Ethanol Extract of Mao Jian Green Tea Attenuates Gastrointestinal Symptoms in a Rat Model of Irritable Bowel Syndrome with Constipation via the 5-hydroxytryptamine Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of MJGT_EE
2.3. Animal Grouping, Construction of the IBS-C Model, and Sample Collection
2.4. Measurement of FWC
2.5. Measurement of Intestinal Sensitivity
2.6. Effects of MJGT_EE on Gastric Emptying and Small Intestinal Propulsion
2.7. Hematoxylin and Eosin (H&E) Staining and Immunohistochemistry
2.8. Western Blot Analysis
2.9. 16S rDNA Sequencing
2.10. Identification of the Four Chemical Component Types in MJGT_EE with HPLC
2.11. Statistical Analysis
3. Results
3.1. Evaluation of the IBS-C Model
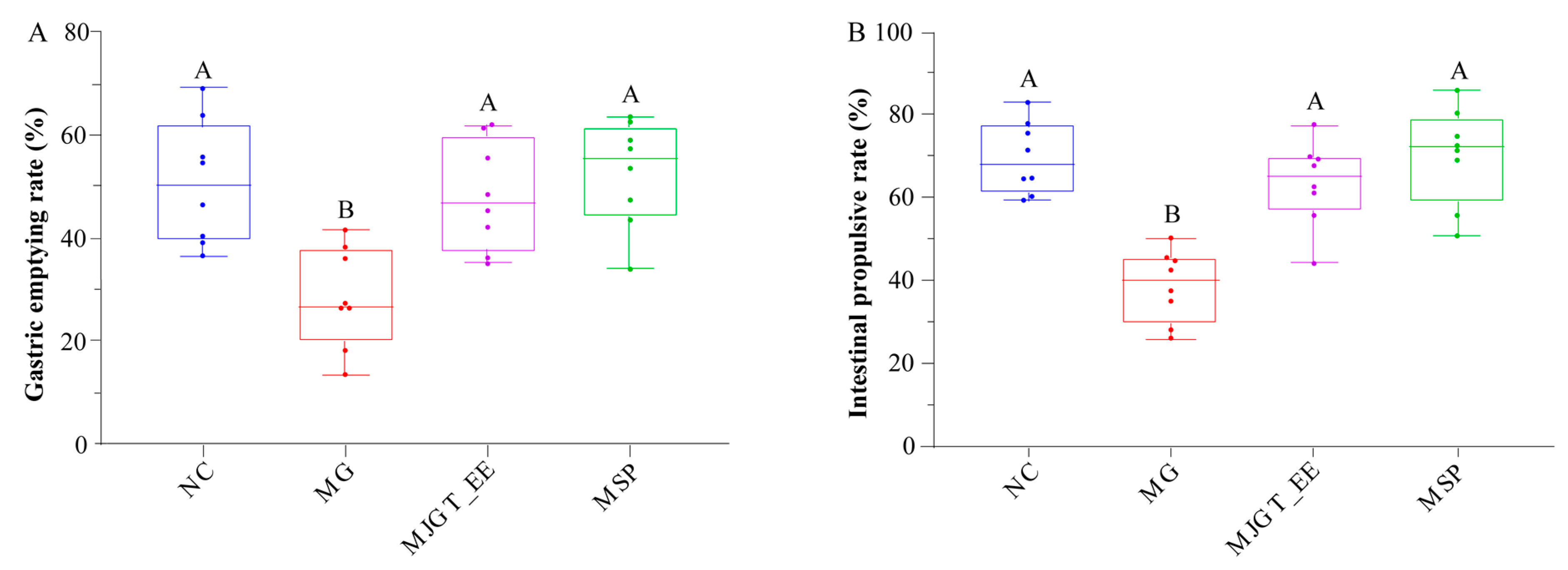
3.2. Effects of MJGT_EE on Gastric Emptying and Small Intestinal Propulsion in IBS-C Rats
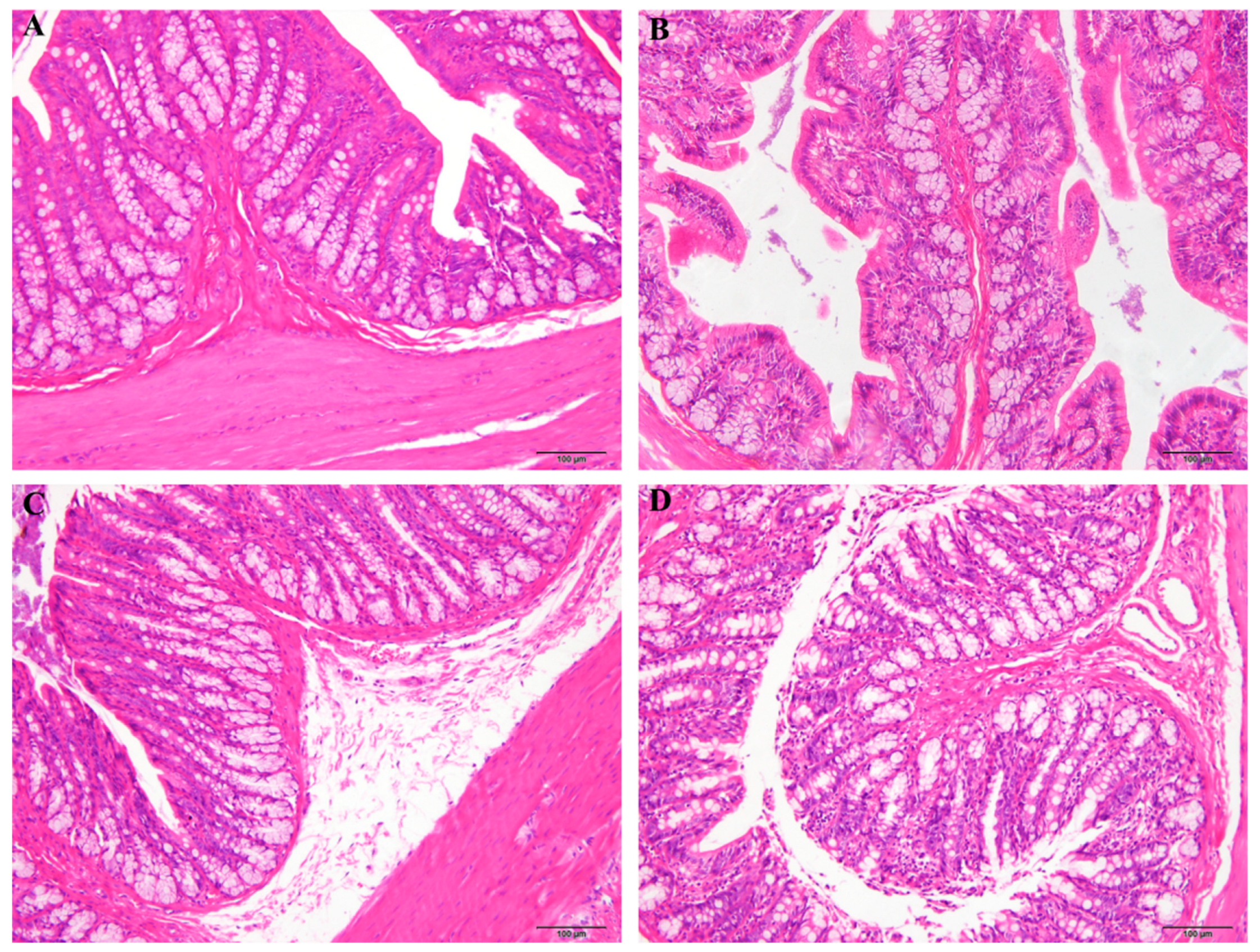
3.3. Effect of MJGT_EE on the Colonic Tissue Morphology
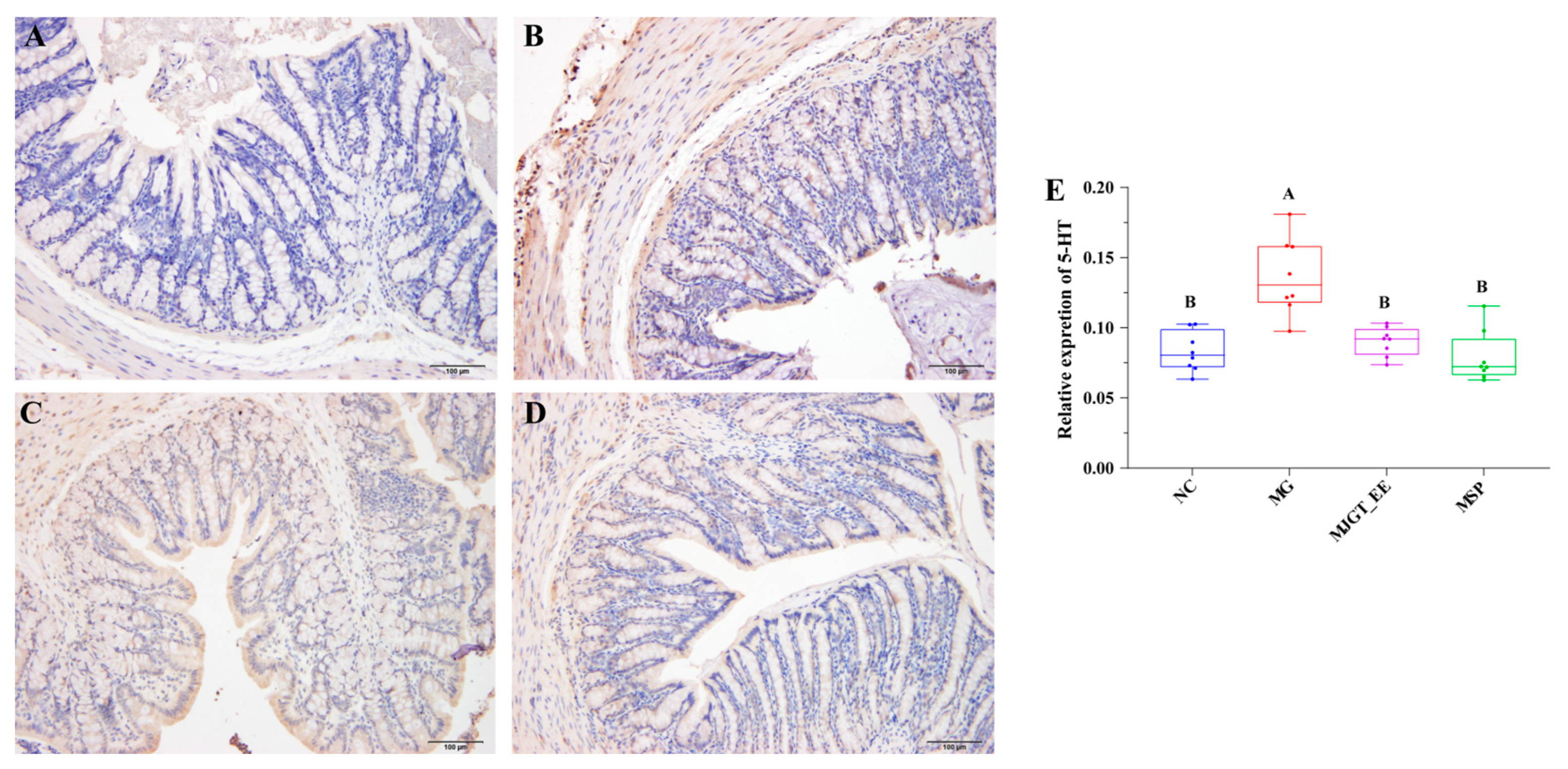
3.4. Evaluation of the Accumulation of 5-HT in the Colonic Tissue Impacted by MJGT_EE
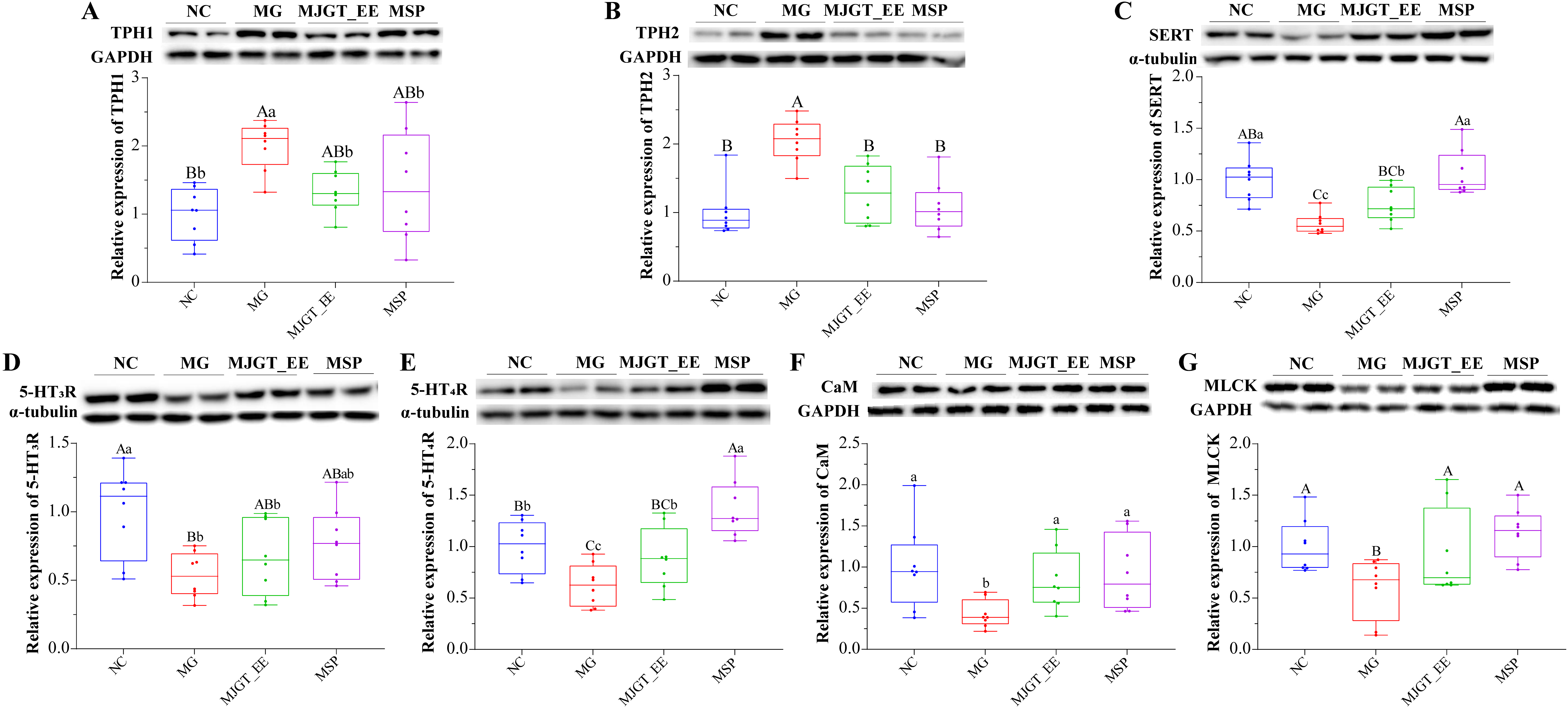
3.5. Evaluation of the Expression of TPH1 and TPH2 in the Colonic Tissue Impacted by MJGT_EE
3.6. Effect of MJGT_EE on SERT Expression in the Colon
3.7. Effect of MJGT_EE on the Expression of 5-HT3R and 5-HT4R in the Colon
3.8. Effect of MJGT_EE on CaM-MLCK Signaling Pathway
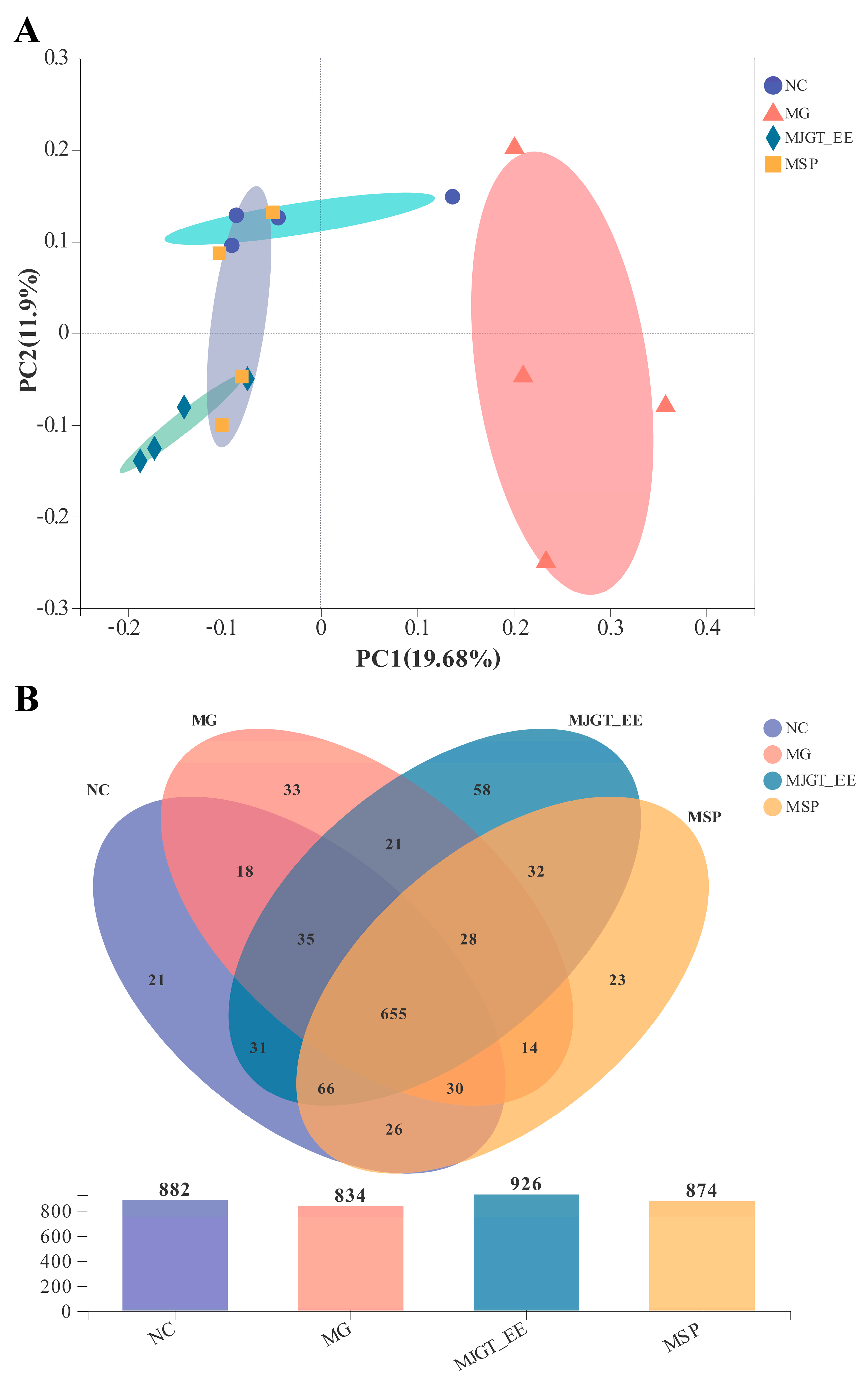
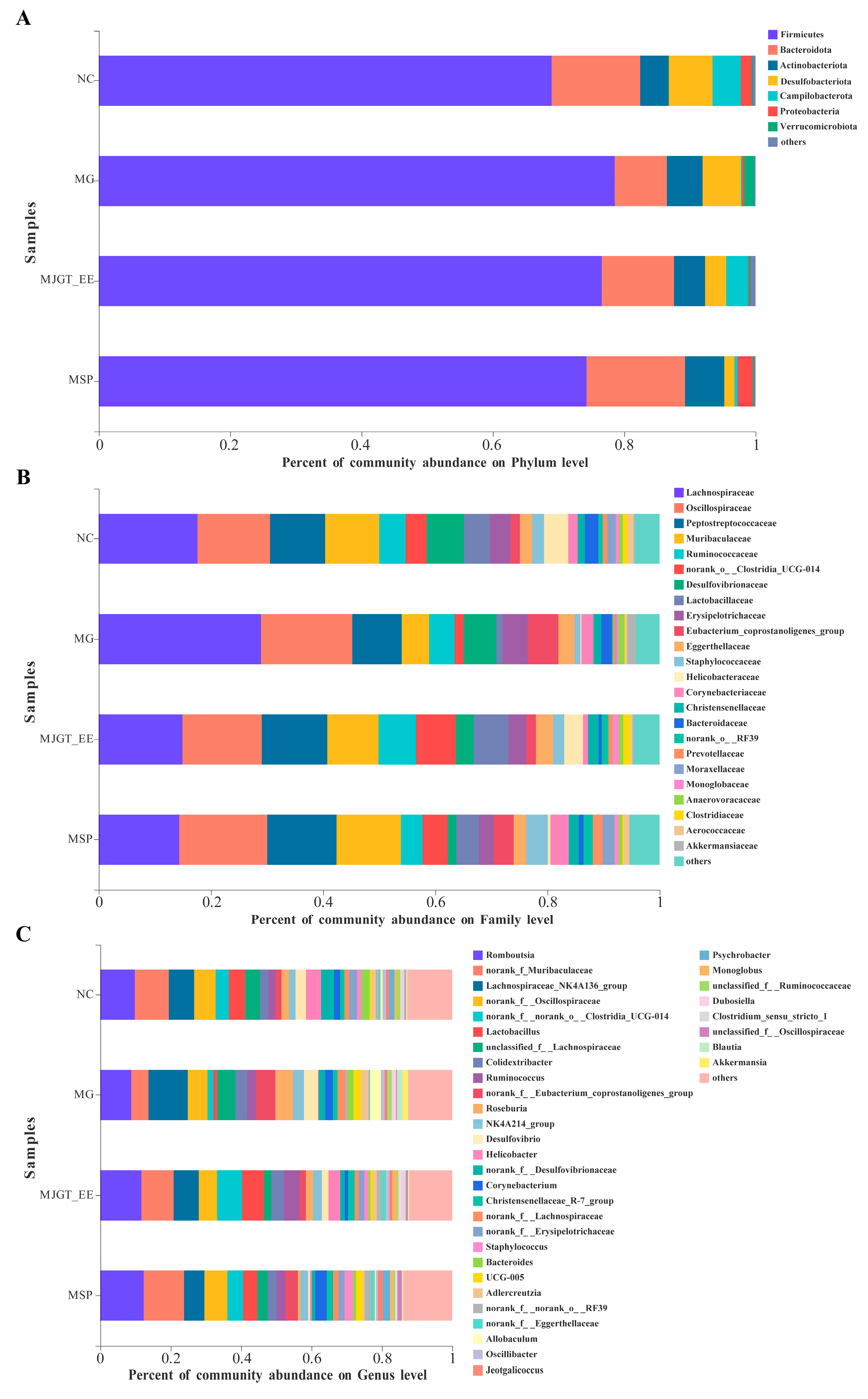
3.9. Effects of MJGT_EE on IBS-C Rat Gut Microbiota
3.10. Identification of the Four Chemical Component Types in MJGT_EE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Harper, L.; Bold, J. An exploration into the motivation for gluten avoidance in the absence of coeliac disease. Gastroenterol. Hepatol. Bed Bench. 2018, 11, 259–268. [Google Scholar] [PubMed]
- Fadgyas-Stanculete, M.; Buga, A.M.; Popa-Wagner, A.; Dumitrascu, D.L. The relationship between irritable bowel syndrome and psychiatric disorders: From molecular changes to clinical manifestations. J. Mol. Psychiatry 2014, 2, 4. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Faseleh Jahromi, M.; Ho, Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed. Res. Int. 2014, 2014, 927268. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.Y.; Fang, X.C.; Li, X.Q.; Fei, G.J. Ethnic differences in genetic polymorphism associated with irritable bowel syndrome. World J. Gastroenterol. 