Electrospray-Assisted Fabrication of Dextran–Whey Protein Isolation Microcapsules for the Encapsulation of Selenium-Enriched Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterizations
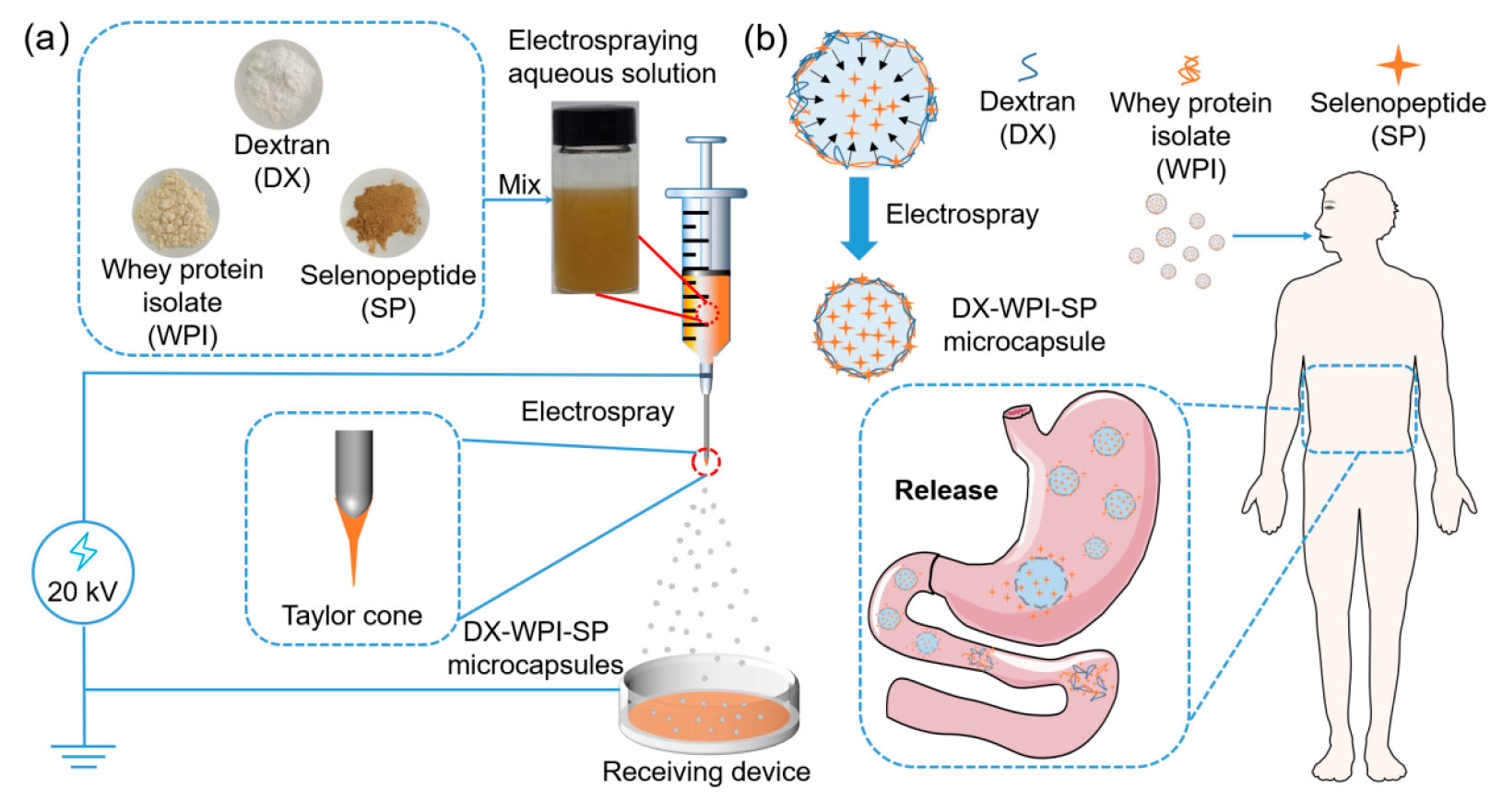
2.3. Preparation of DX-WPI-SP Microcapsules
2.4. Antioxidant Activity Evaluation
2.4.1. DPPH Radical Scavenging Assay
2.4.2. ABTS Radical Scavenging Ability Assay
2.5. Stability of DX-WPI-SP Microcapsules against pH Changes
2.6. Determination of In Vitro Release Properties of DX-WPI-SP Microcapsules
2.7. Cell Culture and Vitality Assay In Vitro
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation of DX-WPI-SP Microcapsules
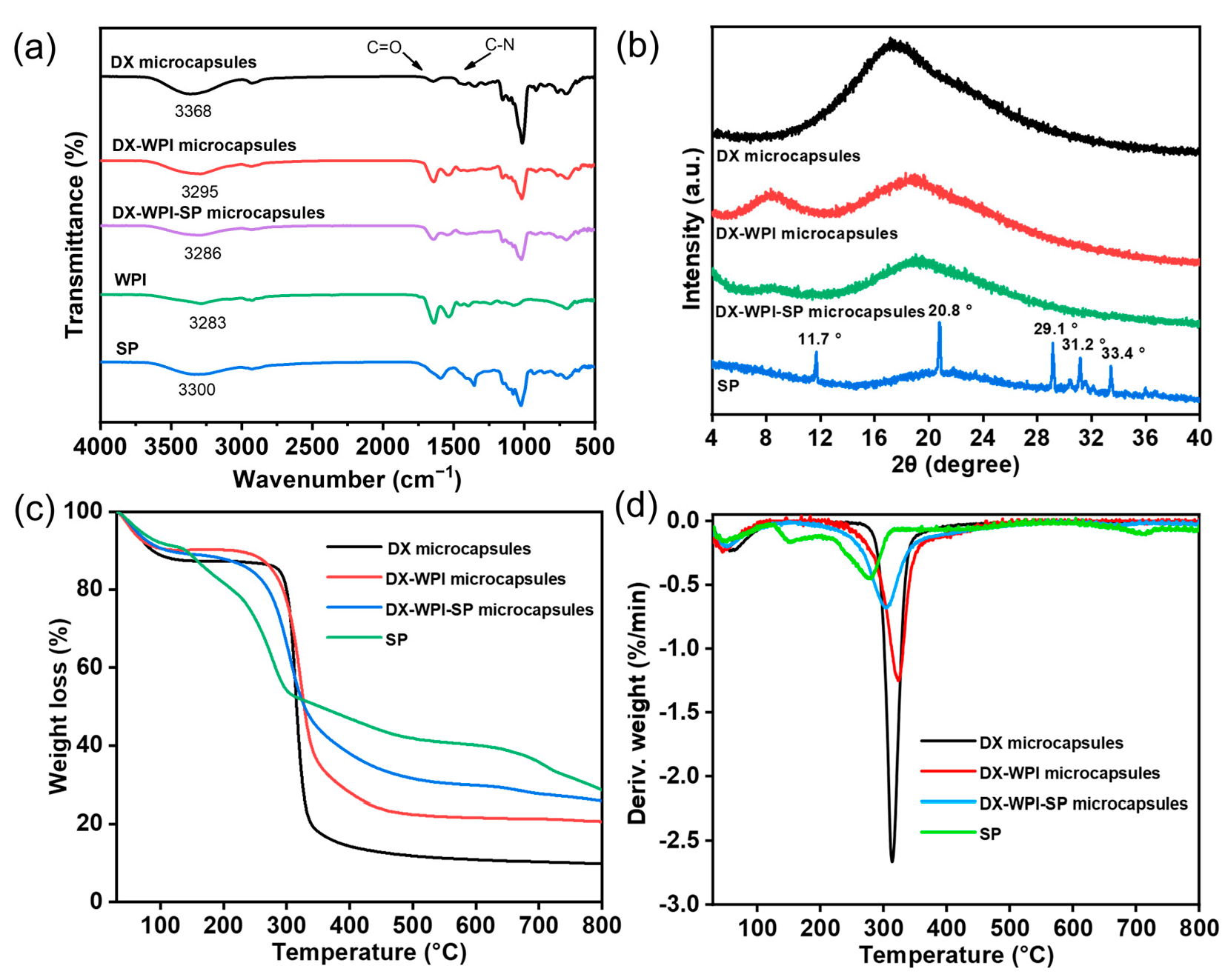
3.2. Structure and Characterization of DX-WPI-SP Microcapsules
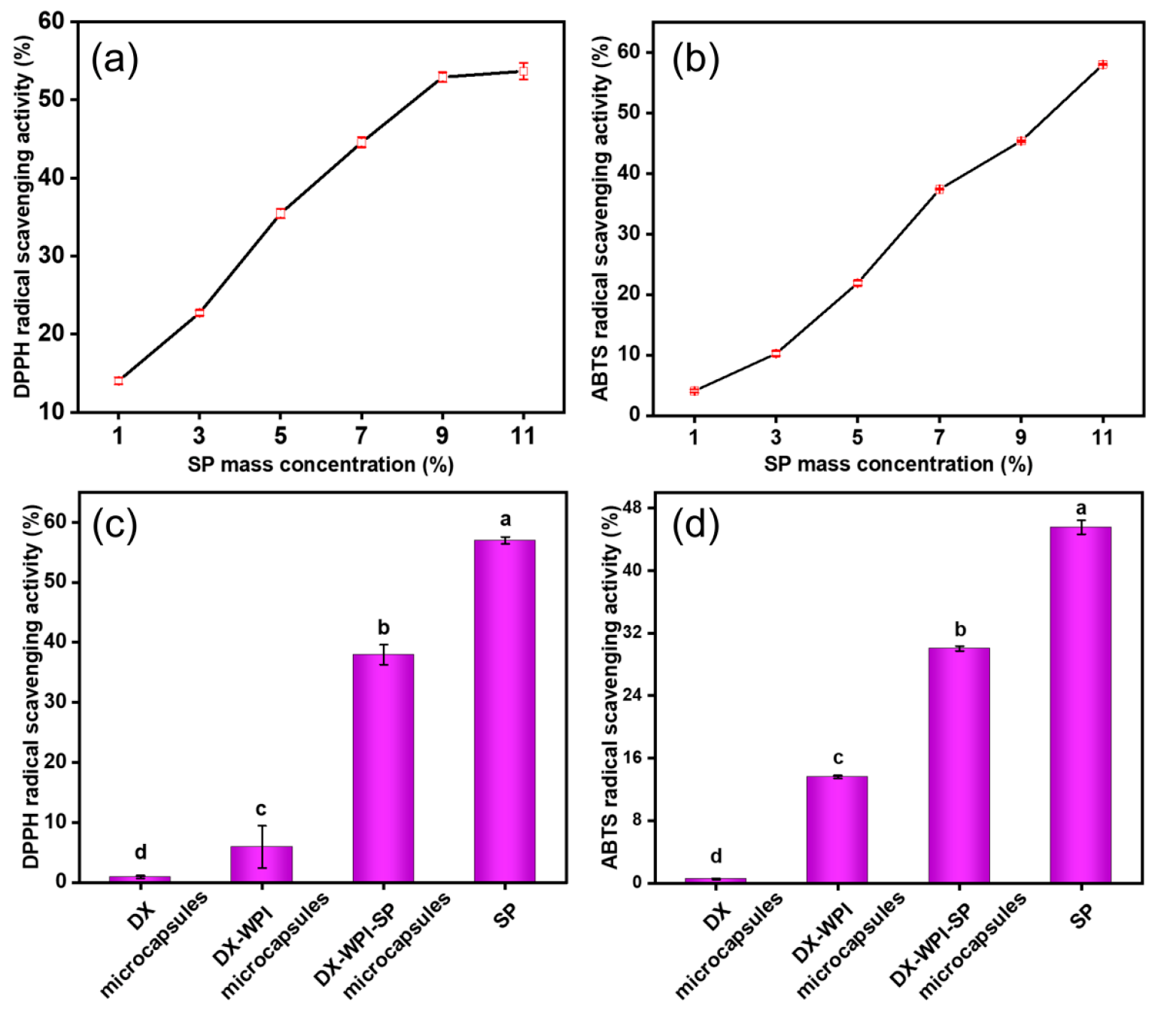
3.3. Antioxidant Activity Evaluation of DX-WPI-SP Microcapsules
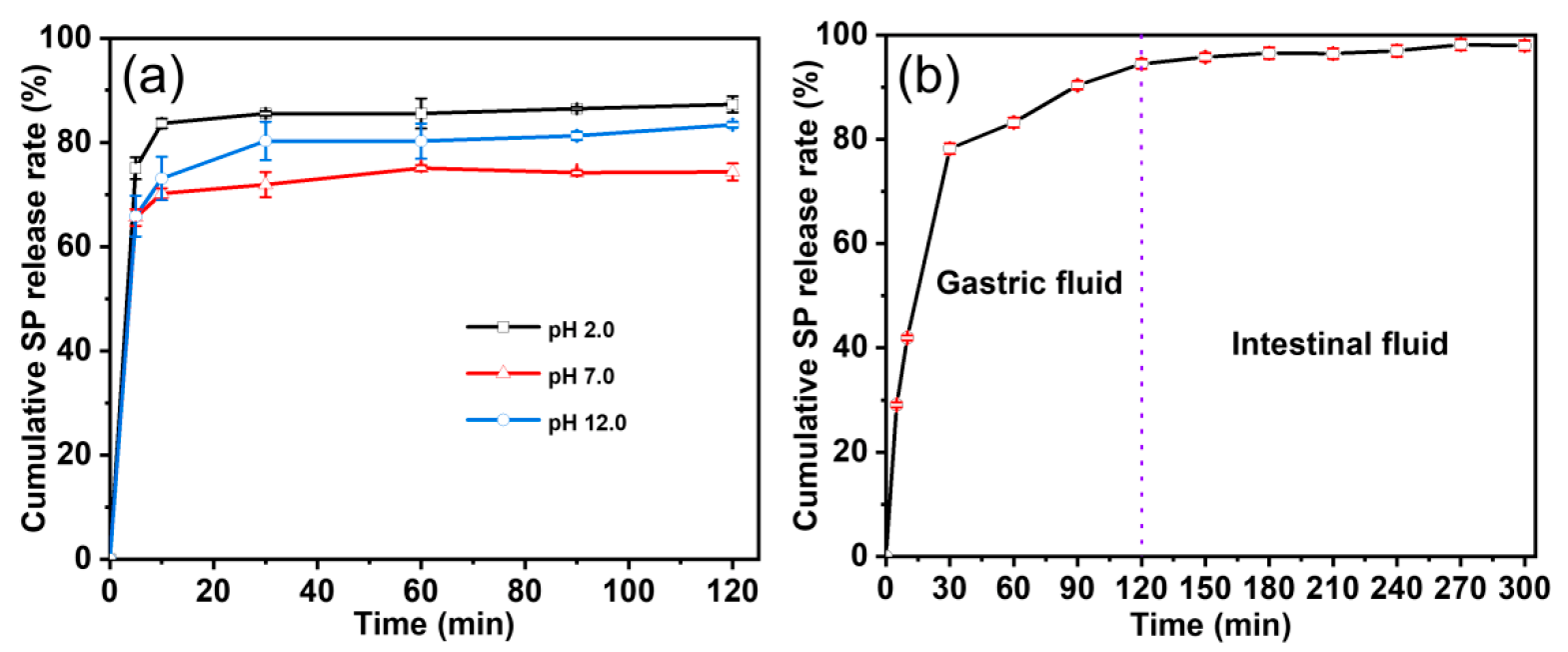
3.4. Controlled-Release Properties Evaluation Results
3.5. Cytotoxicity Study of Digested DX-WPI-SP Microcapsules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, N.; Zhao, C.; Zhang, T. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Raines, A.M.; Sunde, R.A. Selenium toxicity but not deficient or supernutritional selenium status vastly alters the transcriptome in rodents. BMC Genomics 2011, 12, 26. [Google Scholar] [CrossRef]
- Zhu, S.; Du, C.; Yu, T.; Cong, X.; Liu, Y.; Chen, S.; Li, Y. Antioxidant activity of selenium-enriched peptides from the protein hydrolysate of Cardamine violifolia. J. Food Sci. 2019, 84, 3504–3511. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Liu, G.; Xu, Y.; Wu, X.; Yi, R.; Jin, F.; Sa, C.; Su, X. Antioxidant stress and anticancer of peptide chelate selenium element in vitro. Int. J. Mol. Med. 2020, 48, 1–15. [Google Scholar] [CrossRef]
- Feng, M.; Wang, X.; Xiong, H.; Qiu, T.; Zhang, H.; Guo, F.; Jiang, L.; Sun, Y. Anti-inflammatory effects of three selenium-enriched brown rice protein hydrolysates in LPS-induced RAW264.7 macrophages via NF-κB/MAPKs signaling pathways. J. Funct. Foods 2021, 76, 104320. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, X.; Zhao, E.; Shi, Y.; Shen, X.; Wu, J.; Pei, F.; Hu, Q.; Qiu, W. Isolation and identification of immunomodulatory selenium-containing peptides from selenium-enriched rice protein hydrolysates. Food Chem. 2019, 275, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
