H.C.; Kim, S.J. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells through activation of the AMPK pathway. Food Chem. Toxicol. 2013, 59, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Lu, T.Y.; Cheng, M.C.; Lu, H.C.; Wu, M.F.; Hsu, C.L. Deep Sea Water Improves Abnormalities in Lipid Metabolism through Lipolysis and Fatty Acid Oxidation in High-Fat Diet-Induced Obese Rats. Mar. Drugs 2017, 15, 386. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Qiao, L.; Yoo, H.S.; Madon, A.; Kinney, B.; Hay, W.W., Jr.; Shao, J. Adiponectin enhances mouse fetal fat deposition. Diabetes 2012, 61, 3199–3207. [Google Scholar] [CrossRef]
- Zheng, T.; Yang, X.; Li, W.; Wang, Q.; Chen, L.; Wu, D.; Bian, F.; Xing, S.; Jin, S. Salidroside Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via AMPK-Dependent TXNIP/NLRP3 Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 8597897. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Bae, J.H.; Im, S.S.; Hwang, I.; Ha, H.; Song, D.K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflug. Arch 2019, 471, 829–843. [Google Scholar] [CrossRef]
- Carvalho, K.M.; Marinho Filho, J.D.; de Melo, T.S.; Araujo, A.J.; Quetz Jda, S.; da Cunha Mdo, P.; de Melo, K.M.; da Silva, A.A.; Tome, A.R.; Havt, A.; et al. The Resin from Protium heptaphyllum Prevents High-Fat Diet-Induced Obesity in Mice: Scientific Evidence and Potential Mechanisms. Evid Based Complement Altern. Med. 2015, 2015, 106157. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Park, C.; Jin, C.Y.; Kim, G.Y.; Jeong, Y.K.; Kim, W.J.; Choi, Y.H. Induction of apoptosis by ethanol extract of Prunus mume in U937 human leukemia cells through activation of caspases. Oncol. Rep. 2011, 26, 987–993. [Google Scholar] [CrossRef]
- Tada, K.; Kawahara, K.; Matsushita, S.; Hashiguchi, T.; Maruyama, I.; Kanekura, T. MK615, a Prunus mume Steb. Et Zucc (‘Ume’) extract, attenuates the growth of A375 melanoma cells by inhibiting the ERK1/2-Id-1 pathway. Phytother. Res. 2012, 26, 833–838. [Google Scholar] [CrossRef]