2020, 26, 2049–2063. [Google Scholar] [CrossRef]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Barbara, G.; Grover, M.; Bercik, P.; Corsetti, M.; Ghoshal, U.C.; Ohman, L.; Rajilic-Stojanovic, M. Rome Foundation Working Team Report on Post-Infection Irritable Bowel Syndrome. Gastroenterology 2019, 156, 46–58.e47. [Google Scholar] [CrossRef]
- Gros, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Abdollahi, M.; Rahimi, R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J. Neurogastroenterol. Motil. 2016, 22, 558–574. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Qiao, H.; Zhang, Y. A genetic association study of single nucleotide polymorphisms in GNbeta3 and COMT in elderly patients with irritable bowel syndrome. Med. Sci. Monit. 2014, 20, 1246–1254. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef]
- Schoenfeld, P.S. Advances in IBS 2016: A Review of Current and Emerging Data. Gastroenterol. Hepatol. 2016, 12, 1–11. [Google Scholar]
- Grundmann, O.; Yoon, S.L. Irritable bowel syndrome: Epidemiology, diagnosis and treatment: An update for health-care practitioners. J. Gastroenterol. Hepatol. 2010, 25, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Soni, R.; Khan, S. How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome. Cureus 2020, 12, e9871. [Google Scholar] [CrossRef]
- Drossman, D.A.; Chey, W.D.; Johanson, J.F.; Fass, R.; Scott, C.; Panas, R.; Ueno, R. Clinical trial: Lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment. Pharmacol. Ther. 2009, 29, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Lembo, A.J.; Lavins, B.J.; Shiff, S.J.; Kurtz, C.B.; Currie, M.G.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Fitch, D.A.; et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am. J. Gastroenterol. 2012, 107, 1702–1712. [Google Scholar] [CrossRef]
- Rao, S.; Lembo, A.J.; Shiff, S.J.; Lavins, B.J.; Currie, M.G.; Jia, X.D.; Shi, K.; MacDougall, J.E.; Shao, J.Z.; Eng, P.; et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am. J. Gastroenterol. 2012, 107, 1714–1724. [Google Scholar] [CrossRef]
- Brenner, D.M.; Fogel, R.; Dorn, S.D.; Krause, R.; Eng, P.; Kirshoff, R.; Nguyen, A.; Crozier, R.A.; Magnus, L.; Griffin, P.H. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: Results of two phase 3 randomized clinical trials. Am. J. Gastroenterol. 2018, 113, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Burr, N.E.; Ford, A.C. Relative Efficacy of Tegaserod in a Systematic Review and Network Meta-analysis of Licensed Therapies for Irritable Bowel Syndrome With Constipation. Clin. Gastroenterol. Hepatol. 2020, 18, 1238–1239.e1. [Google Scholar] [CrossRef]