- Wehrle, K.; Gallagher, E.; Neville, D.P.; Keogh, M.K.; Arendt, E.K. Microencapsulated high-fat powders in biscuit production. Z. Lebensm-Forsch. A 1999, 208, 388–393. [Google Scholar] [CrossRef]
- Tao, L.; Wang, P.; Zhang, T.; Ding, M.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil. Food Sci. Hum. Wellness 2023, 12, 397–406. [Google Scholar] [CrossRef]
- Comunian, T.; Babazadeh, A.; Rehman, A.; Shaddel, R.; Akbari-Alavijeh, S.; Boostani, S.; Jafari, S.M. Protection and controlled release of vitamin C by different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2022, 62, 3301–3322. [Google Scholar] [CrossRef]
- Gomez-Betancur, A.M.; Carmona-Tamayo, R.; Martínez-Álvarez, O.L.; Casanova-Yepes, H.; Torres-Oquendo, J.D. Effect of fat substitution using long-chain inulin and fortification with microencapsulated calcium in the rheological and sensory properties of yogurt mousse. J. Food Process. Eng. 2020, 45, e13433. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, D.; Wei, L.F.; Zhu, W.J.; Yan, X.Q.; Zhou, R.; Din, Z.U.; Ding, W.P.; Ma, T.Z.; Cai, J. Structural and mechanistic insights into starch microgel/anthocyanin complex assembly and controlled release performance. Int. J. Biol. Macromol. 2022, 213, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Bhandari, B.; Bai, B. Fennel essential oil loaded porous starch-based microencapsulation as an efficient delivery system for the quality improvement of ground pork. Int. J. Biol. Macromol. 2021, 172, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Choi, Y.J.; Jeong, G.J.; Im, G.I. Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 2013, 34, 5359–5368. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Zhang, W.; Fan, Z. Research progress of electrostatic spray technology over the last two decades. J. Energy Eng. 2021, 147, 03121003. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrost. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Xu, G.; Ye, X.; He, D.; Zhong, J. Micropattern of nano-hydroxyapatite/silk fibroin composite onto Ti alloy surface via template-assisted electrostatic spray deposition. Mater. Sci. Eng. C 2012, 32, 390–394. [Google Scholar] [CrossRef]
- Wang, P.; Ding, M.; Zhang, T.; Wu, T.; Qiao, R.; Zhang, F.; Wang, X.; Zhong, J. Electrospraying technique and its recent application advances for biological macromolecule encapsulation of food bioactive substances. Food Rev. Int. 2022, 38, 566–588. [Google Scholar] [CrossRef]
- Dhiman, A.; Suhag, R.; Singh, A.; Prabhakar, P.K. Mechanistic understanding and potential application of electrospraying in food processing: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8288–8306. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a novel process for the synthesis of particles/nanoparticles loaded with poorly water-soluble bioactive molecules. Adv. Colloid Interface Sci. 2021, 290, 102384. [Google Scholar] [CrossRef]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Dry. Technol. Int. J. 2016, 35, 139–162. [Google Scholar] [CrossRef]
- Costamagna, M.S.; Gómez-Mascaraque, L.G.; Zampini, I.C.; Alberto, M.R.; Pérez, J.; López-Rubio, A.; Isla, M.I. Microencapsulated chañar phenolics: A potential ingredient for functional foods development. J. Funct. Foods 2017, 37, 523–530. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Micro- and nano-encapsulation of beta-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Doherty, S.B.; Gee, V.L.; Ross, R.P.; Stanton, C.; Fitzgerald, G.F.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocolloids 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Du, X.; Hu, M.; Liu, G.; Yan, S.; Qi, B.; Zhang, S.; Huang, Y.; Li, Y.; Chen, H.; Zhu, X. Development of high-internal-phase emulsions stabilized by soy protein isolate-dextran complex for the delivery of quercetin. J. Sci. Food Agric. 2022, 102, 6273–6284. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Q.; Fan, D.; Xiao, J.; Zhao, Y.; Cheng, K.-W.; Wang, M. Novel roles of hydrocolloids in foods: Inhibition of toxic maillard reaction products formation and attenuation of their harmful effects. Trends Food Sci. Technol. 2021, 111, 706–715. [Google Scholar] [CrossRef]