- Shin, E.J.; Hur, H.J.; Sung, M.J.; Park, J.H.; Yang, H.J.; Kim, M.S.; Kwon, D.Y.; Hwang, J.T. Ethanol extract of the Prunus mume fruits stimulates glucose uptake by regulating PPAR-gamma in C2C12 myotubes and ameliorates glucose intolerance and fat accumulation in mice fed a high-fat diet. Food Chem. 2013, 141, 4115–4121. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewiecki, J.; Cvikrova, M.; Martincova, O.; Katrich, E.; Trakhtenberg, S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug. Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Yen, G.C. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. J. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Huang, W.H.; Yen, G.C. Antihypertensive effects of Hsian-tsao and its active compound in spontaneously hypertensive rats. J. Nutr. Biochem. 2009, 20, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Sakuraoka, Y.; Sawada, T.; Okada, T.; Shiraki, T.; Miura, Y.; Hiraishi, K.; Ohsawa, T.; Adachi, M.; Takino, J.; Takeuchi, M.; et al. MK615 decreases RAGE expression and inhibits TAGE-induced proliferation in hepatocellular carcinoma cells. World J. Gastroenterol. 2010, 16, 5334–5341. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Yamakawa, T.; Kamei, J.; Kadonosono, K.; Tanaka, S. Anti-hyperglycemic effects of plum in a rat model of obesity and type 2 diabetes, Wistar fatty rat. Biomed. Res. 2005, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]


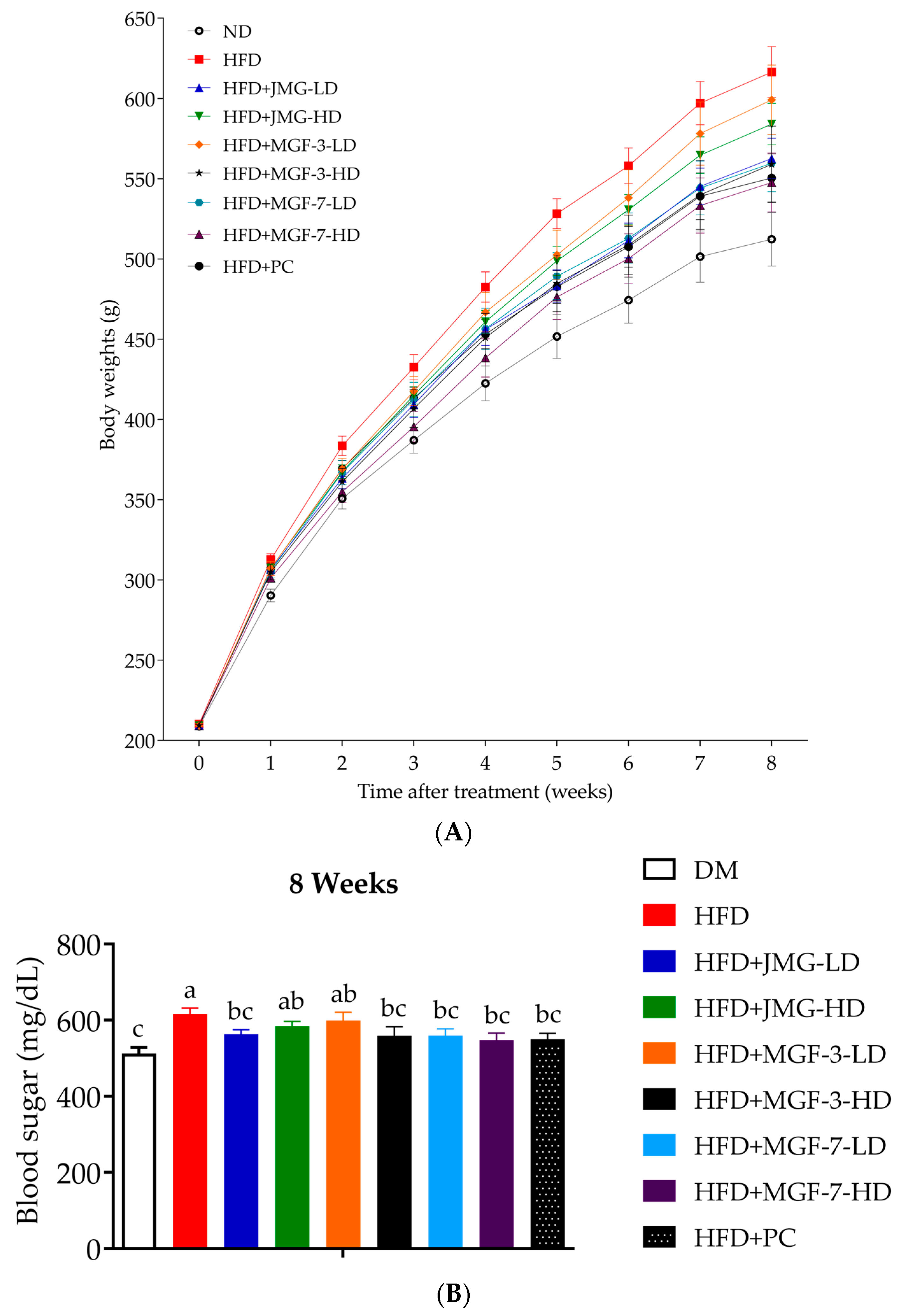
| Dietary Groups | Initial Body Weight (g) | Final Body Weight (g) | Weight Change (g) |
|---|---|---|---|
| ND | 209 ± 2 a | 512 ± 17 c | 304 ± 17 c |
| HFD | 210 ± 2 a | 617 ± 16 a | 406 ± 16 a |
| HFD + JMG-LD | 210 ± 2 a | 563 ± 13 bc | 353 ± 13 bc |
| HFD + JMG-HD | 209 ± 2 a | 584 ± 13 ab | 375 ± 14 ab |
| HFD + MGF-3-LD | 209 ± 2 a | 599 ± 22 ab | 390 ± 20 ab |
| HFD + MGF-3HD | 209 ± 2 a | 559 ± 24 bc | 350 ± 23 bc |
| HFD + MGF-7-LD | 209 ± 2 a | 560 ± 18 bc | 351 ± 17 bc |
| HFD + MGF-7-HD | 209 ± 2 a | 548 ± 18 bc | 338 ± 18 bc |
| HFD + PC | 209 ± 2 a | 551 ± 15 bc | 341 ± 15 bc |