- Bassotti, G.; Usai Satta, P.; Bellini, M. Chronic Idiopathic Constipation in Adults: A Review on Current Guidelines and Emerging Treatment Options. Clin. Exp. Gastroenterol. 2021, 14, 413–428. [Google Scholar] [CrossRef]
- Nakajima, A.; Shoji, A.; Kokubo, K.; Igarashi, A. A Systematic Review and Network Meta-Analysis on the Efficacy of Medications in the Treatment of Chronic Idiopathic Constipation in Japan. Gastroenterol. Res. Pract. 2021, 2021, 5534687. [Google Scholar] [CrossRef]
- Pei, L.; Geng, H.; Guo, J.; Yang, G.; Wang, L.; Shen, R.; Xia, S.; Ding, M.; Feng, H.; Lu, J.; et al. Effect of Acupuncture in Patients With Irritable Bowel Syndrome: A Randomized Controlled Trial. Mayo Clin. Proc. 2020, 95, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, M.; Wang, X.; Ye, K.; Shi, S.; Li, H.; Wang, J.; Chen, X.; Ni, J.; Wei, Q.; et al. Efficacy of acupuncture in refractory irritable bowel syndrome: Study protocol for a randomised controlled trial. BMJ Open 2021, 11, e045655. [Google Scholar] [CrossRef]
- Ma, K.; Liu, Y.; Shao, W.; Sun, J.; Li, J.; Fang, X.; Li, J.; Wang, Z.; Zhang, D. Brain Functional Interaction of Acupuncture Effects in Diarrhea-Dominant Irritable Bowel Syndrome. Front. Neurosci. 2020, 14, 608688. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fu, Q.; Yang, S.; Wang, H.; Li, Y. Efficacy and Safety of Acupoint Catgut Embedding for Diarrhea-Predominant Irritable Bowel Syndrome and Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2020, 2020, 5812320. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.W.; Ha, N.Y.; Kim, J.; Ryu, H.S. Herbal Therapies in Functional Gastrointestinal Disorders: A Narrative Review and Clinical Implication. Front. Psychiatry 2020, 11, 601. [Google Scholar] [CrossRef]
- Alammar, N.; Wang, L.; Saberi, B.; Nanavati, J.; Holtmann, G.; Shinohara, R.T.; Mullin, G.E. The impact of peppermint oil on the irritable bowel syndrome: A meta-analysis of the pooled clinical data. BMC Complement. Altern. Med. 2019, 19, 21. [Google Scholar] [CrossRef]