- Paximada, P.; Kanavou, E.; Mandala, I.G. Effect of rheological and structural properties of bacterial cellulose fibrils and whey protein biocomposites on electrosprayed food-grade particles. Carbohydr. Polym. 2020, 241, 116319. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, G.; Tian, Y.; Kong, F.; Xiong, S.; Zhao, S.; Jia, D.; Manyande, A.; Du, H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Mulye, N.V.; Turco, S.J. A simple-model based on first-order kinetics to explain release of highly water-soluble drugs from porous dicalcium phosphate dihydrate matrices. Drug Dev. Ind. Pharm. 1995, 21, 943–953. [Google Scholar] [CrossRef]
- Laracuente, M.L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Controlled Release 2020, 327, 834–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Desai, K.G.H.; Tang, X.; Chen, X. Drug release kinetics of spray-dried chitosan microspheres. Dry. Technol. Int. J. 2007, 24, 769–776. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparticles containing diazepam: Preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Budincic, J.M.; Petrovic, L.; Dekic, L.; Fraj, J.; Bucko, S.; Katona, J.; Spasojevic, L. Study of vitamin E microencapsulation and controlled release from chitosan/sodium lauryl ether sulfate microcapsules. Carbohydr. Polym. 2021, 251, 116988. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT-Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, W.; Li, Q.; Zhao, J.; Feng, W.; Zhao, T.; Mao, G.; Chen, Y.; Wu, X.; Yang, L.; et al. Investigation of the uptake and transport of polysaccharide from Se-enriched Grifola frondosa in Caco-2 cells model. Int. J. Biol. Macromol. 2020, 158, 1330–1341. [Google Scholar] [CrossRef]