| Dietary Groups | Feed Intake (g/Rat/Day) | Energy Intake (kcal/Rat/Day) | Feed Efficiency (%) | Water Intake (mL/Rat/Day) |
|---|---|---|---|---|
| ND | 25 ± 1 a | 101 ± 2 b | 21 ± 1 c | 33 ± 1 bc |
| HFD | 21 ± 0 b | 110 ± 2 a | 35 ± 1 a | 36 ± 1 bc |
| HFD + JMG-LD | 19 ± 0 c | 101 ± 2 b | 33 ± 1 ab | 40 ± 1 a |
| HFD + JMG-HD | 20 ± 0 bc | 106 ± 2 ab | 33 ± 1 ab | 36 ± 2 bc |
| HFD + MGF-3-LD | 21 ± 1 b | 109 ± 3 a | 33 ± 2 ab | 34 ± 1 ab |
| HFD + MGF-3HD | 20 ± 1 bc | 106 ± 3 ab | 31 ± 2 b | 33 ± 1 c |
| HFD + MGF-7-LD | 20 ± 1 bc | 105 ± 2 ab | 31 ± 1 ab | 36 ± 1 bc |
| HFD + MGF-7-HD | 20 ± 0 bc | 104 ± 2 ab | 30 ± 2 b | 35 ± 1 bc |
| HFD + PC | 21 ± 0 bc | 107 ± 1 a | 30 ± 1 b | 35 ± 0 bc |
| Dietary Groups | Organ Weights (g/Rat) | ||||
|---|---|---|---|---|---|
| Heart | Liver | Spleen | Lung | Kidney | |
| ND | 2 ± 0 a | 15 ± 1 b | 1 ± 0 a | 2 ± 1 a | 3 ± 0 a |
| HFD | 2 ± 0 a | 19 ± 1 a | 1 ± 0 a | 2 ± 0 a | 4 ± 0 a |
| HFD + JMG-LD | 2 ± 0 a | 16 ± 1 b | 1 ± 0 a | 2 ± 0 a | 4 ± 0 a |
| HFD + JMG-HD | 2 ± 0 a | 17 ± 1 a b | 1 ± 0 a | 2 ± 0 a | 4 ± 0 a |
| HFD + MGF-3-LD | 2 ± 0 a | 17 ± 1 a b | 1 ± 0 a | 2 ± 0 a | 4 ± 0 a |
| HFD + MGF-3-HD | 2 ± 0 a | 16 ± 1 b | 1 ± 0 a | 2 ± 0 a | 4 ± 0 a |
| HFD + MGF-7-LD | 2 ± 0 a | 15 ± 1 b | 1 ± 0 a | 2 ± 0 a | 3 ± 0 a |
| HFD + MGF-7-HD | 2 ± 0 a | 15 ± 1 b | 1 ± 0 a | 2 ± 0 a | 3 ± 0 a |
| HFD + PC | 2 ± 0 a | 15 ± 1 b | 1 ± 0 a | 2 ± 0 a | 4 ± 0 a |
| Dietary Groups | Weights (mg/g Rat) | ||||
|---|---|---|---|---|---|
| Perirenal Adipose Tissue | Epididymal Adipose Tissue | Mesenteric Adipose Tissue | Retroperitoneal Adipose Tissue | Inguinal Adipose Tissue | |
| ND | 28 ± 3 c | 23 ± 2 b | 20 ± 1 c | 21 ± 2 c | 14 ± 2 b |
| HFD | 48 ± 2 a | 32 ± 2 a | 31 ± 2 a | 36 ± 2 a | 21 ± 1 a |
| HFD + JMG-LD | 39 ± 2 b | 31 ± 2 a | 25 ± 1 b | 24 ± 2 bc | 16 ± 1 b |
| HFD + JMG-HD | 41 ± 2 ab | 32 ± 2 a | 27 ± 2 ab | 30 ± 4 abc | 17 ± 2 ab |
| HFD + MGF-3-LD | 43 ± 2 ab | 31 ± 2 a | 27 ± 2 ab | 32 ± 3 ab | 18 ± 2 ab |
| HFD + MGF-3-HD | 39 ± 3 b | 30 ± 2 a | 24 ± 3 bc | 25 ± 3 bc | 15 ± 2 b |
| HFD + MGF-7-LD | 39 ± 2 b | 30 ± 2 a | 25 ± 2 bc | 24 ± 3 bc | 13 ± 1 b |