- Jafarzadeh, E.; Shoeibi, S.; Bahramvand, Y.; Nasrollahi, E.; Maghsoudi, A.S.; Yazdi, F.; KarkonShayan, S.; Hassani, S. Turmeric for Treatment of Irritable Bowel Syndrome: A Systematic Review of Population-Based Evidence. Iran. J. Public Health 2022, 51, 1223–1231. [Google Scholar] [CrossRef]
- Xie, Y.; Zhan, X.; Tu, J.; Xu, K.; Sun, X.; Liu, C.; Ke, C.; Cao, G.; Zhou, Z.; Liu, Y. Atractylodes oil alleviates diarrhea-predominant irritable bowel syndrome by regulating intestinal inflammation and intestinal barrier via SCF/c-kit and MLCK/MLC2 pathways. J. Ethnopharmacol. 2021, 272, 113925. [Google Scholar] [CrossRef]
- Editorial Board of the National Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Scientific and Technical Publishers: Shanghai, China, 1999. [Google Scholar]
- Ren, D. Chemical and Bioactive Studies on Two Species of The Genus Dracocephalum. Ph.D. Thesis, Shandong University, Jinan, China, 2007. [Google Scholar]
- Gao, J.; Wang, Z.; Chen, D.; Peng, J.; Xie, D.; Lin, Z.; Lin, Z.; Dai, W. Metabolomic characterization of the chemical compositions of Dracocephalum rupestre Hance. Food Res. Int. 2022, 161, 111871. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Jung, M.; Shankel, D.; Dou, J.H.; Steele, L.; Pillai, S.P. Chemoprotection: A review of the potential therapeutic antioxidant properties of green tea (Camellia sinensis) and certain of its constituents. Med. Res. Rev. 1997, 17, 327–365. [Google Scholar] [CrossRef]
- Korolkiewicz, P.K.; Salloum, A. Commentary on: Impact of drinking Chinese green tea on postoperative short outcomes for gastric cancer: A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 1679–1680. [Google Scholar] [CrossRef]
- Ma, J.-y. Study on Functional Ingredients of Maojian Tea in the Lvliang Mountains. Ph.D. Thesis, Shanxi University, Taiyuan, China, 2020. [Google Scholar]
- Ye, J.-H.; Ye, Y.; Yin, J.-F.; Jin, J.; Liang, Y.-R.; Liu, R.-Y.; Tang, P.; Xu, Y.-Q. Bitterness and astringency of tea leaves and products: Formation mechanism and reducing strategies. Trends Food Sci. Technol. 2022, 123, 14. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Singh, D.P.; Borse, S.P.; Nivsarkar, M. Overcoming the exacerbating effects of ranitidine on NSAID-induced small intestinal toxicity with quercetin: Providing a complete GI solution. Chem. Biol. Interact. 2017, 272, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Hevia, A.; Lopez, P.; Suarez, A.; Sanchez, B.; Margolles, A.; Gonzalez, S. Association of polyphenols from oranges and apples with specific intestinal microorganisms in systemic lupus erythematosus patients. Nutrients 2015, 7, 1301–1317. [Google Scholar] [CrossRef]