- Zhou, Y.; She, X.; Chen, Z.; Wei, Y.; Xiao, Y.; Zhou, X. Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) protein-derived antioxidant peptides: Mechanisms of action and structure-activity relationship in Caco-2 cell models. Food Sci. Hum. Wellness 2022, 11, 1580–1590. [Google Scholar] [CrossRef]
- Ye, Y.; Li, P.; Zhou, J.; He, J.; Cai, J. The improvement of sensory and bioactive properties of yogurt with the introduction of tartary buckwheat. Foods 2022, 11, 1774. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; Lagaron, J.M.; López-Rubio, A. Development and optimization of novel encapsulation structures of interest in functional foods through electrospraying. Food Bioprocess Technol. 2014, 7, 3236–3245. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak fitting applied to Fourier transform infrared and Raman spectroscopic analysis of proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- He, J.; He, Y.; Zhuang, J.; Zhang, H.; Lei, B.; Liu, Y. Luminescence properties of Eu3+/CDs/PVA composite applied in light conversion film. Opt. Mater. 2016, 62, 458–464. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Xu, X.; Zhuang, J.; Zhang, H.; Zhang, X.; Hu, C.; Lei, B.; Kaminski, C.F.; Liu, Y. Carbon dot-silica nanoparticle composites for ultralong lifetime phosphorescence imaging in tissue and cells at room temperature. Chem. Mater. 2019, 31, 9887–9894. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.-H.; Kim, C.S. Electrospun bioactive poly (ɛ-caprolactone)-cellulose acetate-dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Shawki, M.M.; Hereba, A.M.; Ghazal, A. Formation and characterisation of antimicrobial dextran nanofibers. Rom. J. Bio-Phys. 2010, 20, 335–346. [Google Scholar]
- Can, H.K.; Kavlak, S.; ParviziKhosroshahi, S.; Guner, A. Preparation, characterization and dynamical mechanical properties of dextran-coated iron oxide nanoparticles (DIONPs). Artif. Cells Nanomed. Biotechnol. 2018, 46, 421–431. [Google Scholar] [CrossRef]