| HFD + MGF-7-HD | 36 ± 2 b | 29 ± 2 a | 24 ± 2 bc | 24 ± 2 bc | 13 ± 1 b |
| HFD + PC | 38 ± 3 b | 30 ± 2 a | 25 ± 1 bc | 27 ± 3 bc | 14 ± 1 b |
| Dietary Groups | Weights (mg/g Rat) | ||
|---|---|---|---|
| Visceral Adipose Tissue | Subcutaneous Adipose Tissue | Total Body Fat | |
| ND | 71 ± 5 c | 35 ± 3 d | 106 ± 7 c |
| HFD | 111 ± 5 a | 57 ± 3 a | 168 ± 7 a |
| HFD + JMG-LD | 96 ± 4 ab | 40 ± 2 bcd | 135 ± 5 b |
| HFD + JMG-HD | 100 ± 5 ab | 47 ± 5 abc | 146 ± 10 ab |
| HFD + MGF-3-LD | 100 ± 6 ab | 50 ± 4 ab | 150 ± 10 ab |
| HFD + MGF-3-HD | 93 ± 7 b | 40 ± 4 bcd | 132 ± 11 b |
| HFD + MGF-7-LD | 94 ± 5 b | 37 ± 3 cd | 131 ± 7 b |
| HFD + MGF-7-HD | 88 ± 6 b | 37 ± 3 cd | 126 ± 8 bc |
| HFD + PC | 92 ± 5 b | 41 ± 4 bcd | 134 ± 8 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-L.; Chang, W.-T.; Lin, J.-L.; Cheng, M.-C.; Huang, S.-C.; Chen, S.-C.; Wong, Y.-C.; Hsu, C.-L. An Innovative Mei-Gin Formula Exerts Anti-Adipogenic and Anti-Obesity Effects in 3T3-L1 Adipocyte and High-Fat Diet-Induced Obese Rats. Foods 2023, 12, 945. https://doi.org/10.3390/foods12050945
Cheng H-L, Chang W-T, Lin J-L, Cheng M-C, Huang S-C, Chen S-C, Wong Y-C, Hsu C-L. An Innovative Mei-Gin Formula Exerts Anti-Adipogenic and Anti-Obesity Effects in 3T3-L1 Adipocyte and High-Fat Diet-Induced Obese Rats. Foods. 2023; 12(5):945. https://doi.org/10.3390/foods12050945
Chicago/Turabian StyleCheng, Hsin-Lin, Wei-Tang Chang, Jiun-Ling Lin, Ming-Ching Cheng, Shih-Chien Huang, Shiuan-Chih Chen, Yue-Ching Wong, and Chin-Lin Hsu. 2023. "An Innovative Mei-Gin Formula Exerts Anti-Adipogenic and Anti-Obesity Effects in 3T3-L1 Adipocyte and High-Fat Diet-Induced Obese Rats" Foods 12, no. 5: 945. https://doi.org/10.3390/foods12050945
APA StyleCheng, H.-L., Chang, W.-T., Lin, J.-L., Cheng, M.-C., Huang, S.-C., Chen, S.-C., Wong, Y.-C., & Hsu, C.-L. (2023). An Innovative Mei-Gin Formula Exerts Anti-Adipogenic and Anti-Obesity Effects in 3T3-L1 Adipocyte and High-Fat Diet-Induced Obese Rats. Foods, 12(5), 945. https://doi.org/10.3390/foods12050945