- Mendel, M.; Chlopecka, M.; Dziekan, N.; Karlik, W. Modification of abomasum contractility by flavonoids present in ruminants diet: In vitro study. Animal 2016, 10, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jin, X.; Zheng, C.; Ma, F.; Zhang, X.; Gao, P.; Gao, J.; Zhang, L. Bidirectional Effects of Mao Jian Green Tea and Its Flavonoid Glycosides on Gastrointestinal Motility. Foods 2023, 12, 854. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Stickney, J.C.; Northup, D.W. Effect of gastric emptying upon propulsive motility of small intestine of rats. Proc. Soc. Exp. Biol. Med. 1959, 101, 582–583. [Google Scholar] [CrossRef]
- DuPont, A.W.; Jiang, Z.D.; Harold, S.A.; Snyder, N.; Galler, G.W.; Garcia-Torres, F.; DuPont, H.L. Motility abnormalities in irritable bowel syndrome. Digestion 2014, 89, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Posserud, I.; Ersryd, A.; Simren, M. Functional findings in irritable bowel syndrome. World J. Gastroenterol. 2006, 12, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Zeppelin, T.; Ladefoged, L.K.; Sinning, S.; Schiott, B. Substrate and inhibitor binding to the serotonin transporter: Insights from computational, crystallographic, and functional studies. Neuropharmacology 2019, 161, 107548. [Google Scholar] [CrossRef]
- Simren, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G.; Rome Foundation, C. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing Evidence That Irritable Bowel Syndrome and Functional Gastrointestinal Disorders Have a Microbial Pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 468. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Bouin, M.; Poitras, P. Visceral hypersensitivity in irritable bowel syndrome: Does it really normalize over time? Gastroenterology 2007, 132, 464–465. [Google Scholar] [CrossRef]
- Muller, P.A.; Koscso, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.M.; Mucida, D.; et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014, 158, 300–313. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Coleman, N.S.; Blackshaw, E.; Perkins, A.C.; Singh, G.; Marsden, C.A.; Spiller, R.C. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2005, 3, 349–357. [Google Scholar] [CrossRef]