- Bora, A.F.M.; Kouame, K.J.E.-P.; Li, X.; Liu, L.; Sun, Y.; Ma, Q.; Liu, Y. Development, characterization and probiotic encapsulating ability of novel Momordica charantia bioactive polysaccharides/whey protein isolate composite gels. Int. J. Biol. Macromol. 2023, 225, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, R.G.K.; Rahima, M.; Chatterjee, N.S.; Tejpal, C.S.; Anas, K.K.; Vishnu, K.V.; Sarika, K.; Asha, K.K.; Anandan, R.; Suseela, M. Chitosan–Whey protein as efficient delivery system for squalene: Characterization and functional food application. Int. J. Biol. Macromol. 2019, 135, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xiao, Y.; Liu, E.; Su, Z.; Meng, X.; Liu, B. Preparation of Ca-alginate-whey protein isolate microcapsules for protection and delivery of L. bulgaricus and L. paracasei. Int. J. Biol. Macromol. 2020, 163, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Onwulata, C.I.; Isobe, S.; Tomasula, P.M.; Cooke, P.H. Properties of whey protein isolates extruded under acidic and alkaline conditions. J. Dairy Sci. 2006, 89, 71–81. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Li, T.; Sun, D.; Hou, J.; Li, A.; Jiang, Z. Impact of ultrasonic power on the structure and emulsifying properties of whey protein isolate under various pH conditions. Process. Biochem. 2019, 81, 113–122. [Google Scholar] [CrossRef]
- Mine, Y. Recent advances in the understanding of egg white protein functionality. Trends Food Sci. Technol. 1995, 6, 225–232. [Google Scholar] [CrossRef]
- Kim, J.C.; Lund, D.B. Milk protein stainless steel interaction relevant to the initial stage of fouling in thermal processing. J. Food Process. Eng. 1998, 21, 369–386. [Google Scholar] [CrossRef]
- Ge, M.; Li, Y.; Zhu, C.; Liang, G.; Jahangir Alam, S.M.; Hu, G.; Gui, Y.; Junaebur Rashid, M. Preparation of organic-modified magadiite-magnetic nanocomposite particles as an effective nanohybrid drug carrier material for cancer treatment and its properties of sustained release mechanism by Korsmeyer-Peppas kinetic model. J. Mater. Sci. 2021, 56, 14270–14286. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Skalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Enhancing bioactive peptide release and identification using targeted enzymatic hydrolysis of milk proteins. Anal. Bioanal. Chem. 2018, 410, 3407–3423. [Google Scholar] [CrossRef]