- Gershon, M.D. Review article: Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment. Pharmacol. Ther. 1999, 13 (Suppl. S2), 15–30. [Google Scholar] [CrossRef]
- Camilleri, M. Serotonergic modulation of visceral sensation: Lower gut. Gut 2002, 51 (Suppl. S1), i81–i86. [Google Scholar] [CrossRef]
- Wang, C.; Fang, X. Inflammation and Overlap of Irritable Bowel Syndrome and Functional Dyspepsia. J. Neurogastroenterol. Motil. 2021, 27, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hortnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Lee, J.; Lee, J.Y.; Kim, H.; Lee, S.; Oh, C.M. A Systems Biology Approach to Investigating the Interaction between Serotonin Synthesis by Tryptophan Hydroxylase and the Metabolic Homeostasis. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.P.; Bertrand, R.L. Serotonin release and uptake in the gastrointestinal tract. Auton. Neurosci. 2010, 153, 47–57. [Google Scholar] [CrossRef]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology 2017, 158, 1049–1063. [Google Scholar] [CrossRef]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin cells of the human gut: Sensors for spices and odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.C.; Cao, H.L.; Xu, M.Q.; Wang, S.N.; Wang, Y.M.; Yan, F.; Wang, B.M. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J. Gastroenterol. 2016, 22, 8137–8148. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, R.A.; Van Colen, I.; Pauwelyn, V.; De Maeyer, J.H. Synergistic effect between 5-HT4 receptor agonist and phosphodiesterase 4-inhibitor in releasing acetylcholine in pig gastric circular muscle in vitro. Eur. J. Pharmacol. 2016, 781, 76–82. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, T.; Tang, Y.; Xiong, W.; Shen, X.; Jiang, L.; Lin, L. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0172846. [Google Scholar] [CrossRef]
- Zhao, J.M.; Chen, L.; Zhou, C.L.; Shi, Y.; Li, Y.W.; Shang, H.X.; Wu, L.Y.; Bao, C.H.; Dou, C.Z.; Wu, H.G. Comparison of Electroacupuncture and Moxibustion for Relieving Visceral Hypersensitivity in Rats with Constipation-Predominant Irritable Bowel Syndrome. Evid. Based Complement. Altern. Med. 2016, 2016, 9410505. [Google Scholar] [CrossRef]
- Fu, R.; Chen, M.; Chen, Y.; Mao, G.; Liu, S. Expression and clinical significance of 5-HT and 5-HT(3)R in the intestinal mucosa of patient with diarrhea-type irritable bowel syndrome. Exp. Ther. Med. 2019, 17, 3077–3082. [Google Scholar] [CrossRef]