- Majidiyan, N.; Hadidi, M.; Azadikhah, D.; Moreno, A. Protin complex nanoparticles reinforced with industrial hemp essential oil: Characterization and application for shelf-life extension of rainbow trout fillets. Food Chem. X 2022, 13, 100202. [Google Scholar] [CrossRef] [PubMed]
- Hesami, S.; Safi, S.; Larijani, K.; Badi, H.N.; Abdossi, V.; Hadidi, M. Synthesis and characterization of chitosan nanoparticles loaded with greater celandine (Chelidonium majus L.) essential oil as an anticancer agent on MCF-7 cell line. Int. J. Biol. Macromol. 2022, 194, 974–981. [Google Scholar] [CrossRef]
- Ye, Y.Y.; He, J.L.; He, Z.J.; Zhang, N.; Liu, X.Q.; Zhou, J.J.; Cheng, S.Y.; Cai, J. Evaluation of the brewing characteristics, digestion profiles, and neuroprotective effects of two typical Se-enriched green teas. Foods 2022, 11, 2159. [Google Scholar] [CrossRef] [PubMed]
- Anani, J.; Noby, H.; Zkria, A.; Yoshitake, T.; ElKady, M. Monothetic analysis and response surface methodology optimization of calcium alginate microcapsules characteristics. Polymers 2022, 14, 709. [Google Scholar] [CrossRef]
- Wang, P.P.; Li, M.; Wei, D.X.; Ding, M.Z.; Tao, L.N.; Liu, X.W.; Zhang, F.P.; Tao, N.P.; Wang, X.C.; Gao, M.Y.; et al. Electrosprayed soft capsules of millimeter size for specifically delivering fish oil/nutrients to the stomach and intestines. ACS Appl. Mater. Interfaces 2020, 12, 6536–6545. [Google Scholar] [CrossRef]
- Garcia, P.; Vega, J.; Jimenez, P.; Santos, J.; Robert, P. Alpha-tocopherol microspheres with cross-linked and acetylated inulin and their release profile in a hydrophilic model. Eur. J. Lipid Sci. Technol. 2013, 115, 811–819. [Google Scholar] [CrossRef]
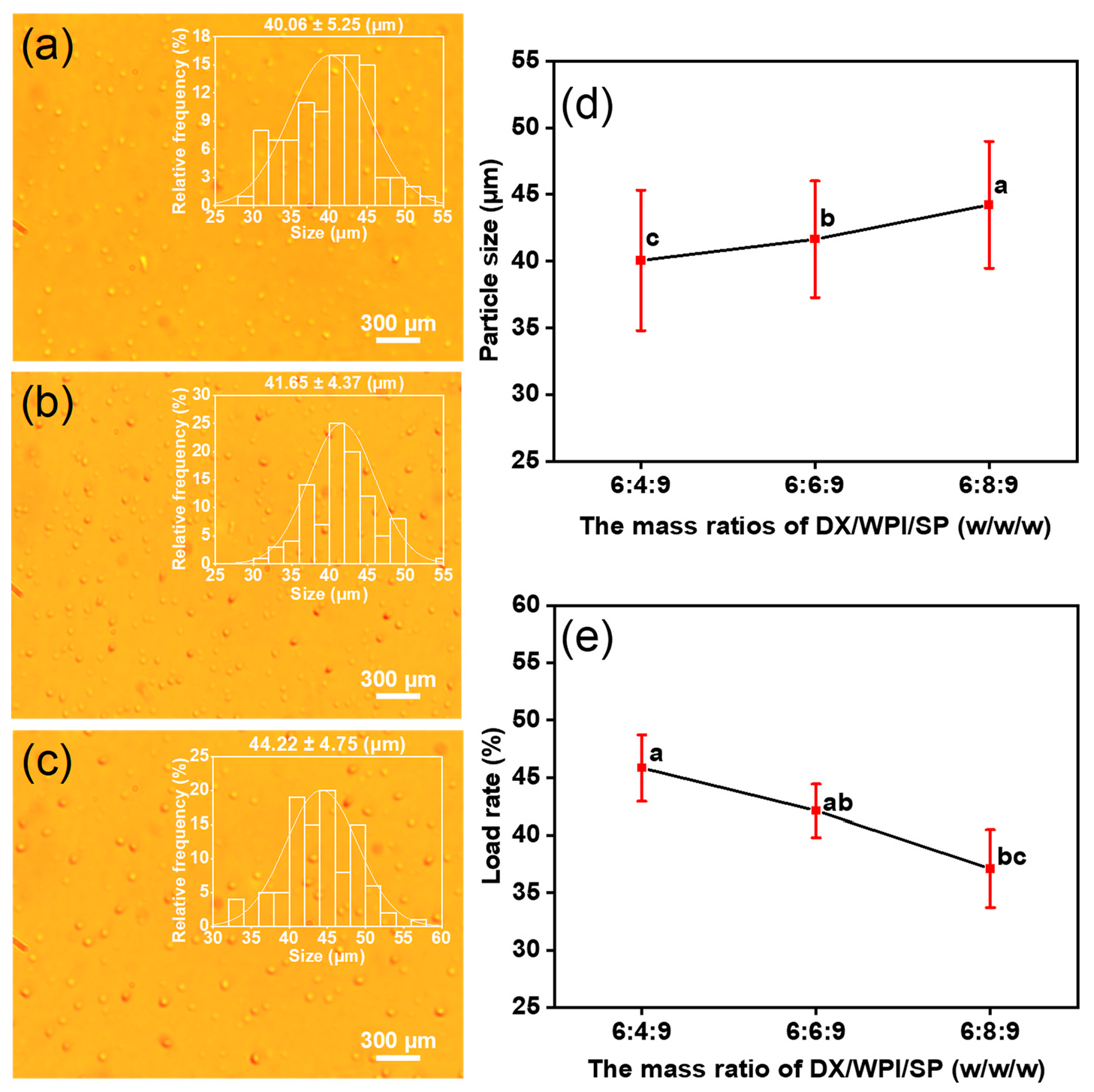

| Microcapsule | DX (g) | WPI (g) | SP (g) | Voltage (kV) | Distance (cm) | Feeding Rate (mL/h) | Diameter (μm) |
|---|---|---|---|---|---|---|---|
| DX microcapsules | 0.6 | - | - | 15 | 15 | 1.0 | 26.47 ± 3.50 |
| DX-WPI microcapsules | 0.6 | 0.2 | - | 15 | 15 | 1.0 | 30.31 ± 2.91 |
| 0.6 | 0.4 | - | 15 | 15 | 1.0 | 30.75 ± 3.64 | |
| 0.6 | 0.6 | - | 15 | 15 | 1.0 | 33.42 ± 3.09 | |
| 0.6 | 0.8 | - | 15 | 15 | 1.0 | 34.38 ± 4.07 | |
| 0.6 | 1.0 | - | 15 | 15 | 1.0 | 35.83 ± 3.64 | |
| DX-WPI-SP microcapsules | 0.6 | 0.4 | 0.9 | 15 | 15 | 1.0 | 40.06 ± 5.25 |
| 0.6 | 0.6 | 0.9 | 15 | 15 | 1.0 | 41.65 ± 4.37 | |
| 0.6 | 0.8 | 0.9 | 15 | 15 | 1.0 | 44.22 ± 4.75 |
| Digestive Solution at Different Dilutions | Total Selenium Content (μg/mL) | Cell Viability (%) | p Value a |
|---|---|---|---|
| Control | - | 100 | - |
| Digestive solution | 45.72 ± 2.98 | 97.21 ± 2.57 a | 0.9127 ns |
| Digestive solution-2 | 22.86 ± 1.49 | 98.33 ± 2.90 a | 0.9925 ns |
| Digestive solution-10 | 4.57 ± 0.30 | 95.94 ± 4.00 a | 0.6711 ns |
| Digestive solution-20 | 2.29 ± 0.15 | 97.94 ± 5.22 a | 0.9783 ns |
| Digestive solution-50 | 1.14 ± 0.06 | 97.58 ± 0.66 a | 0.9533 ns |
| Digestive solution-100 | 0.57 ± 0.03 | 96.69 ± 2.93 a | 0.8298 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wang, Z.; Wei, L.; Ye, Y.; Din, Z.-u.; Zhou, J.; Cong, X.; Cheng, S.; Cai, J. Electrospray-Assisted Fabrication of Dextran–Whey Protein Isolation Microcapsules for the Encapsulation of Selenium-Enriched Peptide. Foods 2023, 12, 1008. https://doi.org/10.3390/foods12051008
He J, Wang Z, Wei L, Ye Y, Din Z-u, Zhou J, Cong X, Cheng S, Cai J. Electrospray-Assisted Fabrication of Dextran–Whey Protein Isolation Microcapsules for the Encapsulation of Selenium-Enriched Peptide. Foods. 2023; 12(5):1008. https://doi.org/10.3390/foods12051008
Chicago/Turabian StyleHe, Jiangling, Zhenyu Wang, Lingfeng Wei, Yuanyuan Ye, Zia-ud Din, Jiaojiao Zhou, Xin Cong, Shuiyuan Cheng, and Jie Cai. 2023. "Electrospray-Assisted Fabrication of Dextran–Whey Protein Isolation Microcapsules for the Encapsulation of Selenium-Enriched Peptide" Foods 12, no. 5: 1008. https://doi.org/10.3390/foods12051008
APA StyleHe, J., Wang, Z., Wei, L., Ye, Y., Din, Z.-u., Zhou, J., Cong, X., Cheng, S., & Cai, J. (2023). Electrospray-Assisted Fabrication of Dextran–Whey Protein Isolation Microcapsules for the Encapsulation of Selenium-Enriched Peptide. Foods, 12(5), 1008. https://doi.org/10.3390/foods12051008