- Hillsley, K.; Kirkup, A.J.; Grundy, D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J. Physiol. 1998, 506, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.P.; Kunze, W.A.; Furness, J.B.; Bornstein, J.C. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 2000, 101, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Grider, J.R.; Kuemmerle, J.F.; Jin, J.G. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am. J. Physiol. 1996, 270, G778–G782. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gershon, M.D. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J. Neurosci. 2000, 20, 3295–3309. [Google Scholar] [CrossRef]
- Lukas, T.J. A signal transduction pathway model prototype II: Application to Ca2+-calmodulin signaling and myosin light chain phosphorylation. Biophys J. 2004, 87, 1417–1425. [Google Scholar] [CrossRef][Green Version]
- Menees, S.; Chey, W. The gut microbiome and irritable bowel syndrome. F1000Res 2018, 7, 1029. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome-A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.E.; Singh, R.; Ayoub, Y.K.; Khairy, A.M.; Mullin, G.E. The gut microbiome and irritable bowel syndrome: State of art review. Arab J. Gastroenterol. 2018, 19, 136–141. [Google Scholar] [CrossRef]
- Vervier, K.; Moss, S.; Kumar, N.; Adoum, A.; Barne, M.; Browne, H.; Kaser, A.; Kiely, C.J.; Neville, B.A.; Powell, N.; et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut 2022, 71, 1821–1830. [Google Scholar] [CrossRef]
- Qi, H.; Li, Y.; Yun, H.; Zhang, T.; Huang, Y.; Zhou, J.; Yan, H.; Wei, J.; Liu, Y.; Zhang, Z.; et al. Lactobacillus maintains healthy gut mucosa by producing L-Ornithine. Commun. Biol. 2019, 2, 171. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, S.; Okamoto, Y.; Okawa, T.; Hisamitsu, M.; Chazono, H.; Kobayashi, K.; Sakurai, D.; Horiguchi, S.; Hanazawa, T. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. 2009, 30, 397–405. [Google Scholar] [CrossRef]
- Rossoni, R.D.; Velloso, M.D.S.; de Barros, P.P.; de Alvarenga, J.A.; Santos, J.D.D.; Santos Prado, A.; Ribeiro, F.C.; Anbinder, A.L.; Junqueira, J.C. Inhibitory effect of probiotic Lactobacillus supernatants from the oral cavity on Streptococcus mutans biofilms. Microb. Pathog. 2018, 123, 361–367. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.F.; Dai, T.Y.; Qi, X.; Wang, Y.; Zheng, T.Z.; Gao, X.Y.; Zhang, Y.J.; Ai, Y.; Ma, L.; et al. Short-Chain Fatty Acids Produced by Ruminococcaceae Mediate alpha-Linolenic Acid Promote Intestinal Stem Cells Proliferation. Mol. Nutr. Food Res. 2022, 66, e2100408. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A.; Kovtun, A.S.; Ushakova, V.M.; Morozova, A.Y.; Kostyuk, G.P.; Danilenko, V.N.; Chekhonin, V.P. Bacterial Metabolites of Human Gut Microbiota Correlating with Depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; Hove, A.S.T.; Brul, S.; Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.S.; Osadchiy, V.; Hsiao, E.Y.; Tap, J.; Derrien, M.; Gupta, A.; Tillisch, K.; Le Neve, B.; Grinsvall, C.; Ljungberg, M.; et al. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Du, Y.; Ren, D.; Yang, X.; Zhao, Y. Gut microbiota-dependent catabolites of tryptophan play a predominant role in the protective effects of turmeric polysaccharides against DSS-induced ulcerative colitis. Food Funct. 2021, 12, 9793–9807. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Gao, L.; Jin, X.; Chen, Z.; Qiao, X.; Cui, X.; Gao, J.; Zhang, L. Ethanol Extract of Mao Jian Green Tea Attenuates Gastrointestinal Symptoms in a Rat Model of Irritable Bowel Syndrome with Constipation via the 5-hydroxytryptamine Signaling Pathway. Foods 2023, 12, 1101. https://doi.org/10.3390/foods12051101
Wu L, Gao L, Jin X, Chen Z, Qiao X, Cui X, Gao J, Zhang L. Ethanol Extract of Mao Jian Green Tea Attenuates Gastrointestinal Symptoms in a Rat Model of Irritable Bowel Syndrome with Constipation via the 5-hydroxytryptamine Signaling Pathway. Foods. 2023; 12(5):1101. https://doi.org/10.3390/foods12051101
Chicago/Turabian StyleWu, Lei, Liming Gao, Xiang Jin, Zhikang Chen, Xutong Qiao, Xiting Cui, Jianhua Gao, and Liwei Zhang. 2023. "Ethanol Extract of Mao Jian Green Tea Attenuates Gastrointestinal Symptoms in a Rat Model of Irritable Bowel Syndrome with Constipation via the 5-hydroxytryptamine Signaling Pathway" Foods 12, no. 5: 1101. https://doi.org/10.3390/foods12051101
APA StyleWu, L., Gao, L., Jin, X., Chen, Z., Qiao, X., Cui, X., Gao, J., & Zhang, L. (2023). Ethanol Extract of Mao Jian Green Tea Attenuates Gastrointestinal Symptoms in a Rat Model of Irritable Bowel Syndrome with Constipation via the 5-hydroxytryptamine Signaling Pathway. Foods, 12(5), 1101. https://doi.org/10.3390/foods12051101





